Polysomaty analysis in diploid and tetraploid
Portulaca grandiflora
Kei-ichiro Mishiba, Masahiro Mii *
Plant Cell Technology Laboratory,Faculty of Horticulture,Chiba Uni6ersity,648Matsudo,Matsudo City,Chiba271-8510, Japan
Received 24 September 1999; received in revised form 11 January 2000; accepted 16 March 2000
Abstract
Polysomaty analysis of the succulent portulaca (Portulaca grandifloraHook.) plant was carried out using flow cytometry. For both diploid and tetraploid plants, mature leaf tissue was found to have a higher level of polysomaty than young leaf tissue. Mesophyll (MP), bundle sheath (BSP) and water storage protoplasts (WSP) were isolated from leaf tissues of diploid portulaca plants. WSP had a higher degree of endopolyploidization than MP and BSP. The ploidy distribution was also variable in different floral organs. Tetraploid plants artificially induced by colchicine treatment showed a decline in the degree of polysomaty compared to diploid plants. Tetraploid plants had more spherical leaves, a larger number of petals and lower pollen fertility than diploid plants. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Portulaca grandifloraHook.; Polysomaty; Succulent plant; Tetraploid; Floral organ; Flow cytometry
www.elsevier.com/locate/plantsci
1. Introduction
Flow cytometry (FCM) is useful for determining ploidy in a large number of samples after chromo-some doubling treatments [1]. Ploidy levels can usually be easily determined by the relative fluorescence intensity of FCM histograms, how-ever, in the case of plant species that can have a variable frequency of polyploid cells in their tis-sues, it is rather complex to determine the ploidy level using FCM.
The term ‘polysomaty’ means a state of a tissue or an organ that consists of cells with different ploidy levels [2]. Somatic polyploidization is caused by cells entering the ‘endo-cycle’ and oc-curs in the differentiation process [3]. Polysomaty in angiosperms has been found in various tissues and organs, especially in reproductive tissues and organs of various species [3,4]. Recently, FCM has been used for the analysis of polysomaty in plant tissue types and is superior to cytofluorometric
methods as FCM analyzes a large number of nuclei in a short period [5]. Tissues exhibiting higher levels of somatic polyploidization include important sources of food as the endosperm of maize and the pericarp of tomato making the information about polysomaty important for im-proving crop production [6,7]. Somatic poly-ploidization can cause cell expansion [8] giving rise to the suggestion that the extent of polysomaty may affect the size and the morphology of the tissues. The polysomaty of floral organs might be also important for ornamental plant species, al-though only a little information has so far been obtained on polysomaty in floral structural organs [9,10].
According to Rocher et al. [9], polysomaty is commonly observed in leaf tissues of succulent crassulacean acid metabolism (CAM) plant species except for some species with a large genome size. They also showed that the intensity of somatic polyploidization in leaf tissues of a succulent CAM plant is inversely related to its genome size. In addition to this concept, some researchers have attempted to confirm whether the extent of polysomaty is affected by the polyploidization * Corresponding author. Tel: +81-47-3088852; fax: +
81-47-3088721.
E-mail address:[email protected] (M. Mii).
[2,11,12]. Hence, comparison between plants with different ploidy levels may offer some valuable information concerning polysomaty.
The objective of our work was to confirm whether endopolyploidization occurs in succulent tissue, especially in water storage cells, of portu-laca (Portulaca grandiflora Hook.), which has suc-culent leaves. Despite being a C4plant [13,14], our
preliminary observations confirmed that portulaca exhibits polysomaty in many organs. The second objective was to efficiently produce tetraploid plants by using colchicine treatment and FCM determination in portulaca and the third to com-pare the polysomaty patterns in different organs, especially in floral organs, of the diploid and tetraploid plants.
