www.elsevier.com / locate / livprodsci
Sow factors affecting voluntary feed intake during lactation
a ,
*
a bJ.J. Eissen
, E. Kanis , B. Kemp
a
Animal Breeding and Genetics Group, Wageningen Institute of Animal Sciences, Wageningen Agricultural University, P.O. Box 338, 6700 AH Wageningen, The Netherlands
b
Animal Health and Reproduction Group, Wageningen Institute of Animal Sciences, Wageningen Agricultural University, P.O. Box 338, 6700 AH Wageningen, The Netherlands
Received 3 March 1999; received in revised form 18 August 1999; accepted 23 September 1999
Abstract
Genetic and environmental changes during the last few decades have resulted in higher milk production and maintenance costs of lactating sows, leading to increased energy requirements, whereas the amount of body fat reserves of, in particular, young immature sows have decreased and voluntary feed intake may have decreased. As a consequence, present voluntary feed intake of sows during lactation is frequently inadequate to meet nutrient demands. This may influence subsequent reproduction. In this paper, it is argued that voluntary feed intake of lactating sows should be included in breeding programmes. To underpin this statement, it is reviewed how the sow factors body weight and body composition at farrowing, litter size during lactation, parity and genotype affect voluntary intake during lactation and possible physiological mechanisms are provided. It is concluded that, for sustainable pig production, the trends of decreasing fat reserves at farrowing and increasing energy requirements during lactation should be accompanied by a higher feed intake capacity during lactation. Genotype seems the most appropriate sow factor that can be used to realise the desired changes and selection for a higher voluntary feed intake during lactation is recommended. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Body composition; Feed intake; Genotype; Lactation; Litter size; Parity; Sows
1. Introduction an attempt to maintain milk production (NRC,
1987). Recent work cited below illustrates that Voluntary feed intake of young and immature nowadays nutrition is more critical for reproduction sows during lactation is frequently inadequate to than in the past. For example, earlier work of Elsley meet nutrient demands for maintenance, milk pro- et al. (1969) and O’Grady et al. (1973) clearly duction and body growth (Noblet et al., 1990). Milk indicated no relationship between lactational feed production has a high priority and, if nutrient intake intake and subsequent reproduction performance. In is insufficient, the sow will mobilise body tissue in recent studies, however, a low feed intake during lactation, accompanied by excessive weight loss, was found to be associated with several common
re-*Corresponding author. Tel.:131-317-482-335; fax:1
31-317-productive problems, including an increased interval
483-929.
E-mail address: [email protected] (J.J. Eissen) from weaning to oestrus (Reese et al., 1982; King
and Williams, 1984; Kirkwood et al., 1987a,b, 1990; illustrations that selection for production traits may Baidoo et al., 1992), an increased incidence of affect voluntary feed intake. Breeding programmes anoestrus (Kirkwood et al., 1987a,b), a lower ovula- putting a high emphasis on production efficiency or tion rate (Zak et al., 1997), a decreased conception leanness rather than on rate of lean growth, can rate (Kirkwood et al., 1987a,b) and a higher em- reduce the appetite of growing pigs (Smith and bryonic mortality (Kirkwood et al., 1987a, 1990; Fowler, 1978; Ellis et al., 1979, 1983; Smith et al., Baidoo et al., 1992). The longer length of lactation in 1991). A reduction in appetite during the growth the earlier works may have contributed to the lower phase can also be reflected by sows during lactation sensitivity of sows in the past, because sows mainly (Kerr and Cameron, 1996b).
lose body reserves during the first 2–3 weeks of Many factors affect spontaneous feed intake dur-lactation and start to recover afterwards (Revell and ing lactation. For convenience they can be grouped Williams, 1993). All of the above-mentioned studies under three main headings, although some of them restricted feed intake during at least part of the interact with each other (Revell and Williams, 1993). lactation in controlled experiments. However, data The three factors are sow (e.g. body weight and analyses of (close to) ad libitum fed sows on composition, litter size, parity, genotype), environ-commercial farms also show that a higher feed intake ment (e.g. temperature, air quality, management, during lactation may improve reproductive perform- length of lactation, stock density, disease incidence) ance (Koketsu et al., 1996b, 1997; Koketsu and Dial, and diet (e.g. digestibility, composition, energy den-1997). sity, protein and amino acid balance, availability of According to Whittemore (1996), this turn of water, feeding frequency). As indicated above, cur-events may have resulted from a change in the pig’s rent selection strategies result in increasing energy genetic make-up. In dam lines, selection has general- requirements of sows due to higher milk production ly been for production and reproduction traits. Selec- and maintenance costs, whereas the amount of fat tion for production traits has resulted in an increase reserves of young sows is decreasing and voluntary in growth rate, a reduction in backfat and an feed intake may be decreasing. Nowadays, inade-improved feed efficiency during the growth phase quate feed intake during lactation is particularly (e.g. Vangen and Kolstad, 1986). These changes evident in primiparous sows (NRC, 1987), sows fed during the growth phase are reflected at later stages generously during gestation (Baker et al., 1968; in a reduction in the amount of fat in the body of Dourmad, 1991) and sows in a hot environment young sows at the times of parturition and weaning (NRC, 1987). Therefore, it can be argued that (Whittemore, 1996). Furthermore, maintenance re- voluntary feed intake during lactation should be quirements at maturity are higher due to a higher considered in breeding programmes. The aim of this mature body weight. Sows also have higher mainte- paper is to review and provide possible physiological nance requirements due to the lower fatness (Camp- mechanisms for the way in which the mentioned sow bell and Taverner, 1988). The common breeding factors affect voluntary feed intake during lactation objective for reproduction focuses on the number of and to investigate if selection for feed intake during piglets weaned per sow per year (Knap, 1990). The lactation should be recommended.
effect of selection for reproduction traits is illustrated by the positive genetic trends for litter size in current
dam lines (Knap et al., 1993). Litter size also tends 2. Voluntary feed intake to increase due to environmental improvements
(Southwood and Kennedy, 1991). As a result of 2.1. Control of voluntary feed intake in general indirect selection, increased litter size and / or
regula-tion; Mayer, 1953), body fatness (lipostatic regula- intake is regulated by mechano-, chemo- and os-tion; Kennedy, 1953) or on a constant body tempera- moreceptors and by release of hormones, e.g. ture (thermostatic control; Brobeck, 1948). Nowa- cholecystokinin (Scharrer, 1991). Stomach disten-days, the various theories are no longer considered as sion is signaled to the brain through vagal afferents alternatives but rather as complementary to each (Gonzales and Deutsch, 1981), whereas humoral and other; together they contribute to a multifactorial nervous signals inform the brain about the presence control system (Forbes, 1988). of nutrients in the small intestinal lumen (Stephens, The control of feed intake is extremely complex 1985). At the post-absorptive level, circulating nu-and involves central as well as peripheral mecha- trients in the blood are important and liver, brain and nisms. The primary site responsible for the integrated body reserves seem to be involved in the regulation control of feed intake and energy balance is the of feed intake (Scharrer, 1991).
central nervous system (CNS), although the specific
mechanisms involved are not well understood (NRC, 2.2. Long-term versus short-term regulation 1987). Peptides found in the CNS have been shown
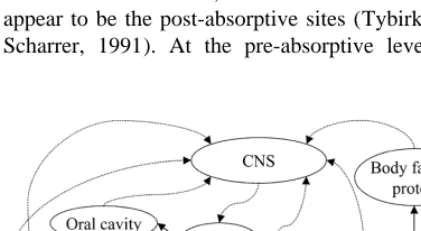
to have a direct effect on the control of metabolism Voluntary feed intake is regulated at two levels and feed intake. For instance, the onset of feeding (Revell and Williams, 1993). The first is short-term may be affected by opioid peptides, whereas termi- regulation, which involves the factors regulating nation of feeding may involve cholecystokinin. A meal eating behaviour, i.e. meal size and meal number of CNS centres and, most likely, peripheral length. The second is long-term regulation, which receptor systems exist that provide information about determines the average daily intake over a period of the animal’s metabolic state. A coordinated feeding time. The daily feed intake of an animal is the behaviour is established via these receptor systems summation of intake during individual meals. While and CNS centres (NRC, 1987). meal size can vary greatly, the total quantity eaten Fig. 1 shows a diagram of a plain general model each day, for example, must be controlled to main-of feed intake regulation main-of a lactating sow. Stimuli tain energy homeostasis. The signals of satiety that that modulate feeding behaviour act at the pre-ab- control meal size must have shorter time constants sorptive (physical regulation) and / or post-absorptive than the signals that regulate long-term energy (metabolic regulation) level. The oral cavity, stomach balance (NRC, 1987). If animals are confronted with and small intestine are the pre-absorptive sites of periodic feed-associated stimuli, variable feed availa-action of these stimuli, whereas the liver and brain bility, changing social situations or novel stimuli, appear to be the post-absorptive sites (Tybirk, 1989; they readily modify their eating pattern while main-Scharrer, 1991). At the pre-absorptive level, feed taining long term energy homeostasis (Woods et al.,
1998).
