Summary We studied carbon and nitrogen allocation in my-corrhizal and non-mymy-corrhizal Scots pine (Pinus sylvestris L.) seedlings grown in a semi-hydroponic system with nitrogen as the growth limiting factor. Three ectomycorrhizal fungi were compared: one pioneer species (Thelephora terrestris Ehrh.: Fr.) and two late-stage fungi (Suillus bovinus (L.: Fr.) O. Kuntze, and Scleroderma citrinum Pers.). By giving all plants in each treatment the same amount of readily available nitrogen, we ensured that the external mycelium could not increase the total nitrogen content of the plants, thereby guaranteeing that any change in carbon or nitrogen partitioning was a direct effect of the mycorrhizal infection itself. Carbon and nitrogen partition-ing were measured at an early and a late stage of mycorrhizal development, and at a low and a high N addition rate.
Although mycorrhizal seedlings had a higher net assimila-tion rate and a higher shoot/root ratio than non-mycorrhizal seedlings, they had a lower rate of shoot growth. The high carbon demand of the mycobionts was consistent with the large biomass of external mycelia and the increased below-ground respiration of the mycorrhizal plants. The carbon cost to the host was similar for pioneer and late-stage fungi. Above-and belowground partitioning of nitrogen was also affected by mycorrhizal infection. The external mycelia of Scleroderma citrinum retained 32% of the nitrogen supplied to the plants, thus significantly reducing nitrogen assimilation by the host plants and consequently reducing their growth rate. By con-trast, the external mycelia of T. terrestris and Suillus bovinus retained less nitrogen than the mycelia of Scleroderma citrinum, hence we attributed the decreased growth rates of their host plants to a carbon drain rather than a nitrogen deficiency.
Keywords: carbon allocation, nitrogen partitioning, root res-piration, Scleroderma citrinum, shoot/root ratio, Suillus bovinus, Thelephora terrestris.
Introduction
Most higher plant species in terrrestrial ecosystems have asso-ciated mycorrhizal fungi, which have direct access to the
assimilates of their hosts and also serve as carriers for mineral nutrients to the host (Finlay and Read 1986, Finlay et al. 1988). These fungi demand large amounts of carbon, and it is this demand that gives rise to mycorrhizal symbiosis, which, in turn, is important not only for the growth of both symbionts but also for the carbon balance of ecosystems. Several studies have shown that mycorrhizal fungi use a significant fraction of the net primary production of natural forests (Vogt et al. 1982, Finlay and Söderström 1992). Laboratory studies suggest that mycorrhizal symbiosis results in significantly higher below-ground carbon allocation than in non-symbiotic conditions. Recent studies have suggested that mycorrhizae impose a higher C cost on their hosts under field conditions than under experimental conditions (Rygiewicz and Andersen 1994, Tinker et al. 1994).
Ectomycorrhizal fungi can reduce the growth rate of their host plant (Nylund and Wallander 1989, Dosskey et al. 1990, Rygiewicz and Andersen 1994). Such yield reductions are usually attributed to an increase in belowground carbon allo-cation. We postulate that this cost of symbiosis is partly com-pensated by an increase in shoot/root ratio in mycorrhizal plants. We also hypothesize that retention of nitrogen in the mycobiont might lead to growth repression in the host plant. Because nitrogen can be a growth limiting factor in forests (Attiwill and Adams 1993), the diversion of nitrogen for fungal growth might have consequences for tree growth and nutrient cycling in natural ecosystems.
We studied growth, nitrogen partitioning, and rates of pho-tosynthesis and respiration in the above- and belowground parts of mycorrhizal and non-mycorrhizal Scots pine (Pinus sylvestris L.) seedlings at an early and a late stage of fungal development. Three ectomycorrhizal fungi were compared: a pioneer species Thelephora terrestris Ehrh.: Fr., and two late-stage fungi, Suillus bovinus (L.: Fr.) O. Kuntze and Sclero-derma citrinum Pers. (Fox 1986). We compared a pioneer fungal species with two late-stage fungal species because there is evidence that there are important physiological differences between pioneer and late-stage ectomycorrhizal fungi, and little is known about the nutrient requirements of the different fungi. Compared to late-stage fungi, pioneer fungi are
charac-Carbon and nitrogen allocation in ectomycorrhizal and
non-mycorrhizal Pinus sylvestris L. seedlings
JAN V. COLPAERT,
1ANDRÉ VAN LAERE,
1and JOZEF A. VAN ASSCHE
21 Laboratory of Developmental Biology, Institute of Botany, Katholieke Universiteit Leuven, K. Mercierlaan, 92, B-3001 Leuven, Belgium 2 Laboratory of Ecology, Institute of Botany, Katholieke Universiteit Leuven, K. Mercierlaan, 92, B-3001 Leuven, Belgium
Received November 29, 1995
terized by a more rapid growth, lower energy investment in biomass and early carpophore development with more ger-minable propagules (Dighton 1991, Deacon and Fleming 1992). Pioneer fungi also tolerate higher concentrations of inorganic nutrients than late-stage fungi.
