www.elsevier.com/locate/ibmb
A third active AKH is present in the pyrgomorphid grasshoppers
Phymateus morbillosus and Dictyophorus spumans
Karl J. Siegert
a,*, Roland Kellner
b, Gerd Ga¨de
aaUniversity of Cape Town, Department of Zoology, Rondebosch 7701, South Africa bMerck KGaA, Biomed Fo/GBT, D-64271 Darmstadt, Germany
Received 7 July 1999; received in revised form 24 March 2000; accepted 30 March 2000
Abstract
The corpora cardiaca of the African pyrgomorphid grasshoppers Phymateus morbillosus and Dictyophorus spumans contain three adipokinetic hormones (AKHs): besides two already known AKHs, Phm–AKH-I and Scg–AKH-II (Ga¨de et al., 1996 [Ga¨de, G., Kellner, R., Rinehart, K.L., 1996. Pyrgomorphid grasshoppers of the genus Phymateus contain species-specific decapeptides of the AKH/RPCH family regulating lipid-mobilisation during flight. Physiol. Entomol. 21, 193–202]), a new AKH-III, denoted Phm– AKH-III, pGlu–Ile–Asn–Phe–Thr–Pro–Trp–Trp–NH2, has been characterised. This is only the second AKH-III identified so far,
thus, only three insect species — all of them grasshoppers — contain three active AKHs. Phm–AKH-III differs from Lom–AKH-III from the migratory locust, Locusta migratoria, only in position 2: isoleucine is present instead of leucine. The structure of the Phm–AKH-III was confirmed by synthesis, subsequent mass determination and reversed-phase high-performance liquid chromato-graphy. The synthetic peptide also induced hyperlipaemia in D. spumans and L. migratoria. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Adipokinetic; Hyperlipaemic; Pyrgomorphid; Grasshopper; Locust
1. Introduction
The corpora cardiaca (CC) of insects synthesise, amongst other peptides, adipokinetic hormones (AKHs) (Hekimi and O’Shea, 1987) which consist of 8–10 amino acids (Ga¨de et al., 1997). AKHs induce the activation of enzymes in the fat body, the main AKH target tissue, and as a result haemolymph lipid, carbohydrate and/or proline levels change in a number of insect species. Steele (1961) reported increased haemolymph carbohydrate concen-trations (hypertrehalosaemic effects) after injection of CC extracts into the American cockroach, Periplaneta amer-icana. In the desert locust, Schistocerca gregaria, however, CC extracts induce increased haemolymph lipid concen-trations (adipokinetic effects)(Mayer and Candy, 1969). In certain beetle species AKHs induce increased proline con-centrations (hyperprolinaemic effects) (Ga¨de, 1997a,b). A
* Corresponding author. Present address: University of Aberdeen, Department of Zoology, Tillydrone Avenue, Aberdeen AB24 2TZ, UK.
E-mail address: [email protected] (K.J. Siegert).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 8 1 - 3
peptide from the CC of locusts, now known as Lom–AKH-I, was the first AKH to be fully characterised (Stone et al., 1976). Lom–AKH-I became the second member of a fam-ily of invertebrate peptides, the AKH/red pigment-concen-trating hormone (RPCH) family. A second AKH was found in S. gregaria (Carlsen et al., 1979) and the migratory locust, Locusta migratoria (Ga¨de et al., 1984); these pep-tides were eventually fully characterised (Siegert et al., 1985; Ga¨de et al., 1986). P. americana also contains two AKH peptides (Scarborough et al., 1984). It is now known that a large number of insect species contain two AKHs whilst others contain only one. The migratory locust, how-ever, is the only insect species known so far to contain three distinct but structurally related active AKHs (Table 1). Interestingly, the closely related species S. gregaria seems to lack this third peptide (Oudejans et al., 1991).