2. Materials and methods
2.1. Plant material
All of the diploid and tetraploid plants used for polysomaty analysis were derived from the same seed source of portulaca (P. grandiflora Hook.; Dai-ichi Seed Co., Ltd., Tokyo, Japan). The phe-notypes of the seed-derived plants were similar except for differences in flower color.
2.2. Tetraploid production
Seeds of portulaca were wrapped in a Miracloth (Chicopee Mills, Inc., NY, USA) and surface ster-ilized with a sodium hypochlorite solution (1% active chlorine) for 15 min and rinsed three times with sterile distilled water. The seeds wrapped in Miracloth were then transferred to a 100 ml Erlen-meyer flask that contained 20 ml of MS liquid medium [15] supplemented with 0.1% (w/v) col-chicine and 3% (w/v) sucrose, at pH 5.8 and maintained for 48 h at 25°C under 24 h illumina-tion (35 mmol m−2 s−1) on a gyratory shaker at
100 cycles min−1. Single colchicine-treated seeds
were then transferred to glass tubes (20×100 mm) containing 10 ml of 0.2% (w/v) gellan gum (Gel-rite; Kelco, Division of Merk and Co. Inc., San Diego, CA), half-strength MS medium supple-mented with 3% sucrose, at pH 5.8. Seeds were maintained at 25°C under 24 h illumination (35
mmol m−2 s−1) with fluorescent lamps.
Germi-nated seedlings were washed to remove the gellan
gum and transferred to pots (9×9 cm) containing vermiculite. Potted plants were acclimatized in a growth chamber at 25°C under 16 h light illumina-tion (45 mmol m−2 s−1) with fluorescent lamps.
After 2 weeks, acclimatized plants were transferred to the greenhouse.
2.3. Plant structures analyzed
For the analysis of ploidy level, greenhouse-grown colchicine-treated plants were used. Young leaves approximately 2 mm long from at least five different branches of each plant were prepared for FCM analysis. The analysis of polysomaty in floral organs consisted of both diploid and te-traploid seed-derived plants obtained by cross-pol-lination between closely related individuals with the same ploidy level since self-pollinated progeny could not be obtained for this species [16]. The diploid and tetraploid seeds were obtained from some flowers of one chimeric individual containing both diploid and tetraploid segments, by cross-pollination with other diploid and tetraploid plants, respectively, and they were germinated and grown in the greenhouse separately. Ploidy level of all progenies including those from a chimeric plant used for the experiment was also confirmed by FCM analysis. Fresh samples were prepared from blooming flower parts, i.e. sepal, petal, filament, anther and stigma together with style. Pollen grains were removed by washing anthers with water carefully just before the FCM analysis.
2.4. Protoplast isolation
Mature leaves (25 – 30 mm length) derived from greenhouse-grown 6 – 8-week old diploid portulaca plants were used for protoplast isolation. Proto-plast isolation, purification and separation were performed according to the method of Ku et al. [13], which was modified for mature leaves.
Leaves were sliced 1-mm thick with a razor blade and incubated in an enzyme solution con-taining 2% (w/v) Cellulase Onozuka R-10 and 0.5% (w/v) Macerozyme R-10 (both Yakult Phar-maceutical Co. Ltd., Japan), 0.2% (w/v) bovine serum albumin (BSA), 1 mM CaCl2, 0.3 M
tube. The crude enzyme solution was then gently overlaid with a 0.2 ml buffer solution containing 0.2% (w/v) BSA, 1 mM CaCl2, 0.3 M sorbitol and
5 mM HEPES-KOH (pH 7.0) and centrifuged at 100×g for 1 min, and the top layer (about 0.2 ml), which mostly contained water storage proto-plasts (WSP), was carefully collected.
The remaining crude enzyme solution was used for the isolation of mesophyll protoplasts (MP) and bundle sheath protoplasts (BSP) according to the procedure of Ku et al. [13]. All three proto-plast fractions were stored on ice before FCM analysis.