Signals from the gastrointestinal tract are likely to be of major importance in the short-term control of voluntary feed intake since feed ingestion ceases before the meal has been completely digested and absorbed (Rayner and Gregory, 1989). The size of each meal appears to be regulated by rapidly acting negative feedback controls initiated by the presence of feed in the gastrointestinal tract involving a quick qualitative and quantitative evaluation of the feed (Houpt, 1984; Le Magnen and Devos, 1984; Forbes, 1988). The products of a meal (nutrients) can be more accurately monitored after the meal
(postpran-Fig. 1. Schematic diagram of a general model of feed intake
dial) and used to determine the onset of the next
regulation of a lactating sow. Solid arrows represent flows of
meal. Meal to meal intervals, and therefore meal
nutrients whereas dashed arrows represent information signals
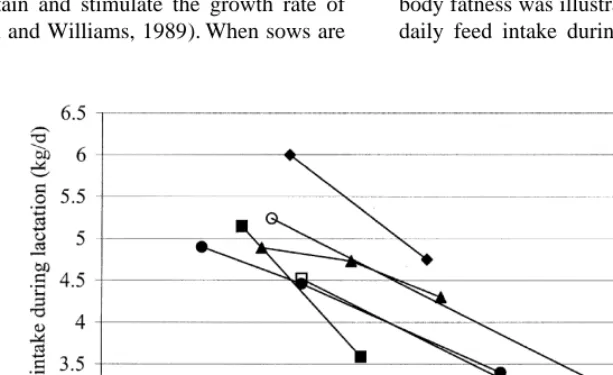
3. Sow factors allowed a high feeding level or ad libitum access to feed during gestation, they consume more feed than The interrelationships between milk production, needed to meet their energy requirement during changes in body weight and composition, and vol- gestation. Sows fed a high gestation feeding level, untary feed intake during lactation (Fig. 1) are however, have a lower voluntary feed intake during complex and the controls of partitioning of nutrients lactation than sows fed according to their require-between milk secretion and deposition or mobilisa- ments during gestation, and mobilise more reserves tion of body reserves are poorly understood. The during lactation (e.g. Mullan and Williams, 1989; physiological drive of lactating sows to produce milk Yang et al., 1989; Dourmad, 1991; Weldon et al., at the expense of other body functions is, however, a 1994a; Xue et al., 1997; Revell et al., 1998a; Fig. 2). key component of the metabolic state of lactating Le Cozler et al. (1998a) showed that a higher feeding sows and is controlled by factors like litter size, level during rearing (ad libitum vs. 80% of ad parity and genotype (Pettigrew et al., 1993). It will libitum) also led to a lower voluntary feed intake be discussed below how the sow factors body weight during lactation (Fig. 2). Points are connected per and body composition at farrowing, parity, litter size study in Fig. 2 to indicate that each study shows an and genotype may affect voluntary feed intake of effect of a similar magnitude.
lactating sows. A higher feeding level during rearing and / or gestation generally results in a higher body weight 3.1. Body weight and body composition at and body fatness of sows at farrowing. The higher
farrowing body fatness at farrowing seems to be associated
with the lower lactation feed intake (Dourmad, 1991; Because feed intake during early lactation is Williams and Smits, 1991; Revell et al., 1998a), generally too low to meet the energy requirements, whereas the effect of body weight at farrowing high producing sows mobilise body reserves to seems to be small (O’Grady et al., 1985; Williams supply energy and nutrients for milk production and and Smits, 1991; Weldon et al., 1994a). The effect of hence to maintain and stimulate the growth rate of body fatness was illustrated by regression analyses of piglets (Mullan and Williams, 1989). When sows are daily feed intake during lactation on backfat
thick-Fig. 2. Relationship between daily feed intake during rearing (♦) or gestation (other symbols) and voluntary feed intake during lactation of a sow:jRevell et al., (1998a);dMullan and Williams (1989);mDourmad (1991);♦Le Cozler et al., (1998a);hXue et al., (1997);s
ness at farrowing: Yang et al. (1989) estimated a directly into the blood circulation and very little
21 21
slope of 218 and 2129 g day mm for glycerol is reesterified into triacylglycerides in adip-primiparous and multiparous sows, respectively, ose cells. Nonesterified fatty acids, on the other
21
Dourmad (1991) estimated a slope of 263 g day hand, may be reesterified into triacylglycerides to a
21
mm for primiparous sows and Koketsu et al. larger extent than glycerol before leaving adipocytes
21 21
(1996a) estimated a slope of 219 g day mm (Revell et al., 1998a). Levels of NEFA are therefore across all parities. better indicators for fat mobilisation than for body
Body weight and fat depots influence feed intake fatness per se.
presumably by modulation of long-term regulation Concentrations in blood of NEFA during late mechanisms. The various control mechanisms are gestation were not significantly affected by rearing or either independently unique in action or synergistic gestation feeding level (Weldon et al., 1994a; Revell and may vary according to the phase of the lactation et al., 1998a; Le Cozler et al., 1998b). Levels of period. How these may interact remains to be glycerol, however, were significantly higher in fat elucidated. Several studies focused on one or two of sows, which supports the mechanism described the mechanisms. Most of these studies used different, above (Revell et al., 1998a). During the first weeks usually two, feeding levels during rearing or gesta- of lactation, levels of NEFA and glycerol were tion resulting in relatively fat and lean sows at always higher in fat than lean sows (Weldon et al., farrowing. During lactation all sows were fed ad 1994a,b; Revell et al., 1998a; Le Cozler et al., libitum. Mechanisms possibly explaining the effect 1998b). However, it is not clear whether these higher of body composition on lactation feed intake of sows levels found post partum in fat sows cause, or are a are turnover of body fat tissue, insulin and leptin consequence of, the lower feed intake of fat sows, levels in blood and cerebrospinal fluid, presence of involving another mechanism.
insulin resistance and glucose intolerance, and levels
of milk production and body protein reserves. These 3.1.2. Insulin and leptin
level during gestation would, therefore, lead to intolerance presumably results in a lower clearance higher levels of both in cerebrospinal fluid at farrow- rate of glucose from the blood after a meal. As a ing, which may inhibit feed intake (Williams, 1998; consequence, use of peripheral glucose is likely Woods et al., 1998). Insulin and leptin then would decreased and voluntary feed intake may be reduced act as a homeostatic mechanism for body fat at the to maintain blood glucose concentrations. Further-brain level. more, lower blood insulin levels as a result of No references were found in which concentrations glucose intolerance may enhance the mobilisation of leptin and insulin in the cerebrospinal fluid or and oxidation of stored adipose tissue as oxidation of concentrations of leptin in blood of fat and lean sows NEFA is depressed to a lesser degree. The latter also were measured. Xue et al. (1997), Revell et al. may reduce voluntary feed intake.
not affect rate of glucose clearance; however, a similarly affected during gestation, which is sup-higher peak insulin secretion was observed for fat ported by the fact that Head et al. (1991), Head and sows, showing that fat sows may be more resistant to Williams (1991, 1995) and Revell et al. (1998b) insulin. During mid lactation, fat sows showed a used suboptimal diets during gestation in order to poorer glucose tolerance and appeared to be more support fat deposition at the expense of lean deposi-resistant to insulin than lean sows after glucose tion. These diets were used to create fat sows at infusion. It seems, therefore, that impaired glucose farrowing that had a similar net weight gain during clearance is more likely caused by body composition gestation compared with lean sows at farrowing, at farrowing per se, than by a high feeding level which received a more optimal diet during gestation. during the preceding gestation.