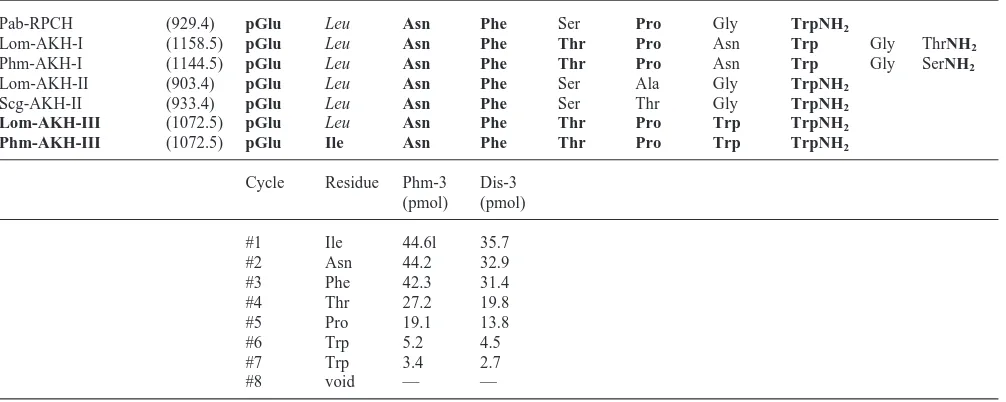
Table 1
Sequences of relevant AKH peptides including the novel AKH-III isolated in the present study. Molecular masses are given in brackets. Residues identical with those in Phm-AKH-III are given in bold type-face, those similar are italicised, e.g. leucine and isoleucine. The sequencing results of deblocked Phm-3 and Dis-3 are also given
Pab-RPCH (929.4) pGlu Leu Asn Phe Ser Pro Gly TrpNH2
Lom-AKH-I (1158.5) pGlu Leu Asn Phe Thr Pro Asn Trp Gly ThrNH2
Phm-AKH-I (1144.5) pGlu Leu Asn Phe Thr Pro Asn Trp Gly SerNH2
Lom-AKH-II (903.4) pGlu Leu Asn Phe Ser Ala Gly TrpNH2
Scg-AKH-II (933.4) pGlu Leu Asn Phe Ser Thr Gly TrpNH2
Lom-AKH-III (1072.5) pGlu Leu Asn Phe Thr Pro Trp TrpNH2
Phm-AKH-III (1072.5) pGlu Ile Asn Phe Thr Pro Trp TrpNH2
Cycle Residue Phm-3 Dis-3 (pmol) (pmol)
#1 Ile 44.6l 35.7
#2 Asn 44.2 32.9
#3 Phe 42.3 31.4
#4 Thr 27.2 19.8
#5 Pro 19.1 13.8
#6 Trp 5.2 4.5
#7 Trp 3.4 2.7
#8 void — —
2. Materials and methods
2.1. Insects
Insects were either taken from a colony at the Depart-ment of Zoology, University of Cape Town, (L. migratoria) or were collected in the field ca. 100 km north of Cape Town near the Langebaan lagoon and kept in a constant temperature room at 25±2°C with bran and oleander leaves ad libitum.
2.2. Extractions
CC were dissected and transferred into Eppendorf tubes which were kept on crushed ice and contained either 50 µl 0.1% trifluoroacetic acid (TFA) or 50 µl 20% acetonitrile in 0.1% TFA. Tissues were then son-icated for 30 s on ice with a Branson sonicator, spun for 10 min (13,000 rpm) and immediately used for reversed-phase high-performance liquid chromatography (RP-HPLC). Other extracts were kept at 270°C until they were taken to Aberdeen for HPLC analysis.
2.3. RP-HPLC
At the University of Cape Town, a Gilson system was used as described elsewhere (Ga¨de, 1985) with an Ultra-sphere C-18 column (4.6 mm×250 mm, plus guard car-tridge; Altex) at a flow rate of 0.8 ml/min; 0.1% TFA (pump A) and acetonitrile (pump B) served as solvents. Peaks were detected in the ultraviolet range at l=206
nm and also with a Shimadzu Model RF 535 fluor-escence HPLC monitor (excitation wavelength 276 nm, emission wavelength 350 nm) to identify
tryptophan-containing peptides. The sensitivities of the spectropho-tometer and fluorometer were selected in a way that pep-tides containing one tryptophan residue had approxi-mately the same peak height in the two detection systems. The output was plotted on conventional chart recorders. Peaks were collected by hand into Eppendorf tubes, dried in vacuo and used for further HPLC analy-ses, mass spectrometry, bioassays or sequencing. Initially, a gradient that started at 20% acetonitrile was used; the percentage of B increased at a rate of 1% B/min (Gradient 1). AKHs were also separated with a gradient that started at 20% B but increased at 0.5% B/min (Gradient 2). For the separation of deblocked pep-tides the gradient started at 30% B and increased at 0.5% B/min (Gradient 3).