2.5. FCM analysis
For FCM analysis, a Partec PA cytometer equipped with a mercury lamp and filter combina-tions of KG1, BG38, UG1, TK420, and GG435, was used. To release nuclei, the sample tissue was chopped with a razor blade in 0.3 ml solution A of Plant High Resolution DNA kit type P (Partec GmbH-Munster) in a plastic Petri dish [5]. For the isolated MP and BSP, 0.3 ml of solution A was added to the pellet of protoplasts after removing the supernatant, and the mixture was then mixed with a pipette. After incubating the crude nuclei suspension for 5 min at room temperature, 1.5 ml of staining solution with the following composi-tion: 10 mM Tris, 50 mM sodium citrate, 2 mM MgCl2, 1% (w/v) PVP, 0.1% (v/v) Triton X-100,
and 2 mg l−1 4%,6-diamidino-2-phenylindole
(DAPI), pH 7.5, was added to the crude suspen-sion and filtered through a 30mm nylon mesh. For
the isolated WSP, solution A was not used and 1 ml of the staining solution was directly added to 0.2 ml of WSP suspension by mixing with a pipette.
After 5 min staining, nuclei suspensions were subjected to the measurement of the relative nu-clear DNA content on a semi-logarithmic scale histogram. Since the histogram peaks on a semi-logarithmic scale are evenly distributed along the abscissa [17], the peak height can be considered as the relative number of nuclei at each ploidy level. At least 5000 nuclei were counted for each sample. The DNA C-values of portulaca used in the present study were estimated by the DNA content relative to the peak for the smallest DNA content for mature pollen, which was regarded as the 1C DNA value.
Statistical analysis (t-test) was performed on the data of percent nuclei distribution at each ploidy level in different organs of both diploid and te-traploid lines.
2.6. Chromosome counts
Chromosome numbers were counted in cells of root tips taken from two tetraploid and two diploid plants, the ploidy levels of which had already been determined by flow cytometry. The collected root tips were pretreated with ice-cold distilled water for 24 h and fixed with a mixture of ethanol and acetic acid (3:1, v/v). The root tips were then rinsed with distilled water, hydrolyzed for 1 min at 60°C with 1 N HCl, squashed under cover glass, and stained with acetocarmine.
3. Results and discussion
3.1. FCM analysis of portulaca lea6es
The results on FCM analysis of diploid portu-laca plants clearly showed that this species has polysomaty in leaf tissues and that the pattern of polysomaty differed according to the developmen-tal stage of the leaves (Fig. 1). There were high ploidy cell populations of up to 64C and a low proportion of the lowest ploidy level (2C) in ma-ture leaf tissues (Fig. 1B). On the contrary, young leaves (2 mm long) mainly consisted of the lowest ploidy cell population (Fig. 1A)
Since P. grandiflora is known to be a succulent C4 plant, it can be considered that the
poly-ploidization in leaf tissues is not directly related to its photosynthetic category (CAM photosynthesis) but may be related to the production of the succu-lent-related cells, namely water storage cells [13].
3.2. Fractionation of protoplasts
were collected from the top layer after centrifuga-tion, whereas other types of protoplasts and debris were pelleted. Further purification and separation of MP and BSP were successfully achieved without
contamination with WSP. The three types of pro-toplasts had different visual characteristics and were clearly distinguished under the light micro-scope as indicated by Ku et al. [13], and purity of
Fig. 1. Typical flow cytometric histograms (log scale) of the nuclei isolated from (A) a diploid young leaf, (B) a diploid mature leaf, (C) a tetraploid young leaf, (D) a tetraploid mature leaf ofP.grandiflora. The peaks correspond to nuclear DNAC-values indicated below each histogram, which were assessed by comparison to the nuclear DNA content of haploid mature pollen.