In conclusion, it seems that differences in glucose 3.1.5. Body protein reserves
intolerance and insulin resistance between fat and Another reason for fat sows to have a lower lean sows may, at least partly, explain the lower voluntary feed intake might be the lower supply of voluntary lactation feed intake of fat sows, although endogenous substrates for milk production (Wil-results are not fully unambiguous. Results of the liams, 1998), which could also be linked to the studies in which glucose was infused may differ due reduced milk output of fat sows in the studies of to the dose used, as Weldon et al. (1994b) and Xue et Head et al. (1991), and Head and Williams (1991, al. (1997) infused 1 g glucose per kg body weight, 1995). Fat sows have less protein reserves to supply whereas Revell et al. (1998a) and Le Cozler (1998b) substrates for milk production compared with lean infused 0.06 g and 0.5 g per kg body weight, animals at a similar weight (Revell et al., 1998a). In respectively. studies that changed feeding level during gestation and not diet composition, fat sows were heavier at 3.1.4. Milk production farrowing (Weldon et al., 1994a,b; Xue et al., 1997; Head et al. (1991) and Head and Williams (1991) Le Cozler et al., 1998a,b) and may have had a reported that fat sows, in comparison with lean sows, similar or even higher amount of body protein had a lower capacity to secrete energy in milk reserves than lean sows. If milk output is limited by because they had fewer milk secretory cells. This the supply of endogenous amino acids, then the may have caused the significantly reduced litter capacity of the animal to produce milk is reduced. growth of the fat sows reported by Head and Limited body protein reserves may therefore reduce Williams (1995). Revell et al. (1998b) reported that milk production and hence the voluntary feed intake milk yield was about 15% higher in lean than fat of sows (Williams, 1998).
sows, which was also reflected in litter growth. A Mahan and Mangan (1975) found that voluntary lower milk production may diminish the drive to eat feed intake during lactation was reduced when sows and reduce voluntary feed intake of sows (Fig. 1). In were fed a diet low in protein during gestation and most other studies, however, litter growth was not lactation. However, voluntary feed intake during affected by a high gestation feeding level and fatness lactation was not reduced when the diet during at farrowing, indicating that the effect of fewer milk gestation was high in protein, indicating that endog-secretory cells of fat sows was probably limited (e.g. enous protein reserves may limit voluntary feed Dourmad, 1991; Weldon et al., 1994a,b; Xue et al., intake under certain circumstances. Mahan (1998)
1997). found that multiparous sows consumed more feed
In dairy cattle, rapid rates of growth in the during the first week of lactation and primiparous prepubertal period are associated with substantial sows during the whole lactation when offered a diet reductions in milk production in all subsequent with a higher protein content during gestation. Revell lactations (Little and Kay, 1979; Sejrsen et al., et al. (1998a) used a low and high protein diet during 1982). Recent reports suggest that the deleterious lactation. The dietary supply of protein increased effect is associated with feeding heifers a diet with voluntary feed intake during weeks 3 and 4 of an inadequate protein:energy balance resulting in an lactation, possibly by increasing milk production and excessive fat deposition during the prepubertal hence the drive to consume feed.
21 21
milk production and, therefore, voluntary feed intake estimated slope was 263 g day mm for aver-of sows during lactation. However, protein reserves age feed intake during the whole lactation, whereas
21 21
only seem a limiting factor for feed intake when the the slope was 295 g day mm when only the protein supply of the lactation diet is not optimal in average feed intake during week 1 of lactation was relation to the body composition of the sow. considered.
These results are not surprising as differences in 3.1.6. Meal eating behaviour fatness between fat and lean sows get smaller during Dourmad (1993) and Weldon et al. (1994a) the course of lactation due to the greater losses of studied meal eating behaviour during lactation of tissue of fat sows during the early phase of lactation sows that were fed (close to) ad libitum or restricted (Le Cozler et al., 1999).
during gestation, respectively. Weldon et al. (1994a)
found that ad libitum fed, and thus fatter sows, had a 3.1.8. Optimum body composition at farrowing lower daily feed intake during lactation by eating It is clear that the sow’s feed intake during fewer meals rather than smaller meals. In contrast, lactation is controlled in some way and that the Dourmad (1993) found that fat sows had smaller ingested nutrients are integrated with body reserves. meals, which were shorter in duration rather than The amount of body reserves at farrowing has an fewer meals, especially during the first 2 weeks of important influence on subsequent reproductive per-lactation. Both used a meal criterion of ,10 min to formance because it determines the extent that summarise information of feeding bouts into meals. reserves can be mobilized during lactation without Results of Weldon et al. (1994a) suggest that gas- affecting the interval between weaning and sub-trointestinal signals mainly affect meal ending, sequent mating (Mullan and Williams, 1989). High whereas results of Dourmad (1993) suggest that a body reserves at farrowing lead to a reduced vol-metabolic control was also partly involved. Both untary feed intake during lactation, as shown above, studies agree that fat sows use more time to absorb and excessive weight loss. Excessive weight loss has and utilise ingested nutrients before starting the next been associated with several common reproductive meal. This is reflected by the larger meal to meal problems as already mentioned in Section 1. Also, interval in case of a similar meal size (Weldon et al., overfeeding during gestation is not recommended 1994a) and a similar meal to meal interval in case of because of the increased frequency of farrowing smaller meals (Dourmad, 1993). These results may problems in fat sows (Dourmad et al., 1994). Low point towards a higher level of insulin resistance and feed allowances during gestation lead to an increased glucose intolerance of fat sows compared with lean voluntary feed intake and consequently less weight sows. loss of sows during lactation. The increase in vol-untary feed intake during lactation, however, gener-3.1.7. Effect of body fatness at farrowing on ally does not compensate for the lower intake during
voluntary lactation feed intake in relation to stage gestation. Therefore, feed intake over the whole
of lactation cycle of gestation and lactation is reduced when a
Revell et al. (1998a) concluded that, during the normal lactation length of about 4 weeks is consid-first 2 weeks of lactation, voluntary feed intake ered (e.g. Dourmad, 1991; Xue et al., 1997; Revell et mainly depends on body fatness whereas, during the al., 1998a). As a result, a low gestation feeding level latter phase of lactation, also other effects like decreases backfat thickness and body weight at protein content of the diet may effect voluntary feed weaning and tends to delay the return to oestrus after intake. Dourmad (1991) and Le Cozler et al. (1999) weaning, especially in high producing sows (Dour-found that voluntary feed intake during the first part mad, 1991).
optimum trait, taking the expected reproductive As litter size increased from 3 to 13 piglets, average daily feed intake of sows during lactation increased performance and feed intake during the following
gradually by 0.6 kg, from 4.4 to 5.0 kg. Weaned litter lactation of sows into account (Dourmad, 1991). For
sizes of 14 and 15 piglets were not associated with a example, Yang et al. (1989) advised a target backfat
higher lactational feed intake relative to litters having thickness (P2) at first parturition of 20 mm.