Materials and methods
Plant and fungus material
Half-sib seeds of Pinus sylvestris were surface sterilized in 30% H2O2 for 15 min, sown in a 1/1 (v/v) mix of vermiculite
and perlite and watered with a balanced nutrient solution (Ingestad et al. 1986). The weight proportions of the macronu-trients in the solution were 100 N/60 K/18 P/6 Ca/6 Mg/9 S. After 10 weeks, plants were selected for uniformity and inocu-lated with a mycorrhizal fungus or left uninocuinocu-lated. Three ectomycorrhizal species were used for inoculation: Thele-phora terrestris (24 plants), Suillus bovinus (24 plants) and Scleroderma citrinum (12 plants). For inoculations, mycorrhi-zal fungi were grown in 10-cm diameter plastic petri dishes containing modified Melin-Norkrans agar medium covered with sterile cellophane sheets. Once the mycelia had covered most of the cellophane surface, the root system of a selected seedling was spread over the young mycelia and a thick filter paper (9 cm in diameter), soaked in Ingestad’s nutrient solu-tion, was used to cover the roots and mycelia. Control plants were treated in the same way, except that their roots were placed on Melin-Norkrans agar medium without mycelium. Three to four days after inoculation, the plants were trans-planted to 4-liter containers to allow the growth of extensive external mycelia.
Growth conditions
After inoculation, two plants were transferred to each 4-liter container (0.40 × 0.20 × 0.05 m) and 250 g of acid-washed perlite was added. The perlite was irrigated with 1.45 dm3 of
nutrient solution, resulting in saturation of the substrate to 80% of its water holding capacity. The perlite in each container was covered with a dark plastic lid to prevent algal growth. The containers were weighed at least twice a week so that water use could be estimated during the experiment. After each weigh-ing, the containers were rearranged on the benches in the growth room.
Plants were maintained in non-sterile conditions in a growth chamber in a 16-h photoperiod and a day/night temperature of 22/15 °C. Relative air humidity was at least 70% and photo-synthetically active radiation (PAR) was 400 µmol m−2 s−1.
The seedlings were grown at two nutrient addition rates. In-itially, nutrients were supplied daily in a single addition but after 8 weeks two additions per day were necessary.
Treatments
Six plant containers of each fungal treatment received bal-anced nutrient solution for Scots pine at a low relative addition rate of 2.6% day−1 (LN treatment). The amount of nitrogen
supplied was based on the nitrogen content of the seedlings
(Ingestad et al. 1986). The nitrogen concentration of the nutri-ent solution was 20 mg l−1. On the first day, 0.4 mg N was
supplied to each plant and the amount was gradually increased to 4 mg on Day 90. Distilled water was supplied to compensate for differences in water use between plants. The initial nitrogen content of the irrigated perlite in the LN treatment was 20 mg per container. The perlite substrate was maintained at a pH of 4.0 by the addition of a 1/1 mix of NO3− and NH4−. Adsorption of added N on the perlite was low (< 10%).
For the second treatment (the HN treatment), control plants and plants inoculated with T. terrestris or Suillus bovinus received exactly twice the amount of nutrients as the plants in the LN treatment (0.8 mg N per plant on Day 1 up to 8 mg on day 90). The initial nitrogen content of the irrigated perlite in the HN treatment was 40 mg per container.
Gas exchange measurements
Measurements of net photosynthesis and respiration were con-ducted on all seedlings before harvest. Carbon dioxide was measured with an infrared gas analyzer (IRGA) in an open system. Outside air was allowed to adjust to the ambient temperature of the growth room. Before measurement, entire plants were transferred to PVC root cuvettes without disturb-ing the root substrate. The inner compartment of each cuvette was exactly the same size as the containers. The bottom and one side wall (0.2 × 0.4 m) of each inner compartment con-sisted of a stainless steel grid, with a 1-cm air gap between the grid and the bottom and side of the root cuvette. Relative humidity of air entering the root compartment was adjusted to 80--85%. Temperature and relative humidity of air entering and leaving the root cuvette were measured. Air was circulated through the root compartment for 4 h before commencing the CO2 measurements. Measurements were recorded at 10-min
intervals.