Table 1
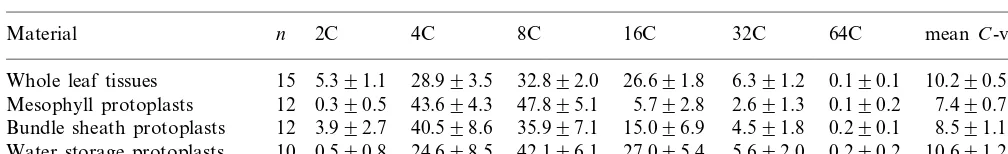
Percent distribution of nuclei in different types of cell from the leaf of diploidP.grandiflora(mean 9S.D.)
2C 4C 8C
Material n 16C 32C 64C meanC-value
5.391.1 28.993.5 32.892.0
Bundle sheath protoplasts 0.290.1 8.591.1
0.590.8 24.698.5 42.196.1 27.095.4
Water storage protoplasts 10 5.692.0 0.290.2 10.691.2
these three types of protoplasts was estimated to be approximately 95% in WSP, 90% in MP and 80% in BSP, respectively (Fig. 2A – C).
3.3. Polysomaty analysis of fractionated protoplasts
Table 1 shows the ploidy distribution of the three types of protoplasts (MP, BSP and WSP) isolated from mature leaves. The original mature leaf (WL) (25 – 30 mm long) from diploid plants had typical pattern of polysomaty that consists of 2C – 64C cells with the main peaks ranging from 4C to 16C. Compared to WL, the ploidy distribu-tion of MP was apparently different and it mostly consisted of 4C and 8C cells with obviously re-duced 2C-, 16C- and 32C-cell proportions. In con-trast, WSP showed a tendency to have a higher ploidy distribution, in particular, nearly five-fold 16C-cell proportion against MP. BSP had an in-termediate ploidy proportion between MP and WSP with higher proportions of 2C and 16C cells than MP.
From the results noted above, there was a ten-dency for the ploidy distribution of WSP to be higher than that of other two types of protoplasts, which was confirmed by the meanC-values (Table 1). This suggests that the higher level of endopoly-ploidization might be beneficial for the functioning of water storage cells. Since the mesophyll and bundle sheath cells also exhibited endopoly-ploidization, though to a lower extent than the water storage cells, further investigation is needed to clarify the significance of the endopolyploidiza-tion in these three types of cells in portulaca.
3.4. Production of tetraploid portulaca plants
Portulaca seeds had already germinated during the colchicine treatment culture period. They were transferred onto MS-solidified medium for further growth. About half (14/30) of the seedlings grew
satisfactorily in vitro and were then acclimatized in growth chamber. The remaining seedlings (16/
30) exhibited initial growth, but then grew with aberrant morphology, having extreme hyper-trophic leaves, expanded roots and hyperhydra-tion. This aberrant morphology was probably due to the antimitotic and toxic effects of colchicine and/or other in vitro culture conditions.
The ploidy level of acclimatized plants was de-termined by FCM analysis. By comparing the FCM histograms of young leaves, diploids and tetraploids could be clearly distinguished by the position of the lowest peak (Fig. 1A and C). The histograms for mature leaves were much more complex than for young leaves, and it was difficult to determine their ploidy levels (Fig. 1B and D). In the previous study on tomato, nearly the same difference in polysomaty pattern was observed between diploid and tetraploid leaf tissues [2] al-though they used only leaves at an early stage of development. Based on these results, we used leaf material that was as young as possible (B2 mm long) to precisely determine the ploidy levels of 14 normally grown plants by FCM analysis. Two plants were revealed to be tetraploid, and two other plants were chimeras of diploid and tetraploid.
3.5. Characteristics of tetraploids
rearranged from stamens (Fig. 2E). The mature pollen size of all tetraploid plants was larger than that of diploid plants, while the pollen fertility and the frequency of seed set of tetraploid plants was reduced as compared with diploid plants.