CC extracts and collected peptides were also analysed at the University of Aberdeen using identical HPLC equipment. The same column was employed, however, a fluorometer was not available. Chromatograms were stored by the UniPoint software (Version 1.40) which also controlled the HPLC pumps. Data were exported as text files, imported into Microsoft Excel (Version 5.0a for the Power Macintosh) and then copied into CA-Cricket Graph software (Version 1.5) and plotted.
2.4. Bioassays
concentrations were measured with the anthrone reagent (Spik and Montreuil, 1964).
2.5. Deblocking of peptides
Pyroglutamate aminopeptidase from Pyrococcus furiosus was used according to the manufacturer’s instructions (Takara Shuzo). Peptides were dissolved in 25µl of the buffer (5×diluted) supplied with the enzyme and briefly sonicated. Enzyme (0.5 mU) was added, the Eppendorf tube vortexed, spun and incubated for 2 h at 70°C with occasional vigorous agitation. Twenty-five microlitres of HPLC starting solvent (30% acetonitrile/0.1% TFA) was added and the mixture kept on ice until injection onto the RP-HPLC column (Gradient 3).
2.6. Mass spectrometry
Intact and deblocked peptides were analysed by mass spectrometry using a Voyager Elite matrix-assisted laser desorption/ionisation time-of-flight instrument (PerSeptive Biosystems). Samples were prepared in α -cyano-4-hydroxycinnamic acid and spectra acquired in positive, linear mode.
2.7. Peptide sequencing
Deblocked peptides were subjected to automated Edman degradation using a model 477A sequencer con-nected to an on-line model 120 phenylhydantoin amino acid analyser (both from Applied Biosystems).
2.8. Synthetic peptides
Lom–AKH-III and Phm–AKH-III were synthesised by standard solid-phase chemistry, purified on RP-HPLC, and the identities verified by sequence determi-nation and mass spectrometry.
3. Results
3.1. Identification of novel AKH-IIIs
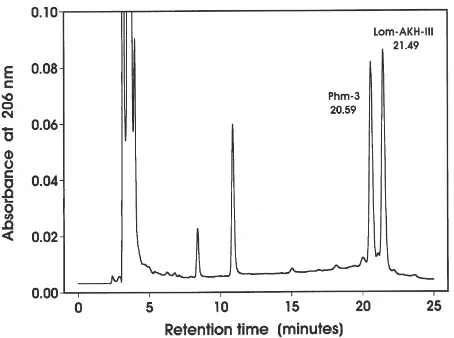
Three main peaks were detected at 206 nm and by fluorometry when TFA CC extracts from adult grass-hoppers Phymateus morbillosus were chromatographed on Gradient 1. The first two peaks corresponded to the two AKHs (Phm–AKH-I and Scg–AKH-II; Table 1) already characterised for this species (Ga¨de et al., 1996), the third peak (Phm-3) eluted much later indicating that the peptide was more hydrophobic than the first two. The fluorometric trace of Phm-3 showed a larger peak than the UV trace which may indicate the presence of two tryptophan residues per peptide and points to Phm-3
Fig. 1. Chromatogram of a TFA extract from 5 pairs of P.
mor-billosus CC (Gradient 2). A chromatogram for extracts of D. spumans
CC is not shown but was generally very similar to the one obtained for P. morbillosus.
being Lom–AKH-III-like or e.g. Lom-MIP-like (Schoofs et al., 1991). The retention time of synthetic Lom–AKH-III on Gradient 1 was very similar to that of Phm-3 (not shown). Using Gradient 2 the two already known AKHs of P. morbillosus had retention times of ca. 24.9 and 26.8 min, respectively. On this gradient Phm-3 had a retention time of ca. 41.9 min (Fig. 1) eluting consist-ently earlier than Lom–AKH-III. When co-chromato-graphed on Gradient 3, Phm-3 eluted after 20.6 min and natural Lom–AKH-III after 21.5 min (Fig. 2).