Immediately after root respiration was recorded, the air stream through the root compartment was stopped. To deter-mine photosynthetic rate, a shoot cuvette (3.8 or 8.5 dm3, with a maximal flow rate through the cuvette of 14 dm3 min−1),
which enclosed both seedlings, was mounted on the root com-partment and measurements of CO2 were continued until
steady-state conditions were obtained. Temperature in the shoot compartment was about 1.0 °C higher than ambient temperature. Aboveground respiration was determined imme-diately after the photosynthesis measurements were com-pleted. Steady-state conditions were achieved in the dark after about 15 min.
Harvest
Plants were harvested at Weeks 5 and 12 after transfer of the seedlings to the 4-liter containers. Two subsamples of rooting substrate (approximately 20 g), free of root material, were transferred to 20 ml of distilled water, stirred for 15 min and subsequently vacuum-filtered over a 0.45 µm membrane filter. Conductivity and nitrogen concentration of the filtrate were determined. The filtered perlite was subsequently washed with two 20-ml aliquots of distilled H2O, dried at 50 °C, weighed
defined the external mycelium as the fungal biomass that was not associated with roots.
Shoots were separated from roots, dried at 80 °C for at least 4 days and weighed. Carpophores and roots were rinsed free of perlite on a 1-mm sieve and subsequently treated in the same way as the shoots. For the calculation of shoot/root ratio and relative growth rate (RGR), the fungal biomass of the myce-lium in the substrate was excluded. The RGR, expressed as % day−1 was calculated by fitting seedling weight (y) at different
days (x; start day and both harvests) to the equation y = aeRGRx.
Nitrogen determinations
Nitrogen in oven-dried shoots, roots and rooting substrate was extracted by the Kjeldahl procedure. The NH3 was steam
distilled into H2SO4 (0.05 M) and determined colorimetrically
with Nessler’s reagent. Nitrogen was also determined in the carpophores of T. terrestris and Scleroderma citrinum formed during the experiment as well as in a Suillus bovinus mycelium cultured on a modified Melin-Norkrans medium without malt extract. Data from the Suillus mycelium were used to calculate fungal biomass from the N concentration of the washed perlite substrate (Colpaert et al. 1992). Perlite from the non-mycorrhi-zal treatment was used to determine the background concen-tration of the non-mycorrhizal N pool. The N concenconcen-tration of the Suillusbovinus mycelium might be slightly overestimated because the N concentration of the MMN medium (53 mg l−1)
was higher than that of the plant nutrient solution. This error could result in slight underestimations of fungal biomass, especially in the LN treatment.
Statistical analyses
Significant differences between means within a single treat-ment were determined with a one-way ANOVA and the Tukey’s studentized range test.
Results
Plant and fungal growth
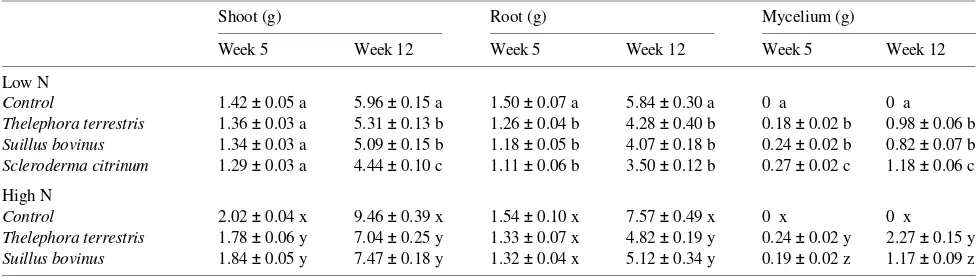
Dry weights of shoots, roots and external mycelia are shown in Table 1. Decreased shoot growth in the mycorrhizal plants
was already apparent 5 weeks after inoculation. Twelve weeks after inoculation, non-mycorrhizal seedlings had significantly larger shoots and roots than mycorrhizal seedlings in both nutrient treatments. Although relative growth rates of mycor-rhizal plants decreased during the study, their shoot/root ratios increased, indicating that mycorrhizal infection suppressed root growth more than shoot growth (Table 2).
In the LN treatment, plant water use increased exponentially throughout the experiment (r2 > 0.98), whereas in the HN treatment, water use plateaued in the last month of the experi-ment, indicating that seedlings had entered a more linear growth phase. The conductivity of the solution in the perlite was between 20 and 45 µS cm−1 at Week 5 and between 10 and 25 µS cm−1 at Week 12. The amount of nitrogen that could be washed from the perlite was always less than the amount of nitrogen added. These observations indicate that plant nutrient uptake rates were high in both treatments, and there was no oversupply of nutrients.