7 to 13 piglets. O’Grady et al. (1985) estimated in a multiple regression analysis a linear (0.22) and a 3.2. Litter size
2 21 21
quadratic response (20.01 kg (pig ) day ) for In response to greater suckling intensity, sows litter size, indicating a maximum feed intake at a nursing more piglets produce more milk (Auldist and litter size of 14 piglets. According to the formulae of King, 1995; Toner et al., 1996; Auldist et al., 1998; O’Grady et al. (1985), daily feed intake increases by Revell et al., 1998b). Auldist et al. (1998) estimated 0.96 kg when litter size increases from 3 to 13 a significant linear relationship between milk yield piglets. The intercept A in the study of O’Grady et (Y; kg / day) and litter size (LS: 8, 10, 12 or 14 al. (1985) was arbitrarily taken as 5.2 to avoid an piglets): Y55.9810.6893LS and Y58.201 overlap of data points of this study with other studies 0.3243LS for early (day 10 to 14) and late (day 24 in Figs. 3 and 4. Auldist et al. (1998) studied feed to 28) lactation, respectively. Toner et al. (1996) intake of sows nursing 6, 8, 10, 12 or 14 piglets and studied milk yield of sows nursing 6, 7, 8 or 10 did not find a linear relationship between feed intake piglets and also estimated a significant linear rela- and litter size. However, feed intake of sows nursing tionship between milk yield and litter size. Sows six piglets was lowest and feed intake of sows with a greater litter size and milk production have a nursing eight piglets was also lower than feed intake greater need to use energy and may, therefore, have a of sows nursing larger litters. It should be noted that larger voluntary feed intake (Fig. 1). Auldist et al. (1998) limited daily feed intake to
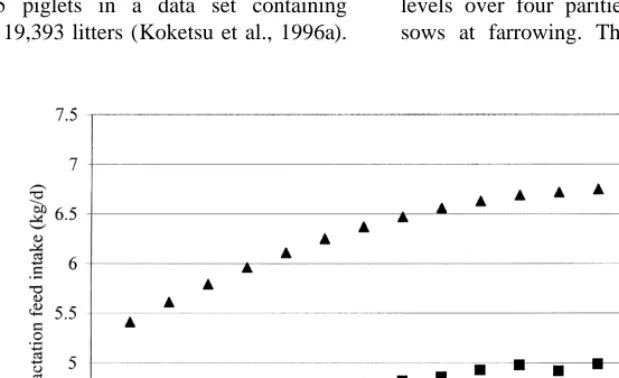
Fig. 3 shows relationships between lactation feed maximally 5 kg.
intake and litter size. Litter size at weaning ranged Yang et al. (1989) used different gestation feeding from 3 to 15 piglets in a data set containing levels over four parities, resulting in lean and fat information of 19,393 litters (Koketsu et al., 1996a). sows at farrowing. They studied ad libitum feed
Fig. 3. Comparison of the relationships between lactation feed intake and litter size:jKoketsu et al., (1996a);dAuldist et al., (1998);m
2
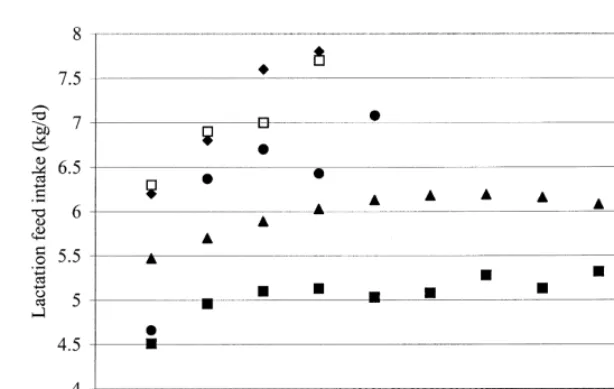
Fig. 4. Comparison of the relationships between lactation feed intake and parity:jKoketsu et al., (1996a);dMahan (1998);mO’Grady et
2 al., (1985);♦,hNeil et al., (1996). Within the study of O’Grady et al., (1985): lactation feed intake5A10.2973P20.0223P where A
corresponds to the intercept and effects of other factors (A arbitrarily taken as 5.2) and P stands for parity. Within the study of Neil et al., (1996):♦, sows received a simplified diet during the gestation period;h, sows received a conventional diet during the gestation period.
intake (maximally 7 kg / day) of sows nursing 6 or 10 compensate completely for the increased energy piglets but did not find a significant effect of litter demand. Dourmad (1991), using equations proposed size on voluntary feed intake. This was especially by Noblet and Etienne (1989), calculated that an clear in the lean sows. Fat sows at farrowing nursing increase in milk energy output of 1 MJ / day induces 10 piglets, however, had a higher feed intake than fat a 45 g / day increase in feed intake, which represents sows nursing six piglets at parities 1 to 4. This only about 40% of supplemental energy required for indicates that body condition at farrowing might milk production. Koketsu et al. (1996a) used a affect a sow’s response in feed intake to increasing general rule of thumb ‘1.8 kg / day plus 0.45 kg
21 21
litter sizes. piglet day ’, which has been recommended by In summary, voluntary feed intake of lactating Tokach and Dial (1992; cited by Koketsu et al., sows nursing relatively small litters increases with 1996a) as a guideline for producers to use in feeding increasing litter size. Apparently, factors that limit lactating sows. Sows nursing small litters (,7 feed intake of sows nursing small litters can be piglets) consumed more than suggested by the guide-overridden or terminated when litter size and there- line, but most sows consumed decidedly less. This fore milk production is increased. The increase in illustrates that high producing sows consume an feed intake, however, seems to be following a insufficient amount of feed to meet the energy diminishing increment-type pattern, indicating that needed to adequately support lactation (Koketsu et limiting factors can only be overridden to a certain al., 1996a).
extent and / or other factors become limiting for sows
nursing larger litters. 3.3. Parity Auldist et al. (1998) studied the effect of litter size
larger energy and protein requirements for body shows the relationships between lactation feed intake growth than higher parity sows. Parity number may, and parity in four studies. Again, the intercept A in therefore, affect the partitioning of energy and / or the study of O’Grady et al. (1985) was taken as 5.2, protein between maternal tissue and milk during to avoid overlap of data points of this study with the lactation. This is supported by Pluske et al. (1998) others. O’Grady et al. (1985) estimated in a multiple
21
who made primiparous sows anabolic during lacta- regression analysis a linear (0.30 kg parity number
21
tion by super-alimentation (provide sows with nutri- day ) and a quadratic response (20.02 kg (parity
2 21 21
tion via stomach cannulae to achieve a feed intake of number ) day ) for parity, with a maximum feed about 125% of ad libitum). They concluded that intake between parity 6 and 7. According to this primiparous sows seem to partition extra energy into formula, daily feed intake of sows increases by 0.73 body growth rather than milk production, whereas kg from parity 1 to 7. Koketsu et al. (1996a) found a multiparous sows show an increase in milk yield significant lower feed intake for primiparous sows (Matzat et al., 1990; cited by Pluske et al., 1998). than for older sows in their study about factors Results presented by Cole (1990) suggest that affecting lactation feed intake on commercial herds. primiparous sows need a higher dietary energy intake Feed intake increased by 0.81 kg, from 4.51 kg to avoid maternal losses in live weight and condition (parity 1) to 5.32 kg (parity 9). Mahan (1998) found during lactation than second parity sows. This would a significant quadratic increase in weekly and total indicate that primiparous sows have higher mainte- lactation feed intake by parity (parities 1 to 5); nance requirements. In particular, primiparous sows however, litter size during lactation was linearly are of relatively low absolute size in relation to their affected by parity in this study. Mahan (1998) also productivity and nutrient demands (Whittemore, found an effect of parity that was related to protein 1996), which may cause gastrointestinal limitations content of the diet. A higher protein content in the to be more severe for primiparous than for higher diet increased voluntary feed intake of primiparous parity sows. For example, Sinclair et al. (1996) sows during the full lactation, whereas this was not recorded feed intake of primiparous and parity three the case for multiparous sows. This may be a sows. Sows of both parities had a similar feed intake reflection of higher maternal protein requirements of during the first 2 weeks of lactation; however, primiparous sows; however, lower protein body primiparous sows had a lower feed intake during reserves of primiparous sows may also play a role. weeks 3 to 5. Feed intake increased significantly with increasing Differences in milk yield have been found be- parity in the study of Neil et al. (1996). They did not tween parities. However, experiments in which clear present information about levels of litter size; how-conclusions are drawn are scarce, since estimates ever, they mentioned that litter size increased with must be made during successive cycles. Summa- parity and that ad libitum feed intake of sows was rising the results presented by Etienne et al. (1998), not affected by litter size.