Mass spectrometry of Phm-3 revealed two signals: m/z=1095.3 (sodium adduct of Phm-3) and m/z=1111.3 (potassium adduct). These values point to a mass for Phm-3 of 1072 which is very close to or identical with the mass of Lom–AKH-III (see Table 1). Comparison
of peak heights of Phm-3 with Lom–AKH-III standards revealed that ca. 40 pmol were present per pair of CC. TFA CC extracts from the grasshopper Dictyophorus spumans were also analysed on Gradient 2. The two major UV and fluorometric peaks, eluting with retention times of 25.0 and 26.8 min respectively, showed masses identical with Phm–AKH-I and Scg–AKH-II. Because of their abundance, both peptides could easily be isolated in sufficient amounts. Edman sequencing after treatment with pyroglutamate aminopeptidase showed that the two peptides were indeed identical with Phm–AKH-I and Scg–AKH-II respectively isolated from P. morbillosus (data not shown in detail). The result was confirmed by co-chromatography of natural and synthetic peptides. A peak, tentatively named Dis-3, with a retention time of 41.8 min was also observed. The peak was smaller than Phm-3 but the UV/fluorescence ratio was again indica-tive of the possible presence of two tryptophan residues. D. spumans contained ca. 10–20 pmol Dis-3 per pair of CC.
Comparative RP-HPLC runs of Dis-3 with Lom– AKH-III or Phm-3 were not performed because of the scarcity of natural peptide: D. spumans was found only in two bushes adjacent to one another ca. 100 km north of Cape Town and it was not at all certain whether more specimens could be collected for sequence analysis. The mass of Dis-3 was determined as m/z=1095.0 (sodium adduct) and 1111.9 (potassium adduct) indicative of the peptide being either identical with Lom–AKH-III or Phm-3 (see above) or very similar.
3.2. Biological activities of Phm-3 and Dis-3
The two peptides isolated above were tested for adipo-kinetic activity using the heterologous locust bioassay. Both peptides were active at doses of 1 pair of CC equiv-alents per insect (Table 2), but the peptides did not pro-duce the same response as 10 pmol Lom–AKH-I which was used as a positive control. The equivalent of 1 pair of P. morbillosus CC produced a significant increase of ca. 15.9 mg lipid/ml (p=0.0001), whilst the same dose
Table 2
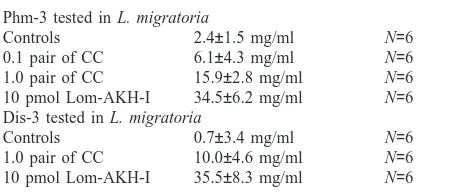
Adipokinetic effects of Phm-3 and Dis-3 isolated from P. morbillosus and D. spumans. Peptides were isolated by RP-HPLC and then tested in L. migratoria. 10 pmol Lom-AKH-I was injected as positive control, saline as negative control
Phm-3 tested in L. migratoria
Controls 2.4±1.5 mg/ml N=6
0.1 pair of CC 6.1±4.3 mg/ml N=6
1.0 pair of CC 15.9±2.8 mg/ml N=6
10 pmol Lom-AKH-I 34.5±6.2 mg/ml N=6
Dis-3 tested in L. migratoria
Controls 0.7±3.4 mg/ml N=6
1.0 pair of CC 10.0±4.6 mg/ml N=6
10 pmol Lom-AKH-I 35.5±8.3 mg/ml N=6
of Dis-3 resulted in an increase of ca. 10.0 mg/ml (p=0.062). Ten picomoles of Lom–AKH-I increased lipid concentrations by 35 mg/ml. Since both peptides had adipokinetic activity and retention times different from Lom–AKH-III, it was concluded that they may indeed represent AKHs of unknown structure.