3.6. Comparison of polysomaty in diploid and tetraploid flower organs
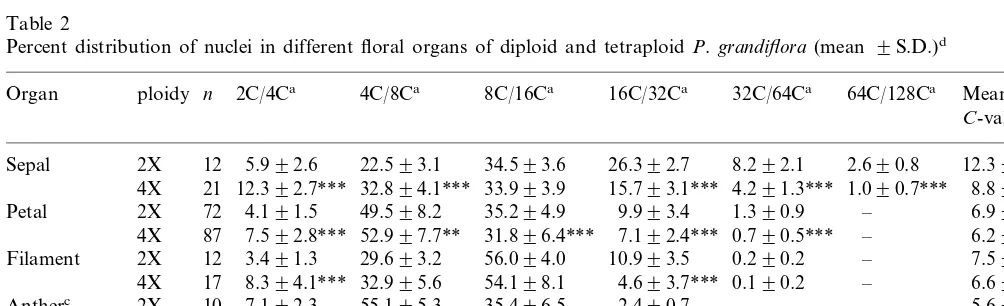
The polysomaty analysis of various floral or-gans with respect to the percentages of polyploid nuclei in both diploid and tetraploid portulaca progenies was performed (Table 2). Polysomaty was found in all organs observed, and each organ showed a characteristic pattern in the distribution of polyploid cells. Genetic variability would be expected among replicate samples (due to individ-ual samples being segregants from outcrosses) yet the S.D.s for ploidy levels of the particular cell types was small, indicating that genotype had little effect on the ploidy distribution. The sepal tissue particularly had the highest ploidy levels, 64C in diploid and 128C in tetraploid plants, respectively. The results on the FCM analysis of other floral organs also showed the same number of peaks in both diploid and tetraploid plants. These results suggest that the development of polysomaty is regulated genetically as suggested by Smulders et al. [2]. Nevertheless, the frequency of the endo
-cy-cle event in each organ was different between diploid and tetraploid plants. There was a signifi-cant reduction in the higher ploidy cells (mainly those higher than 32C) and a significant increase in the lower ploidy cells in tetraploid plants as compared with the diploid plants in each organ (Table 2). These phenomena have also been repre-sented in the previous studies [2,12]. Birader et al. [12] suggested two possible causes for these phe-nomena; (1) the progress of tissue differentiation in tetraploid plants was merely lesser than that in diploid plants or (2) genetic regulation of polyso-maty had occurred according to the ploidy levels. In our present study, a difference in the extent of the development of flower organ was not observed between diploid and tetraploid plants whereas a decline in the degree of polysomaty was shown in the tetraploid plant. Therefore, this decline might be caused by the difference in ploidy.
From the results noted above, portulaca was shown to have a higher degree of polysomaty in succulent leaf tissues and in some floral organs. Since these floral organs, in particular, sepal and petal might not have a specific function for a higher rate of synthetic activity by endopoly-ploidization as shown in suspensors, endosperms, etc [18], the high degree of polysomaty in these floral structural organs might be for the purpose of morphology [8].
Table 2
Percent distribution of nuclei in different floral organs of diploid and tetraploidP.grandiflora(mean 9S.D.)d
64C/128Ca
Organ ploidy n 2C/4Ca 4C/8Ca 8C/16Ca 16C/32Ca 32C/64Ca Mean
C-valueb
aNuclear DNAC-value represented in diploid/tetraploid.
bMean C-values for diploid and tetraploid organs. For comparison, half-values are indicated with tetraploid organs. cPollen grains were not contained in this experiment.
dIn each column, statistical analysis was made between diploid and tetraploid plants for each organ.
* Significant at a=0.05.
** Significant at a=0.01.
References
[1] D.E. Costich, R. Ortiz, T.R. Meagher, L.P. Bruederle, N. Vorsa, Determination of ploidy level and nuclear DNA content in blueberry by flow cytometry, Theor. Appl. Genet. 86 (1993) 1001 – 1006.