maintenance and milk production of sows with intake, despite a leaner body composition, seems to increasing parity. Differences in glucose tolerance be contradictory to the previous conclusion that, between lower and higher parity sows, however, may within genotype, lean sows have a higher feed also play a role. Kemp et al. (1996) orally adminis- intake. An explanation for this apparent paradox tered glucose after an overnight fast at day 105 of could be that feed intake during lactation is depend-gestation to sows of different parities. This resulted ing on the difference between the actual body fatness in significant higher blood peak levels and area under of a sow at farrowing, which mainly depends on the the curve (up to 75 min after administration) of feeding strategy during gestation, and the potential glucose for lower parity sows compared with higher body fatness at farrowing, which depends on the parity sows, indicating that lower parity sows may be genotype. The smaller the difference between actual more glucose intolerant or insulin resistant. and potential body fatness, the lower the feed intake of a sow during lactation would be. This would mean 3.4. Genotype that the sows selected for a low daily feed intake were indeed absolutely leaner, but, compared to their 3.4.1. Selection genetic potential for body fatness, relatively fatter In several species, feed intake data of lactating than the sows selected for a high daily feed intake. animals were recorded to estimate genetic parameters The results of Kerr and Cameron (1996b) are in for voluntary feed intake. In a small data set, Van Erp agreement with the estimated genetic correlation et al. (1998) estimated a heritability of 0.19 for between voluntary daily feed intake during the voluntary feed intake of lactating sows. In dairy growth phase and during lactation for pigs (rg5 cattle, Van Arendonk et al. (1991) estimated a 0.9260.50; Van Erp et al., 1998). Archer et al. heritability of 0.46 for dry matter intake, and Koenen (1998) estimated a genetic correlation of 0.51 be-and Veerkamp (1998) be-and Van Elzakker be-and Van tween post weaning voluntary feed intake and feed Arendonk (1993) estimated heritabilities varying intake at maturity in mice.
In conclusion, voluntary feed intake of sows general, pigs of the high gain lines have a higher during lactation can directly be changed by selection. daily feed intake than pigs of the low gain or control In practice, feed intake during lactation may indirect- lines. Clutter et al. (1998) found that the high weight ly be changed by selection for production traits, gain line had a significantly lower concentration of which may also affect litter performance. the putative satiety hormone cholecystokinin per unit of feed consumed compared with the low line. This 3.4.2. Background of genetic differences in supports the hypothesis that cholecystokinin may
lactation feed intake play a role in genetic differences between lines for In general, genetic differences in lactation feed feed intake. Norton et al. (1989) reported higher intake of sows will, to some extent, reflect differ- blood glucose and insulin concentrations for a high ences in body weight and composition at farrowing, weight gain line whereas blood growth hormone and and in litter size and milk production during lacta- NEFA concentrations were higher for a low line. In tion. Meishan synthetic sows consumed significantly contrast, Arbona et al. (1988) showed greater basal more feed than Large White and Landrace sows blood growth hormone concentrations for pigs select-(Sinclair et al., 1998). In that study, Meishan sows ed for high weight gain than for control pigs. Clutter were significantly lighter, had more backfat at day 1 et al. (1995) reported greater concentrations of of lactation and had a larger litter during lactation. circulating IGF-I for high weight gain line pigs Grandhi (1997) did two experiments with Hamshire compared with low line pigs throughout periods of and Yorkshire sows. During one experiment, Ham- feed deprivation and refeeding. Altogether, these shire sows ate significantly more and, in the second results indicate that selection for post weaning experiment, Hamshire sows tended to eat more. weight gain resulted in concomitant changes in These breed differences could at least partly be due endocrine and metabolic status of growing pigs. to the higher body weight of the Hamshire sows It is generally assumed that feed intake regulatory (Grandhi, 1997). Landrace sows produced milk mechanisms are similar for mammalian species and significantly higher in protein content than Duroc chickens, although there are also some differences sows, whereas fat content was not different (Shurson (Barbato, 1994). Chickens selected for a high gain and Irvin, 1992), which may have contributed to the voluntarily consumed a volume of feed approaching higher lactation feed intake of Landrace sows in their the full capacity of their gastrointestinal tract, where-study. as chickens selected for a low daily gain consumed a Differences in glucose tolerance and insulin resist- small percentage of total capacity (Barbato et al., ance between lines of sows may also affect voluntary 1984). Selection for body weight gain increased feed intake. Sows of a dam line had significantly villus surface area by 20-fold due to increases in higher peak levels of glucose and a higher area under crypt size and enterocyte migration rates (Smith et the curve after oral administration of glucose in late al., 1990; cited by Barbato, 1994). The latter results, gestation compared with sows of a sire line (Kemp et however, could also be due to the positive relation-al., 1996). This may point towards a higher level of ship between feed intake and villus surface area glucose intolerance and / or insulin resistance of the (Goodlad et al., 1987; cited by Barbato, 1994). dam line sows. Breed may also affect the partitioning O’Sullivan et al. (1992) found that high gain line of nutrients between maternal growth and lactation chickens had significantly higher levels of the en-(Sinclair et al., 1996, 1998) and some breeds may be zyme trypsin than low gain line chickens of similar able to withstand heat stress more effectively than body weight or age. Furthermore, data reported by others (Forbes, 1995). Barbato (1994) suggest a genetic basis for CNS Feed intake regulatory mechanisms that affect neurotransmitter levels, which seems to be related to voluntary feed intake during early life may, at least selection for weight gain.
and body condition of sows and litter size (milk at farrowing and genotype are the two sow factors production) during lactation. Though there is a dearth that best can be used to increase feed intake capacity of information regarding the genetics of central and during lactation. Of these, genotype seems the most peripheral feed intake control mechanisms, it seems appropriate as body composition at farrowing should that mechanisms may act at the pre- and post- be considered as an optimum trait and, from this absorptive level, which shows that each factor men- point of view, voluntary feed intake during lactation tioned in Fig. 1 could be involved. An increase in should not be maximised per se, for example, by voluntary feed intake by selection is likely accom- reducing feeding level during gestation. These re-plished by cancelling or reducing effects of the most sults, therefore, suggest that voluntary feed intake limiting factor(s), whereas a decrease is likely ac- during lactation should be included in breeding complished by introducing new or intensifying ef- programmes. A higher feed intake during lactation fects of existing limiting factor(s). may be accomplished by direct selection for lactation feed intake or indirect selection, for example, for daily gain or daily feed intake during the growth
4. Conclusions and implications phase.
This review showed that the sow factors body
composition at farrowing, litter size during lactation, Acknowledgements parity and genotype affect the voluntary feed intake
of lactating sows. As mentioned in Section 1, fatness Stamboek and Dumeco Breeding in cooperation at farrowing of young sows tends to decrease due to with the Institute for Pig Genetics BV are acknowl-selection, while the number of piglets to be weaned edged for providing funding for this study. We thank per sow per litter and milk production of lactating Dr. Dean Revell for helpful comments.
sow tend to increase. These trends result in higher energy requirements of the sow during lactation, but
body fat reserves to support these extra requirements References are reduced. As illustrated, lactating sows
compen-sate for the larger energy requirements due to Aherne, F.X., Kirkwood, R.N., 1985. Nutrition and sow prolifica-cy. J. Reprod. Fert. Suppl. 33, 169–183.
increasing litter size (milk production) during
lacta-Aherne, F.X., Williams, I.H., 1992. Nutrition for optimizing
tion by increasing their feed intake. The
compensa-breeding herd performance. Vet. Clin. North Am. Food Anim.
tion, however, is not complete for small and medium Practice 8, 589–608.
litters and seems to be absent for large litters. These Arbona, J.R., Marple, D.N., Russell, R.W., Rahe, C.H., Mulvaney,
effects are illustrated by larger weight and backfat D.R., Sartin, J.L., 1988. Secretory patterns and metabolic clearance rate of porcine growth hormone in swine selected for
losses of sows with increasing litter size.
growth. J. Anim. Sci. 66, 3068–3072.