3.3. Sequencing of the new AKH-IIIs
Because Phm-3 and Dis-3 appear to be AKH peptides, it was concluded that their N-termini are probably blocked by pyroglutamate residues. Phm-3 and Dis-3 were both treated with pyroglutamate aminopeptidase to remove the N-terminal residue as described above and gave rise to shorter peptides with longer retention times (not shown). The deblocked P. morbillosus peptide had a mass of 984 (interpreted as the sodium adduct of the deblocked peptide). The same result was found for Dis-3. Both peptides were subjected to N-terminal Edman sequencing and were found to have the same sequence, Ile–Asn–Phe–Thr–Pro–Trp–Trp (see Table 1 for com-plete structure and yield during sequencing) which is in agreement with their respective masses. The peptide was given the acronym Phm–AKH-III since CC extracts from P. morbillosus were analysed first (see above).
Table 3
Adipokinetic effects of different doses of synthetic Phm-AKH-III in
L. migratoria
Controls 0.4±2.4 mg/ml N=7
10 pmol Phm-AKH-III 8.1±2.9 mg/ml N=7
100 pmol Phm-AKH-III 20.5±3.5 mg/ml N=7
200 pmol Phm-AKH-III 18.1±4.3 mg/ml N=7
10 pmol Lom-AKH-III 15.0±2.2 mg/ml N=7
10 pmol Lom-AKH-I 24.3±2.7 mg/ml N=7
3.4. Biological activity of synthetic Phm–AKH-III
In L. migratoria 10 pmol synthetic Phm–AKH-III induced a moderate but significant increase in haemo-lymph lipid levels, whilst the effect of 100 and 200 pmol was comparable to that of 10 pmol Lom–AKH-I (Table 3).
In D. spumans 10 pmol of synthetic Phm–AKH-III had a reduced adipokinetic effect (Table 4) when com-pared to its effect in L. migratoria (Table 3). The same dose of Phm–AKH-III had no significant effect on hae-molymph carbohydrate levels in D. spumans. These results clearly confirm that the peptide is, by biological activity and structure, a new AKH-III.
4. Discussion
The pyrgomorphid grasshoppers P. morbillosus and D. spumans are only the second and third insect species found to date to contain three active AKHs. Lom–AKH-III was found in L. migratoria but not in the closely related species S. gregaria (Oudejans et al., 1991). The sequence of Phm–AKH-III could easily be assigned after isolation from 30 pairs of P. morbillosus and 50 pairs of D. spumans CC, respectively. Since Lom–AKH-III and Phm-3 had different retention times in RP-HPLC but identical molecular masses, the difference was very likely due to the introduction of isoleucine for leucine or aspartate for asparagine or both changes occurred. Only point mutations in the nucleotide sequence of the respective genes are required to progress from one amino acid to the other. These mutations, however, have con-siderable consequences for the biological activities of the analogues. Pea-CAH-II of P. americana (Scarborough
Table 4
Effects of 10 pmol Phm-AKH-III on haemolymph lipid and carbo-hydrate concentrations in D. spumans
Lipids
Controls 22.5±3.5 mg/ml N=6
10 pmol Phm-AKH-III 4.3±2.6 mg/ml N=8 Carbohydrates
Controls 20.4±2.3 mg/ml N=6
10 pmol Phm-AKH-III 0.6±1.4 mg/ml N=7
et al., 1984) is approximately 65× more active in the adipokinetic assay in locusts (Ga¨de, 1990) than its iso-leucine analogue Poa-HrTH (Ga¨de, 1993), and the aspartate-containing AKH of the fruitfly, Drosophila melanogaster, is 10×more active in the same assay than its uncharged, asparagine-containing analogue (Schaffer et al., 1990).
The fact that three active AKHs have been found in grasshoppers may indicate that only this group of insects contains this number of active AKH peptides. The regu-lation of intermediary metabolism in grasshoppers may be very sophisticated. In this respect it will be of interest to substantiate that S. gregaria really does not contain a third AKH: Lom–AKH-III is synthesised more rapidly than the other two Lom–AKHs (Oudejans et al., 1993) and its half-life in the haemolymph is also much shorter (Oudejans et al., 1996). The peptide’s turnover in the S. gregaria CC may be very high and its titre consequently almost zero. If an AKH-III is really absent from S. gre-garia, this species may have different strategies to regu-late intermediary metabolism than those species contain-ing three active AKHs. Definitive proof for the absence of an AKH-III from S. gregaria, however, will be diffi-cult to obtain and the absence of the gene may have to be established.