By Week 5, T. terrestris had completely colonized the growth substrate, infecting almost all short roots. Suillus bovinus and Scleroderma citrinum had colonized 75% of the growth substrate, infecting 80 and 90% of short roots, respec-tively. By Week 12, substrate colonization and short root infec-tion were close to 100% in all fungal treatments, indicating that the nutrient treatments did not affect the colonization rates of substrate and roots.
Carpophores formed in all containers with T. terrestris and in two containers with Scleroderma citrinum. The N concen-tration of the T. terrestris carpophores was 2.5% in the LN treatment and 2.7% in the HN treatment, whereas the N con-centration in Scleroderma citrinum carpophores was 3.8%. Suillus bovinus formed no carpophores and contained 31 mg N per g dry weight. When we used these N values to calculate fungal biomass in the perlite substrate, we found that Scleroderma citrinum produced the largest external mycelial biomass, and that T. terrestris (in the LN treatment) formed the least dense mycelium, 31% of which was made up of small carpophores. The mycelial biomass of T. terrestis was greater in the HN treatment than in the LN treatment, and carpophores accounted for 53% of its biomass.
Table 1. Dry weights of shoots, roots and external mycelia (including carpophores) of mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings grown at a low or a high nutrient addition rate. Standard errors are shown beside values; for each treatment, means within a column followed by different letters are significantly different (n = 6, one-way ANOVA, Tukey’s test, α = 0.05).
Shoot (g) Root (g) Mycelium (g)
Week 5 Week 12 Week 5 Week 12 Week 5 Week 12
Low N
Control 1.42 ± 0.05 a 5.96 ± 0.15 a 1.50 ± 0.07 a 5.84 ± 0.30 a 0 a 0 a
Thelephora terrestris 1.36 ± 0.03 a 5.31 ± 0.13 b 1.26 ± 0.04 b 4.28 ± 0.40 b 0.18 ± 0.02 b 0.98 ± 0.06 b Suillus bovinus 1.34 ± 0.03 a 5.09 ± 0.15 b 1.18 ± 0.05 b 4.07 ± 0.18 b 0.24 ± 0.02 b 0.82 ± 0.07 b Scleroderma citrinum 1.29 ± 0.03 a 4.44 ± 0.10 c 1.11 ± 0.06 b 3.50 ± 0.12 b 0.27 ± 0.02 c 1.18 ± 0.06 c
High N
Nitrogen partitioning
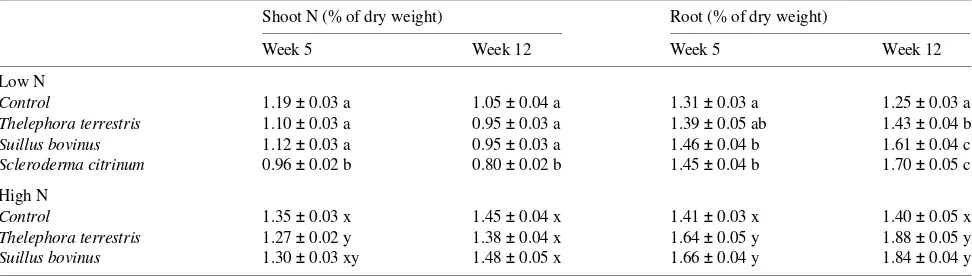
Although mycorrhizal plants had a lower dry weight than non-mycorrhizal plants, the reduction did not result in an increase in shoot N concentration (Table 3). Plants infected with Scleroderma citrinum had a lower shoot N concentration than non-mycorrhizal plants, and this finding was confirmed by a slight yellowing of the needles. The N concentration of mycorrhizal roots was significantly higher than that of non-mycorrhizal roots.
Partitioning of N in above- and belowground biomass is shown in Figure 1. Mycorrhizal root systems retained a larger proportion of N than non-mycorrhizal root systems, indicating that the external mycelia function as an N sink. By Week 12, mycelia had retained between 15 and 32% of all N assimilated (Table 4). These values would be higher if the N contained in the mycorrhizas was also included. Moreover, the recovery of all N added to the plants was similar or slightly higher in mycorrhizal plants than in non-mycorrhizal plants, whereas the amount of N transported to the shoots was considerably
less in mycorrhizal plants than in non-mycorrhizal plants in both nutrient treatments (Figure 1).
Net photosynthesis and respiration
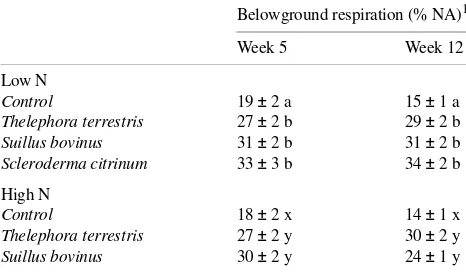
Plants infected with Suillus bovinus and T. terrestris had slightly higher assimilation rates than uninfected plants or plants inoculated with Scleroderma citrinum (Table 5). Al-though the presence of mycorrhizal fungi had no effect on dark respiration of shoots, it significantly increased respiration of roots. The percentage of fixed carbon respired belowground by mycorrhizal plants was twice that respired by non-mycorrhizal plants (Tables 5 and 6).