Continued selection for increasing energy
require-Archer, J.A., Pitchford, W.S., Hughes, T.E., Parnell, P.F., 1998.
ments during lactation will result in an increasing Genetic and phenotypic relationships between food intake, number of sows that consume an insufficient amount growth, efficiency and body composition of mice post weaning
of feed to adequately support lactation. In addition, and at maturity. Anim. Sci. 67, 171–182.
Auldist, D.E., King, R.H., 1995. Piglet’s role in determining milk
continued selection for production traits may further
production in the sow. In: Hennessy, D.P., Cranwell, P.D.
reduce body fatness of young sows. This will
(Eds.), Manipulating Pig Production V, APSA, Werribee, pp.
probably increase early culling of young sows due to 114–118.
reproductive failures and reduce lifetime perform- Auldist, D.E., Morrish, L., Eason, P., King, R.H., 1998. The
ance of sows. For a sustainable production, the influence of litter size on milk production of sows. Anim. Sci. 67, 333–337.
trends of increasing energy requirements and
de-Baidoo, S.K., Aherne, F.X., Kirkwood, R.N., Foxcroft, G.R.,
creasing body fat reserves should be accompanied by
1992. Effect of feed intake during lactation and after weaning
a higher feed intake of sows during lactation. This on sow reproductive performance. Can. J. Anim. Sci. 72, may be realised by changing sow, environmental 911–917.
A.H., Harmon, B.G., 1968. Reproductive performance and some sow and piglet characteristics and of environmental progeny development in swine as influenced by feed intake conditions on milk production. In: Verstegen, M.W.A., during pregnancy. J. Nutr. 97, 489–495. Moughan, P.J., Schrama, J.W. (Eds.), The Lactating Sow, Barbato, G.F., 1994. Genetic control of food intake in chickens. J. Wageningen Pers, Wageningen, pp. 285–299.
Nutr. 124 (Suppl. 8), 1341–1348. Farmer, C., Robert, S., Matte, J.J., 1996. Lactation performance of Barbato, G.F., Siegel, P.B., Cherry, J.A., Nir, I., 1984. Selection sows fed a bulky diet during gestation and receiving growth for body weight at eight weeks of age. 17. Overfeeding. hormone-releasing factor during lactation. J. Anim. Sci. 74,
Poultry Sci. 63, 11–18. 1298–1306.
Black, J.L., Mullan, B.P., Lorschy, M.L., Giles, L.R., 1993. Ferreira, A.S., De Costa, P.M., Pereira, J.A.A., Gomes, J.C., Lactation in the sow during heat stress. Livest. Prod. Sci. 35, Soares-Ferreira, A., De Assuncao Costa, P.M., Alves Pereira, 153–170. J.A., 1988. Estimation of milk yield of sows. Rev. Soc. Brasil. Brobeck, J.R., 1948. Food intake as a mechanism of temperature Zootec. 17, 203–211.
regulation. Yale J. Biol. Med. 20, 545–552. Forbes, J.M., 1988. Metabolic aspects of the regulation of Cameron, N.D., Curran, M.K., 1994. Selection for components of voluntary food intake and appetite. Nutr. Res. Rev. 1, 145–168. efficient lean growth rate in pigs. 4. Genetic and phenotypic Forbes, J.M., 1995. Voluntary Food Intake and Diet Selection in parameter estimates and correlated responses in performance Farm Animals, CAB International, Wallingford.
test traits with ad libitum feeding. Anim. Prod. 59, 281–291. Gonzales, M.F., Deutsch, J.A., 1981. Vagotomy abolishes cues of Campbell, R.G., Taverner, M.R., 1988. Genotype and sex effects satiety produced by gastric distension. Science 212, 1283–
on the relationship between energy intake and protein deposi- 1284.
tion in growing pigs. J. Anim. Sci. 66, 676–686. Goodlad, R.A., Plumb, J.A., Wright, N.A., 1987. The relationship Clutter, A.C., Spicer, L.J., Woltmann, M.D., Grimes, R.W., Ham- between intestinal crypt cell production and intestinal water mond, J.M., Buchanan, D.S., 1995. Plasma growth hormone, absorption measured in vitro in the rat. Clin. Sci. 72, 297–304. insulin-like growth factor I, and insulin-like growth factor Grandhi, R.R., 1997. Effect of selection for lower backfat, and binding proteins in pigs with divergent genetic merit for increased dietary lysine level to digestible energy with supple-postweaning average daily gain. J. Anim. Sci. 73, 1776–1783. mental threonine and methionine on lactation performance of Clutter, A.C., Jiang, R., McCann, J.P., Buchanan, D.S., 1998. Yorkshire and Hamshire sows. Can. J. Anim. Sci. 77, 479–485. Plasma cholecystokinin-8 in pigs with divergent genetic po- Head, R.H., Williams, I.H., 1991. Mammogenesis is influenced by tential for feed intake and growth. Dom. Anim. Endo. 15, pregnancy nutrition. In: Batterham, E.S. (Ed.), Manipulating
9–21. Pig Production IV, APSA, Werribee, p. 33.
Cole, D.J.A., 1982. Nutrition and reproduction. In: Cole, D.J.A., Head, R.H., Williams, I.H., 1995. Potential milk production in Foxcroft, G.R. (Eds.), Control of Pig Reproduction, Butter- gilts. In: Hennessy, D.P., Cranwell, P.D. (Eds.), Manipulating worths, London, pp. 603–619. Pig Production V, APSA, Werribee, p. 134.
Cole, D.J.A., 1990. Nutritional strategies to optimize reproduction Head, R.H., Bruce, N.W., Williams, I.H., 1991. More cells might in pigs. J. Reprod. Fert. (Suppl. 40), 67–82. lead to more milk. In: Batterham, E.S. (Ed.), Manipulating Pig Dourmad, J.Y., 1991. Effect of feeding level in the gilt during Production IV, APSA, Werribee, p. 76.
pregnancy on voluntary feed intake during lactation and Houpt, T.R., 1984. Controls of feeding in pigs. J. Anim. Sci. 59, changes in body composition during gestation and lactation. 1345–1353.
Livest. Prod. Sci. 27, 309–319. Kemp, B., Soede, N.M., Knol, E.F., Berkvens, P.C.H., Van Eerden, Dourmad, J.Y., 1993. Standing and feeding behaviour of the E., 1996. Breed and parity differences in glucose tolerance in lactating sow: effect of feeding level during pregnancy. Appl. pregnant sows. In: 13th International Congress on Animal Anim. Behav. Sci. 37, 311–319. Reproduction, Sydney, vol. 3, p. P16.13.
Dourmad, J.Y., Etienne, M., Prunier, A., Noblet, J., 1994. The Kennedy, G.C., 1953. The role of depot fat in the hypothalamic effect of energy and protein intake of sows on their longevity: a control of food intake in the rat. Proc. R. Soc. B 140, 706–718. review. Livest. Prod. Sci. 40, 87–97. Kerr, J.C., Cameron, N.D., 1995. Reproductive performance of Ellis, M., Smith, W.C., Laird, R., 1979. Correlated responses in pigs selected for components of efficient lean growth. Anim.
feed intake to selection for economy of production and carcass Sci. 60, 281–290.
lean content in Large White pigs. Anim. Prod. 28, 424. Kerr, J.C., Cameron, N.D., 1996a. Genetic and phenotypic rela-Ellis, M., Smith, W.C., Henderson, R., Whittemore, C.T., Laird, tionships between performance test and reproduction traits in
R., Phillips, P., 1983. Comparative performance and body Large White pigs. Anim. Sci. 62, 531–540.
composition of control and selection line Large White pigs. 3. Kerr, J.C., Cameron, N.D., 1996b. Responses in gilt post-farrow-Three low feeding scales for a fixed time. Anim. Prod. 37, ing traits and pre-weaning piglet growth to divergent selection 253–258. for components of efficient lean growth rate. Anim. Sci. 63, Elsley, F.W.H., Bannerman, M., Bathurst, E.V.J., Bracewell, E.G., 523–531.