Our knowledge of the physiological significance of Phm–AKH-III is extremely limited, but we can look to Lom–AKH-III to assess its possible physiological importance. All three AKHs are released simultaneously from the CC of L. migratoria (Flanigan and Ga¨de, 1999), Lom–AKH-I and Lom–AKH-II are co-localised in gran-ules (Diederen et al., 1987) and therefore differential release does not seem to be an option for the regulation of metabolism. The adipokinetic assay uses male L. migratoria ca. 14 days after the adult moult; in these animals Lom–AKH-III (ED50=1.9 pmol per animal) is not as active as Lom–AKH-I (ED50=0.7 pmol) but far more potent than Lom–AKH-II (ED50=6.5 pmol) (Goldsworthy et al., 1995). This means that considerably less Lom–AKH-I is required to induce the haemolymph lipid levels observed during flight (Mayer and Candy, 1969; Orchard and Lange, 1983) than of the other two peptides. The ED50values, however, have to be seen in relation to the peptides’ half-lives in haemolymph. Lom– AKH-III has a half-life of 3–5 min during rest or flight, whilst Lom–AKH-I and Lom–AKH-II have values of 35–50 min (Oudejans et al., 1996). This may indicate that Lom–AKH-III is removed from the haemolymph or inactivated preferentially when compared to the other two peptides and Lom–AKH-III may be considered almost as potent in the adipokinetic assay as Lom– AKH-I.
Lom–AKH-III is the most potent peptide in this assay (Lee and Goldsworthy, 1995). It may be concluded that it is indeed the most important AKH for overall regu-lation of intermediary metabolism. No reliable quantitat-ive haemolymph titre data are available for the three Lom–AKHs to prove or disprove this hypothesis but the amount of Lom–AKH-III mRNA increased 4.2-fold in locusts during flight whilst the mRNA for Lom–AKH-I and Lom–AKH-II increased only 2-fold (Bogerd et al., 1995); this may indicate that the relative amounts released during flight may change in favour of Lom-AKH-III. We have started to investigate the physiologi-cal function of Phm–AKH-III in pyrgomorphid grass-hoppers. Our first, very preliminary results show that lip-ids increased slightly after injection of 10 pmol Phm– AKH-III into D. spumans whilst carbohydrates remained unchanged. Former studies with synthetic Phm–AKH-I and Scg–AKH-II in P. morbillosus only resulted in very moderate increases in haemolymph lipid concentrations (Ga¨de et al., 1996). A closer investigation into the bio-logical potencies of Phm–AKH-III in African pyrgomor-phid grasshoppers will show whether this peptide is as active as Lom–AKH-III in L. migratoria; such an analy-sis is particularly important because both P. morbillosus sexes have normal wings but only males fly (Ga¨de et al., 1996) and D. spumans is a flightless grasshopper.
Acknowledgements
The authors would like to thank Mr Joey Jacobs who looked after the locust colony, Ms Michelle Alexander for help with the dissections and Ms Heather Marco for performing the mass analyses. Partial financial support was provided by the Foundation for Research Develop-ment, now the National Research Foundation (Pretoria, South Africa), a UCT staff award, a grant from the UCT Visitor’s Fund (to KJS), the Volkswagen Foundation (Hannover, Germany, to RK and GG project number 73024) and the Biotechnology and Biological Sciences Research Council (UK).
References
Bogerd, J., Kooiman, F.P., Pijnenburg, M.A.P., Hekking, L.H.P., Oudejans, R.C.H.M., Van der Horst, D.J., 1995. Molecular cloning of three distinct cDNAs, each encoding a different adipokinetic hormone precursor, of the migratory locust Locusta migratoria. Differential expression of the distinct adipokinetic hormone precur-sors genes during flight activity. J. Biol. Chem. 270, 23038–23043. Carlsen, J., Herman, W.S., Christensen, M., Josefsson, L., 1979. Characterisation of a second peptide with adipokinetic and red-pigment-concentrating activity from the locust corpora cardiaca. Insect Biochem. 9, 497–501.