Discussion
A difficulty associated with comparative growth studies of mycorrhizal and non-mycorrhizal plants is that such studies only provide a measure of the net benefit of mycorrhizal infection, including nutritional effects, and thus give no direct information about carbon allocation to the fungus. We over-came this difficulty by using a semi-hydroponic technique. This technique greatly reduces the nutritional benefit to the
Table 2. Shoot/root ratio and relative growth rate of mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings grown at a low or a high
nutrient addition rate. Standard errors are shown beside values; for each treatment, means within a column followed by different letters are significantly different (n = 6, one-way ANOVA, Tukey’s test, α = 0.05).
Shoot/root ratio RGR
Week 5 Week 12 % day−1 r2
Low N
Control 0.95 ± 0.02 a 1.02 ± 0.02 a 2.8 0.97
Thelephora terrestris 1.08 ± 0.03 b 1.24 ± 0.03 b 2.6 0.98 Suillus bovinus 1.14 ± 0.03 b 1.25 ± 0.03 b 2.6 0.97
Scleroderma citrinum 1.16 ± 0.03 b 1.27 ± 0.04 b 2.5 0.97 High N
Control 1.31 ± 0.04 x 1.25 ± 0.03 x 3.2 0.96 Thelephora terrestris 1.34 ± 0.03 x 1.46 ± 0.04 y 2.9 0.93
Suillus bovinus 1.39 ± 0.04 x 1.46 ± 0.05 y 2.9 0.95
Table 3. Nitrogen concentration in shoots and roots of mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings grown at a low or a high nutrient
addition rate. Standard errors are shown beside values; for each treatment, means within a column followed by different letters are significantly different (n = 6, one-way ANOVA, Tukey’s test, α = 0.05).
Shoot N (% of dry weight) Root (% of dry weight)
Week 5 Week 12 Week 5 Week 12
Low N
Control 1.19 ± 0.03 a 1.05 ± 0.04 a 1.31 ± 0.03 a 1.25 ± 0.03 a Thelephora terrestris 1.10 ± 0.03 a 0.95 ± 0.03 a 1.39 ± 0.05 ab 1.43 ± 0.04 b Suillus bovinus 1.12 ± 0.03 a 0.95 ± 0.03 a 1.46 ± 0.04 b 1.61 ± 0.04 c Scleroderma citrinum 0.96 ± 0.02 b 0.80 ± 0.02 b 1.45 ± 0.04 b 1.70 ± 0.05 c
High N
Control 1.35 ± 0.03 x 1.45 ± 0.04 x 1.41 ± 0.03 x 1.40 ± 0.05 x Thelephora terrestris 1.27 ± 0.02 y 1.38 ± 0.04 x 1.64 ± 0.05 y 1.88 ± 0.05 y Suillus bovinus 1.30 ± 0.03 xy 1.48 ± 0.05 x 1.66 ± 0.04 y 1.84 ± 0.04 y
Table 4. The proportion of assimilated nitrogen in the external myce-lia, including carpophores, of mycorrhizal Pinus sylvestris seedlings.
Standard errors are shown beside values; for each treatment, means within a column followed by different letters are significantly different (n = 6, one-way ANOVA, Tukey’s test, α = 0.05).
Fungal nitrogen (% of total)
Week 5 Week 12
Low N
Thelephora terrestris 13 ± 2 a 19 ± 2 a Suillus bovinus 16 ± 2 a 18 ± 2 a Scleroderma citrinum 26 ± 3 b 32 ± 2 b
High N
plant of the mycorrhizal infection by ensuring that both in-fected and non-inin-fected plants have good access to the same nutrients, without affecting the cost of the symbiosis to the host plant (cf. Kähr and Arveby 1986, Nylund and Wallander 1989, Kamminga-Van Wijk et al. 1992).