Cunningham, J.M.M., Dodsworth, T.L., Dodds, P.A., Forbes, King, R.H., Williams, I.H., 1984. The effect of nutrition on the T.J., 1969. The effect of level of feed intake in pregnancy and reproductive performance of first-litter sows. 1. Feeding level in lactation upon the productivity of sows. Anim. Prod. 11, during lactation, and between weaning and mating. Anim.
225–241. Prod. 38, 241–247.
The influence of feeding level during lactation on the occur- J.W. (Eds.), The Lactating Sow, Wageningen Pers, Wageningen, rence and endocrinology of the post weaning estrus in sows. pp. 97–112.
Can. J. Anim. Sci. 67, 405–415. Mahan, D.C., 1998. Relationship of gestation protein and feed Kirkwood, R.N., Lythgoe, E.S., Aherne, F.X., 1987b. Effect of intake level over a five-parity period using a high-producing
lactation feed intake and gonadotrophin-releasing hormone on sow genotype. J. Anim. Sci. 76, 533–541.
the reproductive performance of sows. Can. J. Anim. Sci. 67, Mahan, D.C., Mangan, L.T., 1975. Evaluation of various protein
715–719. sequences on the nutritional carry-over from gestation to
Kirkwood, R.N., Baidoo, S.K., Aherne, F.X., 1990. The influence lactation with first-litter sows. J. Nutr. 105, 1291–1298. of feeding level during lactation and gestation on the endocrine Matzat, P.D., Hogberg, M.G., Fogwell, R.L., Miller, E.R., 1990. status and reproductive performance of second parity sows. Lactation performance in high producing gilts fed in excess of Can. J. Anim. Sci. 70, 1119–1126. ad libitum. In: Report of Swine Research, AS-SW-8904, Knap, P.W., 1990. Pig herdbook breeding in the Netherlands. 3. Michigan State University, East Lansing, pp. 36–40.
Dam line breeding. World Rev. Anim. Prod. 25, 59–64. Mayer, J., 1953. Glucostatic regulation of food intake. N. Engl. J. Knap, P.W., Van Alst, G.J.M., Versteeg, J.G., Kanis, E., 1993. Med. 249, 13–16.
Realised genetic improvement of litter size in Dutch Pig Messias de Braganc¸a, M., Mounier, A.M., Prunier, A., 1998. Does Herdbook breeding. Pig News Inform. 14, 119N–121N. feed restriction mimic the effects of increased ambient tem-Koenen, E.P.C., Veerkamp, R.F., 1998. Genetic covariance func- perature in lactating sows? J. Anim. Sci. 76, 2017–2024.
tions for live weight, condition score, and dry-matter intake Mullan, B.P., Williams, I.H., 1989. The effect of body reserves at measured at different lactation stages of Holstein Friesian farrowing on the reproductive performance of first-litter sows. heifers. Livest. Prod. Sci. 57, 67–77. Anim. Prod. 48, 449–457.
Koketsu, Y., Dial, G.D., 1997. Factors influencing the postweaning Murray, R.K., Mayes, P.A., Granner, D.K., Rodwell, V.W., 1990. reproductive performance of sows on commercial farms. In: 27th ed, Harper’s Biochemistry, Appleton and Lange,
Theriogenology 47, 1445–1461. London.
Koketsu, Y., Dial, G.D., Pettigrew, J.E., March, W.E., King, V.L., NCR-89, 1990. Feeding frequency and the addition of sugar to the 1996a. Characterization of feed intake patterns during lactation diet for the lactating sow. J. Anim. Sci. 68, 3498–3501. in commercial swine herds. J. Anim. Sci. 74, 1202–1210. Neil, M., 1996. Ad libitum lactation feeding of sows introduced Koketsu, Y., Dial, G.D., Pettigrew, J.E., King, V.L., 1996b. Feed immediately before, at, or after farrowing. Anim. Sci. 63,
intake pattern during lactation and subsequent reproductive 497–505.
´
performance of sows. J. Anim. Sci. 74, 2875–2884. Neil, M., Ogle, B., Anner, K., 1996. A two-diet system and ad Koketsu, Y., Dial, G.D., Pettigrew, J.E., King, V.L., 1997. Influence libitum lactation feeding of the sow. 1. Sow performance.
of feed intake during individual weeks of lactation on re- Anim. Sci. 62, 337–347.
productive performance of sows on commercial farms. Livest. Noblet, J., Etienne, M., 1989. Estimation of sow milk nutrient Prod. Sci. 49, 217–225. output. J. Anim. Sci. 67, 3352–3359.
Kronfield, D.S., 1971. Hypoglycemia in ketotic cows. J. Diary Sci. Noblet, J., Dourmad, J.Y., Etienne, M., 1990. Energy utilization in 54, 949–961. pregnant and lactating sows: modeling of energy requirements. Le Cozler, Y., David, C., Beaumal, V., Hulin, J.C., Neil, M., J. Anim. Sci. 68, 562–572.
Dourmad, J.Y., 1998a. Effect of feeding level during rearing on Noblet, J., Etienne, M., Dourmad, J.Y., 1998. Energetic efficiency performance of Large White gilts. Part 1: growth, reproductive of milk production. In: Verstegen, M.W.A., Moughan, P.J., performance and feed intake during lactation. Reprod. Nutr. Schrama, J.W. (Eds.), The Lactating Sow, Wageningen Pers,
Dev. 38, 363–375. Wageningen, pp. 113–130.
Le Cozler, Y., David, C., Beaumal, V., Johansen, S., Dourmad, J.Y., Norton, S.A., Zavy, M.T., Maxwell, C.V., Buchanan, D.S., 1998b. Effect of feeding level during rearing on performance Breazile, J.E., 1989. Insulin, growth hormone, glucose, and of Large White gilts. Part 2: effect on metabolite profiles fatty acids in gilts selected for rapid vs. slow growth rate. Am. during gestation and lactation, and on glucose tolerance. J. Physiol. 257, E554–E560.
Reprod. Nutr. Dev. 38, 377–390. NRC, 1987. Predicting Feed Intake of Food Producing Animals, Le Cozler, Y., Ringmar-Cederberg, E., Rhydmer, L., Lundeheim, National Academy Press, Washington.
N., Dourmad, J.Y., Neil, M., 1999. Effect of feeding level O’Grady, J.F., Elsley, F.W.H., MacPherson, R.M., MacDonald, I., during rearing and mating strategy on performance of Swedish 1973. The response of lactating sows and their litters to Yorkshire sows. 2. Reproductive performance, food intake, different dietary energy allowances. Anim. Prod. 17, 65–74. backfat changes and culling rate during the first two parities. O’Grady, J.F., Lynch, P.B., Kearney, P.A., 1985. Voluntary feed Anim. Sci. 68, 365–377. intake by lactating sows. Livest. Prod. Sci. 12, 355–365. Le Magnen, J., Devos, M., 1984. Meal to meal energy balance in O’Sullivan, N.P., Dunnington, E.A., Larsen, A.S., Siegel, P.B.,
rats. Phys. Behav. 32, 39–44. 1992. Correlated responses in lines of chickens divergently Little, W., Kay, R.M., 1979. The effect of rapid rearing and early selected for fifty-six-day body weight. 3. Digestive enzymes.
calving on the subsequent performance of dairy heifers. Anim. Poultry Sci. 71, 610–617.
Prod. 29, 131–141. Owen, J.B., Ridgman, W.J., 1967. The effect of dietary energy Mackenzie, D.D.S., Revell, K.D., 1998. Genetic influences on content on the voluntary intake of pigs. Anim. Prod. 9, 107–
Owen, J.B., Ridgman, W.J., 1968. Further studies of the effect of Smith, W.C., Ellis, M., Chadwick, J.P., Laird, R., 1991. The dietary energy content on the voluntary intake of pigs. Anim. influence of index selection for improved growth and carcass Prod. 10, 85–91. characteristics on appetite in a population of Large White pigs. Pettigrew, J.E., McNamara, J.P., Tokach, M.D., King, R.H., Anim. Prod. 52, 193–199.