[2] M.J.M. Smulders, W. Rus-Kortekaas, L.J.W. Gilissen, Development of polysomaty during differentiation in diploid and tetraploid tomato (Lycopersicon esculentum) plants, Plant Sci. 97 (1994) 53 – 60.
[3] W. Nagl, J. Pohl, A. Radler, The DNA endoreduplica-tion cycles, in: J.A. Bryant, D. Francis (Eds.), The Cell Division Cycle in Plants, Cambridge University Press, New York, 1985, pp. 217 – 232.
[4] F. D’Amato, Role of polyploidy in reproductive organs and tissues, in: B.M. Johri (Ed.), Embryology of An-giosperms, Springer, Berlin, 1984, pp. 519 – 566. [5] D.W. Galbraith, K.R. Harkins, J.M. Maddox, N.M.
Ayres, D.P. Sharma, E. Firoozabady, Rapid flow cyto-metric analysis of the cell cycle in intact plant tissues, Science 220 (1983) 1049 – 1051.
[6] L. Schweizer, G.L. Yerk-Davis, R.L. Phillips, F. Srienc, R.J. Jones, Dynamics of maize endosperm development and DNA endoreduplication, Proc. Natl. Acad. Sci. U.S.A. 92 (1995) 7070 – 7074.
[7] J.H.W. Bergervoet, H.A. Verhoeven, L.J.W. Gilissen, R.J. Bino, High amounts of nuclear DNA in tomato (Lycopersicon esculentum Mill.) pericarp, Plant Sci. 116 (1996) 141 – 145.
[8] J.E. Melaragno, B. Mehrotra, A.W. Coleman, Relation-ship between endopolyploidy and cell size in epidermal tissue of Arabidopsis, Plant Cell 5 (1993) 1661 – 1668. [9] E.J. De Rocher, K.R. Harkins, D.W. Galbraith, H.J.
Bohnert, Developmentally regulated systemic endopoly-ploidy in succulents with small genomes, Science 250 (1990) 99 – 101.
[10] D.W. Galbraith, K.R. Harkins, S. Knapp, Systemic en-dopolyploidy in Arabidopsis thaliana, Plant Physiol. 96 (1991) 985 – 989.
[11] H.R. Owen, R.E. Veilleux, D. Levy, D.L. Ochs, Environ-mental, genotypic, and ploidy effects on endoreduplica-tion within a genotype of Solanum phureja and its derivatives, Genome 30 (1988) 506 – 510.
[12] D.P. Birader, A.L. Rayburn, D.G. Bullock, Endopoly-ploidy in diploid and tetraploid maize (Zea mays L.), Ann. Bot. 71 (1993) 417 – 421.
[13] S.-B. Ku, Y.-J. Shieh, B.J. Reger, C.C. Black, Photosyn-thetic characteristics ofPortulaca grandiflora, a succulent C4dicot, Plant Physiol. 68 (1981) 1073 – 1080.
[14] D. Nishioka, H. Miyake, T. Taniguchi, Suppression of granal development and accumulation of rubisco in dif-ferent bundle sheath chloroplasts of the C4 succulent
plant Portulaca grandiflora, Ann. Bot. 77 (1996) 629 – 637.
[15] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassay with tobacco tissue cultures, Phys-iol. Plant. 15 (1962) 473 – 497.
[16] C.A. Zimmerman, A comparison of breeding systems and seed physiologies in three species of Portulaca L, Ecology 58 (1977) 860 – 868.
[17] L.J.W. Gilissen, M.J. Staveren, J.C. Hakkert, M.J.M. Smulders, H.A. Verhoeven, J. Creemers-Molenaar, The competence of cells for cell division and regeneration in tobacco explants depends on cellular location, cell cycle phase and ploidy level, Plant Sci. 103 (1994) 81 – 91. [18] W. Nagl, Nuclear organization, Ann. Rev. Plant Physiol.
27 (1976) 39 – 69.