Inoculation with ectomycorrhizal fungi of Scots pine seed-lings growing in a semi-hydroponic system resulted in de-creased plant growth. All three mycorrhizal fungi inhibited root development more than shoot growth. Harley and Smith (1983) concluded that the shoot/root ratio of mycorrhizal plants increased because of their enhanced ability to absorb nutrients, whereas Smith (1980) argued that the mycorrhizal-induced increase in shoot/root ratio was a feed-back response to an increased nutrient uptake by the mycorrhizae. Hetrick (1991) observed that increases in shoot/root ratio are greater in strongly mycotropic plants. Because we used a semi-hydro-ponic system, the increase in shoot/root ratio of the mycorrhi-zal plants in our study cannot be attributed to increased nutrient uptake or increased growth rate of the mycorrhizal plants: we conclude, therefore, that the increase is a result of the mycor-rhizal infection itself. Decreased retention of carbon in the host roots indicates that the fungus was a stronger sink for carbon than the host roots. A shift in carbon allocation from root growth to fungal growth could explain the increase in
shoot/root ratio of the mycorrhizal plants. Miller et al. (1989) demonstrated that the extramatrical hyphae of ectomycorrhizal pine seedlings constitute a strong sink for carbon, especially when a new network of external hyphae was developing. In
Table 5. Rates of respiration and net assimilation in non-mycorrhizal and mycorrhizal Pinus sylvestris seedlings. Units are: aboveground, nmol
CO2 gDWS−1 s−1; belowground (roots and mycelium), nmol CO2 gDWR−1 s−1. Standard errors are shown beside values; for each treatment, means
within a column followed by different letters are significantly different (n = 6, one-way ANOVA, Tukey’s test, α = 0.05).
Net assimilation rate Aboveground respiration Belowground respiration
Week 5 Week 12 Week 5 Week 12 Week 5 Week 12
Low N
Control 20.2 ± 0.9 ab 14.9 ± 0.5 a 6.5 ± 0.5 a 6.1 ± 0.5 a 3.6 ± 0.3 a 2.3 ± 0.2 a Thelephora terrestris 19.6 ± 0.8 ab 17.4 ± 0.8 ab 6.9 ± 0.6 a 6.3 ± 0.3 a 5.0 ± 0.5 ab 5.1 ± 0.3 b Suillus bovinus 22.1 ± 0.8 a 17.6 ± 0.8 b 7.0 ± 0.5 a 6.4 ± 0.4 a 6.5 ± 0.4 b 5.7 ± 0.4 b Scleroderma citrinum 18.5 ± 1.0 b 14.7 ± 0.7 a 6.4 ± 0.4 a 6.1 ± 0.4 a 5.7 ± 0.3 b 4.7 ± 0.3 b
High N
Control 16.9 ± 1.0 x 14.0 ± 0.7 x 6.2 ± 0.6 x 5.7 ± 0.4 x 4.0 ± 0.3 x 2.5 ± 0.2 x Thelephora terrestris 17.8 ± 1.0 xy 16.1 ± 0.6 x 5.9 ± 0.4 x 5.3 ± 0.3 x 5.5 ± 0.3 y 4.8 ± 0.2 y Suillus bovinus 20.8 ± 0.8 y 14.4 ± 1.0 x 6.2 ± 0.5 x 6.0 ± 0.5 x 7.6 ± 0.4 z 4.1 ± 0.3 y
Figure 1. Above- and belowground ni-trogen partitioning in Pinus sylvestris
seedlings uninoculated (NM) or inocu-lated with Thelephora terrestris (T te),
Suillus bovinus (S bo) or Scleroderma citrinum (S ci). Data were obtained 12 weeks after inoculation. For each plant part, bars with a different letter are sig-nificantly different (n = 6, Tukey’s test, α = 0.05). LN = low nutrient addition rate, HN = high nutrient addition rate.
Table 6. The proportion of net fixed carbon respired by the root systems of non-mycorrhizal and mycorrhizal Pinus sylvestris
seed-lings. Standard errors are shown beside values; for each treatment, means within a column followed by different letters are significantly different (n = 6, one-way ANOVA, Tukey’s test, α = 0.05).
Belowground respiration (% NA)1
Week 5 Week 12
Low N
Control 19 ± 2 a 15 ± 1 a Thelephora terrestris 27 ± 2 b 29 ± 2 b Suillus bovinus 31 ± 2 b 31 ± 2 b Scleroderma citrinum 33 ± 3 b 34 ± 2 b
High N
Control 18 ± 2 x 14 ± 1 x Thelephora terrestris 27 ± 2 y 30 ± 2 y Suillus bovinus 30 ± 2 y 24 ± 1 y
natural ecosystems, roots of young seedlings probably inte-grate into the existing soil mycelia of late-stage fungi so that they do not have to sustain an extensive ectomycorrhizal my-celium as would occur on pioneer sites where no active myce-lia of late-stage fungi are present. For these reasons, we expected that the carbon demand of the pioneer fungus T. ter-restris would differ from that of the late-stage fungus Suillus bovinus. However, we found that host plants of both fungi had similar root respiration rates and similar shoot growth, al-though the fungi did exhibit different carbon investment strate-gies. Thelephora terrestris developed a rather sparse substrate mycelium connected with numerous sporulating carpophores, whereas Suillus bovinus produced a more slow-growing, dense substrate mycelium. The pioneer species was more sensitive to the nutrient regime than the late-stage fungus, and the HN treatment caused a doubling of its biomass compared with the LN treatment.