Crooker, B.A., 1993. Metabolic connections between nutrient Southwood, O.I., Kennedy, B.W., 1991. Genetic and environmen-intake and lactational performance in the sow. Livest. Prod. tal trends for litter size in swine. J. Anim. Sci. 69, 3177–3182. Sci. 35, 137–152. Stephens, D.B., 1985. Influence of intraduodenal glucose on meal Pluske, J.R., Williams, I.H., Zak, L.J., Clowes, E.J., Cegielski, size and its modification by 2-deoxy-D-glucose or vagotomy in
A.C., Aherne, F.X., 1998. Feeding lactating primiparous sows hungry pigs. Q. J. Exp. Phys. 70, 129–135.
to establish three divergent metabolic states: III. Milk pro- Stricker, E.M., McCann, M.J., 1985. Visceral factors in the control duction and pig growth. J. Anim. Sci. 76, 1165–1171. of food intake. Brain Res. Bull. 14, 687–692.
Rayner, D.V., Gregory, P.C., 1989. The role of the gastrointestinal Tokach, M.D., Dial, G.D., 1992. Managing the lactating sow for tract in the control of voluntary food intake. In: Forbes, J.M., optimal weaning and rebreeding performance. Vet. Clin. North Varley, M.A., Lawrence, T.L.J. (Eds.), The Voluntary Food Am. Food Anim. Practice 8, 559–573.
Intake of Pigs, Occasional Publication BSAP no. 13, Edin- Toner, M.S., King, R.H., Dunshea, F.R., Dove, H., Atwood, C.S., burgh, pp. 27–39. 1996. The effect of exogenous somatotropin on lactation Reese, D.E., Moser, B.D., Peo, Jr. E.R., Lewis, A.J., Zimmerman, performance of first-litter sows. J. Anim. Sci. 74, 167–172.
D.R., Kinder, J.E., Stroup, W.W., 1982. Influence of energy Tybirk, P., 1989. A model of feed intake regulation in the growing intake during lactation on the interval from weaning to first pig. In: Forbes, J.M., Varley, M.A., Lawrence, T.L.J. (Eds.), estrus in sows. J. Anim. Sci. 55, 590–598. The Voluntary Food Intake of Pigs, Occasional Publication Revell, D.K., Williams, I.H., 1993. Physiological control and BSAP no. 13, Edinburgh, pp. 105–109.
manipulation of voluntary food intake. In: Batterham, E.S. Van Arendonk, J.A.M., Nieuwhof, G.J., Vos, H., Korver, S., 1991. (Ed.), Manipulating Pig Production IV, APSA, Werribee, pp. Genetic aspects of feed intake and efficiency in lactating dairy
55–80. heifers. Livest. Prod. Sci. 29, 263–275.
Revell, D.K., Williams, I.H., Mullan, B.P., Ranford, J.L., Smits, Van Elzakker, P.J.M., Van Arendonk, J.A.M., 1993. Feed intake, R.J., 1998a. Body composition at farrowing and nutrition body weight and milk production: genetic analysis of different during lactation affect the performance of primiparous sows: I. measurements in lactating dairy heifers. Livest. Prod. Sci. 37, Voluntary feed intake, weight loss, and plasma metabolites. J. 37–51.
Anim. Sci. 76, 1729–1737. Van Erp, A.J.M., Molendijk, R.J.F., Eissen, J.J., Merks, J.W.M., Revell, D.K., Williams, I.H., Mullan, B.P., Ranford, J.L., Smits, 1998. Relation between ad libitum feed intake of gilts during R.J., 1998b. Body composition at farrowing and nutrition rearing and feed intake capacity of lactating sows. In: 49th during lactation affect the performance of primiparous sows: II. Annual Meeting EAAP, Warsaw, Poland, G5.10 (abstract). Milk composition, milk yield, and pig growth. J. Anim. Sci. 76, Vangen, O., Kolstad, N., 1986. Genetic control of growth, 1738–1743. composition, appetite and feed utilization in pigs and poultry. Scharrer, E., 1991. Peripheral mechanisms controlling voluntary In: Proceedings 3rd World Congress on Genetic Applications food intake in the pig. Pig News Inform. 12, 377–379. to Livestock Production, Lincoln, USA, vol. 11, pp. 367–380. Sejrsen, K., Huber, J.T., Tucker, H.A., Akers, R.M., 1982. Vanschoubroek, F., Van Spaendonck, R., 1966. Nutrition des truies
´ ´ Influence of nutrition on mammary development in pre- and en lactation en fonction de leurs besoins energetique et
´ post pubertal heifers. J. Dairy Sci. 65, 793–800. protidique. In: L’alimentation de la truie, Journee d’etude
´
Shurson, G.C., Irvin, K.M., 1992. Effects of genetic line and organisee par l’Association professionnelle des fabricants ´
supplemental dietary fat on lactation performance of Duroc and d’aliments composes pour animaux, Bruxelles, pp. 31–55. Landrace sows. J. Anim. Sci. 70, 2942–2949. Weldon, W.C., Lewis, A.J., Louis, G.F., Kovar, J.L., Giesemann, Sinclair, A.G., Edwards, S.A., Hoste, S., McCartney, A., Fowler, M.A., Miller, P.S., 1994a. Postpartum hypophagia in primipar-V.R., 1996. Partitioning of dietary protein during lactation in ous sows I. Effects of gestation feeding level on feed intake, the Meishan synthetic and European white breeds of pig. Anim. feeding behavior, and plasma metabolite concentrations during Sci. 62, 355–362. lactation. J. Anim. Sci. 72, 387–394.
Sinclair, A.G., Cia, M.C., Edwards, S.A., Hoste, S., 1998. Weldon, W.C., Lewis, A.J., Louis, G.F., Kovar, J.L., Miller, P.S., Response to dietary protein during lactation of Meishan 1994b. Postpartum hypophagia in primiparous sows II. Effects synthetic. Large White and Landrace gilts given food to of feeding level during gestation and exogenous insulin on achieve the same target backfat level at farrowing. Anim. Sci. lactation feed intake, glucose tolerance, and epinephrine-stimu-67, 349–354. lated release of nonesterified fatty acids and glucose. J. Anim. Smith, C., Fowler, V.R., 1978. The importance of selection criteria Sci. 72, 395–403.
and feeding regimes in the selection and improvement of pigs. Whittemore, C.T., 1996. Nutrition reproduction interactions in Livest. Prod. Sci. 5, 415–423. primiparous sows: a review. Livest. Prod. Sci. 46, 65–83. Smith, M.W., Mitchell, M.A., Peacock, M.A., 1990. Effects of Williams, I.H., 1998. Nutritional effects during lactation and
genetic selection on growth rate and intestinal structure in the during the interval from weaning to oestrus. In: Verstegen, domestic fowl (Gallus domesticus). Comp. Biochem. Physiol. M.W.A., Moughan, P.J., Schrama, J.W. (Eds.), The Lactating
Williams, I.H., Smits, R.J., 1991. Body protein losses can be 1997. Glucose tolerance, luteinizing hormone release, and minimized during lactation. In: Batterham, E.S. (Ed.), Man- reproductive performance of first-litter sows fed two levels of ipulating Pig Production III, APSA, Werribee, p. 73. energy during gestation. J. Anim. Sci. 75, 1845–1852. Woods, S.C., Porte, D., Bobbioni, E., Ionescu, E., Sauter, J.F., Yang, H., Eastham, P.R., Phillips, P., Whittemore, C.T., 1989.
Rohner-Jeanrenaud, F., Jeanrenaud, B., 1985. Insulin: its Reproductive performance, body weight and body condition of relationship to the central nervous system and to the control of breeding sows with differing body fatness at parturition, food intake and body weight. Am. J. Clin. Nutr. 42, 1063– differing nutrition during lactation, and differing litter size.
1071. Anim. Prod. 48, 181–201.