Although it is well known that mycorrhizal fungi have a large carbon demand, other details of the nutrient requirement for fungal development are sparse. However, such information may be important because tree growth is often limited by nitrogen or phosphorus in natural ecosystems (Attiwill and Adams 1982). In the field, concentrations of minerals in ec-tomycorrhizal carpophores are consistently higher than the concentrations in most plant tissues (Vogt and Edmonds 1980, Vogt et al. 1982). We found that nitrogen use by the mycorrhi-zal fungi was extensive. The relatively small biomass of the external mycelium of the fungi (6--16% of total biomass) retained a large proportion of the total amount of assimilated N (12--32%), a proportion that remained high even in the LN treatment. It is not known how much of this fungal N will become available to the host plant or how much will be used by the fungus for cell wall production, metabolism or the formation of reproductive organs. However, in natural forest soils, the fungal N pool has a rapid turnover because ectomy-corrhizal mycelia are a food source for many organisms (Ek et al. 1994). Also, only a proportion of the hyphae are metabo-lically active. Rygiewicz and Andersen (1994) reported that less than 10% of external hyphae in the roots of 6-month-old Pinus ponderosa Laws. seedlings were active.
Mycelial biomass production of T. terrestris was positively correlated with the nutrient status of the culture solution. Wallander (1995) has postulated that more host carbohydrate becomes available for production of fungal mycelium and fruit bodies under N-limiting conditions than under conditions of adequate N supply. Retention of N for fungal growth is likely to be species dependent. Abuzinadah and Read (1989) reported that the mycorrhizal fungus Paxillus involutus (Batsch: Fr) Fr. retained more N than did the mycorrhizal species Hebeloma crustuliniforme (Bull.: St. Amans) Quél. and Amanita mus-caria (L.: Fr.) Hooker and concluded that fungal retention of N could account for the decrease in growth and N content of the birch seedlings infected with P. involutus. We have demon-strated that N retention in the external mycelia of Scleroderma citrinum was responsible for a significant reduction in N con-centration of the host plant shoots. This reduction, in turn, reduced the relative growth rate of the seedlings (cf. Ingestad
et al. 1986).
Thus, reduced seedling growth in response to ectomycorrhi-zal infection may be the result of increased belowground carbon allocation or it may be a consequence of high nutrient retention by the mycobiont. However, accessibility to nutrient pools will determine the outcome of nutrient retention by ectomycorrhizal fungi. Under natural conditions, the external mycelium of mycorrhizas will likely have better access than uninfected roots to larger or different nutrient pools (Dighton 1991).
Our data on carbon allocation confirmed earlier observa-tions (Reid et al. 1983, Nylund and Walander 1989, Dosskey et al. 1990, Finlay and Soderstrom 1992). At Week 5, when differences in shoot size were small, a slight increase in net photosynthetic rate was observed in seedlings inoculated with Suillus bovinus. Plants colonized with Scleroderma citrinum showed no increase in net assimilation rate; however, this lack of response might be associated with their low shoot N concen-tration. Stimulation of net photosynthesis in mycorrhizal seed-lings can be explained on the basis that photosynthesis is partly controlled by sink demand. However, the increase in net pho-tosynthesis in plants infected with Suillus bovinus did not result in increased shoot growth and failed to compensate for the cost of symbiosis. Belowground respiration in all mycor-rhizal plants was higher than in non-mycormycor-rhizal plants in both nutrient treatments at both harvests. According to Rygiewicz and Andersen (1994), the increased respiration rate of mycor-rhizal plants is associated with a higher fungal respiration rate, a colonization respiration for ectomycorrhizal host roots and a greater proportion of active short roots. The maintenance cost per unit biomass is larger for hyphae than for roots (Fitter 1991). We conclude that the benefit of mycorrhizas to their host plants is not necessarily or exclusively a nutritional bene-fit (Fitter 1991); furthermore, the benebene-fit to the host plant cannot be measured only in terms of an increase in RGR (Harley 1989).
Acknowledgments
We thank Ms. A. Wijnants and Mr. R. Laermans for their technical assistance. Jan Colpaert thanks the Belgian National Fund for Scien-tific Research (N.F.W.O.) for providing a post-doctoral fellowship.
References
Abuzinadah, R.A. and D.J. Read. 1989. The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. V. Nitrogen transfer in birch (Betula pendula) grown in association with mycorrhizal and
non-mycorrhizal fungi. New Phytol. 112:61--68.
Attiwill, P.M. and M.A. Adams. 1993. Nutrient cycling in forests. Tansley Review No. 50. New Phytol. 124:561--582.
Colpaert, J.V., J.A. Van Assche and K. Luijtens. 1992. Relationship between the growth of the extramatrical mycelium of ectomycorrhi-zal fungi and the growth response of Pinus sylvestris plants. New Phytol. 120:127--135.
Deacon, J.W. and L.V. Fleming. 1992. Interactions of ectomycorrhizal fungi. In Mycorrhizal Functioning. An integrative Plant-Fungal
Process. Ed. M.J. Allen. Chapman and Hall, New York, pp 134--160. Dighton, J. 1991. Acquisition of nutrients from organic resources by
Dosskey, M.G., R.G. Linderman and L. Boersma. 1990. Carbon-sink stimulation of photosynthesis in Douglas fir seedlings by some ectomycorrhizas. New Phytol. 115:269--274.
Ek, H., M. Sjögren, K. Arnebrant and B. Söderström. 1994. Extrama-trical mycelial growth, biomass allocation and nitrogen uptake in ectomycorrhizal systems in response to collembolan grazing. Appl. Soil Ecol. 1:155--169.
Finlay, R.D. and D.J. Read. 1986. The structure and function of the vegetative mycelium of ectomycorrhizal plants. II. The uptake and distribution of phosphorus by mycelial strands interconnecting host plants. New Phytol. 103:157--165.
Finlay, R.D. and B. Söderström. 1992. Mycorrhiza and carbon flow to the soil. In Mycorrhizal Functioning. An Integrative Plant-Fungal
Process. Ed. M.J. Allen. Chapman and Hall, New York, pp 134--160. Finlay, R.D., H. Ek, G. Odham and B. Söderström. 1988. Mycelial uptake, translocation and assimilation of nitrogen from 15N-labelled ammonium by Pinus sylvestris plants infected with four different ectomycorrhizal fungi. New Phytol. 110:59--66.
Fitter, A.H. 1991. Costs and benefits of mycorrhizas: implications for functioning under natural conditions. Experientia 47:350--355. Fox, F.M. 1986. Groupings of ectomycorrhizal fungi of birch and pine,
based on establishment of mycorrhizas on seedlings from spores in unsterile soil. Trans. Brit. Mycol. Soc. 87:371--380.
Harley, J.L. 1989. The significance of mycorrhiza. Mycol. Res. 92:129--139.
Harley, J.L. and S.E. Smith. 1983. Mycorrhizal symbiosis. Academic Press Inc., London and New York, 483 p.
Hetrick, B.A.D. 1991. Mycorrhizas and root architecture. Experientia 47:355--362.
Ingestad, T., A.S. Arveby and M. Kähr. 1986. The influence of ectomy-corrhiza on nitrogen nutrition and growth of Pinus sylvestris seed-lings. Physiol. Plant. 68:575--582.
Kähr, M. and A.S. Arveby. 1986. A method for establishing ectomy-corrhiza on conifer seedlings in steady-state conditions of nutrition. Physiol. Plant. 67:333--339.
Kamminga-Van Wijk, C., H.B.A. Prins and P.J.C. Kuiper. 1992. My-corrhizal and non-myMy-corrhizal Douglas fir grown in hydroculture. The effect of nutrient concentration on the formation and function-ing of mycorrhiza. Acta Bot. Neerl. 41:481--495.
Miller, S.L., D.M. Durall and P.T. Rygiewicz. 1989. Temporal alloca-tion of 14C to extramatrical hyphae of ectomycorrhizal ponderosa pine seedlings. Tree Physiol. 5:239--249.
Nylund, J.-E. and H. Wallander. 1989. Effects of ectomycorrhiza on host growth and carbon balance in a semi-hydroponic cultivation system. New Phytol. 112:389--398.
Reid, C.P.P., F.A. Kidd and S.A. Ekwebelam. 1983. Nitrogen nutrition, photosynthesis and carbon allocation in ectomycorrhizal pine. Plant Soil 71:415--432.
Rygiewicz, P.T. and C.P. Andersen. 1994. Mycorrhizae alter quality and quantity of carbon allocated below ground. Nature 369:58--60. Smith, S.E. 1980. Mycorrhizas of autotrophic higher plants. Biol. Rev.
55:475--510.
Tinker, P.B., D.M. Durall and M.D. Jones. 1994. Carbon use efficiency in mycorrhizas: theory and sample calculations. New Phytol.
128:115--122.
Vogt, K.A. and R.L. Edmonds. 1980. Patterns of nutrient concentra-tion in basidiocarps in western Washington. Can. J. Bot. 58:694--698.