Diederen, J.H.B., Maas, H.A., Pel, H.J., Schooneveld, H., Jansen, W.F., Vullings, H.G.B., 1987. Co-localisation of the adipokinetic hormones I and II in the same glandular cells and in the same
secretory granules of corpus cardiacum of Locusta migratoria and
Schistocerca gregaria. An immuno-electron-microscopic study.
Cell Tiss. Res. 249, 379–389.
Flanigan, J.E., Ga¨de, G., 1999. On the release of the three locust (Locusta migratoria) adipokinetic hormones: effect of crustacean cardioactive peptide and inhibition by sugars. Z. Naturforsch. 54C, 110–118.
Ga¨de, G., 1980. Further characteristics of adipokinetic and hyperglyca-emic factor(s) of stick insects. J. Insect Physiol. 26, 351–360. Ga¨de, G., 1985. Isolation of the hypertrehalosaemic factors I and II
from the corpus cardiacum of the Indian stick insect, Carausius
morosus, by reversed-phase high-performance liquid
chromato-graphy, and amino-acid composition of factor II. Biol. Chem. Hoppe-Seyler 366, 195–199.
Ga¨de, G., 1990. Structure–function studies on hypertrehalosaemic and adipokinetic hormones: activity of naturally occurring analogues and some N- and C-terminal analogues. Physiol. Entomol. 15, 299–316.
Ga¨de, G., 1993. Structure–activity relationships for the lipid-mobilis-ing action of further bioanalogues of the adipokinetic hormone/red pigment-concentrating hormone family of peptides. J. Insect Phy-siol. 39, 375–383.
Ga¨de, G., 1997a. The explosion of structural information on insect neuropeptides. In: Herz, W., Kirby, G.W., Moore, R.E., Steglich, W., Tamm, C. (Eds.), Progress in the Chemistry of Organic Natural Products. Springer Verlag, Vienna/New York, pp. 1–128. Ga¨de, G., 1997b. Hyperprolinaemia caused by novel members of the
adipokinetic hormone/red pigment-concentrating hormone family of peptides isolated from onitine beetles. Biochem. J. 321, 201– 206.
Ga¨de, G., Goldsworthy, G.J., Kegel, G., Keller, R., 1984. Single step purification of locust adipokinetic hormones I and II by reversed-phase high-performance liquid chromatography and amino-acid composition of the hormone II. Hoppe-Seyler’s Z. Physiol. Chem. 365, 393–398.
Ga¨de, G., Goldsworthy, G.J., Schaffer, M.H., Cook, J.C., Rinehart, K.L. Jr, 1986. Sequence analyses of adipokinetic hormones II from corpora cardiaca of Schistocerca nitans, Schistocerca gregaria and
Locusta migratoria by fast atom bombardment mass spectrometry.
Biochem. Biophys. Res. Commun. 134, 723–730.
Ga¨de, G., Hoffmann, K.H., Spring, J.H., 1997. Hormonal regulation in insects: facts, gaps, and future directions. Physiol. Rev. 77, 963–1032.
Ga¨de, G., Kellner, R., Rinehart, K.L., 1996. Pyrgomorphid grass-hoppers of the genus Phymateus contain species-specific decapep-tides of the AKH/RPCH family regulating lipid-mobilisation during flight. Physiol. Entomol. 21, 193–202.
Gokuldas, M., Hunt, P.A., Candy, D.J., 1988. The inhibition of lipid synthesis in vitro in the locust, Schistocerca gregaria, by factors from the corpora cardiaca. Physiol. Entomol. 13, 43–48. Goldsworthy, G.J., Lee, M.J., Luswata, R., 1995. Adipokinetic
hor-mones: interassay variations in potencies as clues to hormone-receptor interactions in locust. In: First International Conference on Insects, Chemical, Physiological and Environmental Aspects. University of Wroclaw, Zadek-Zdroj, Poland, pp. 17–27. Hekimi, S., O’Shea, M., 1987. Identification and purification of two
precursors of the insect neuropeptide adipokinetic hormone. J. Neu-rosci. 7, 2773–2784.
Lee, M.J., Goldsworthy, G.J., 1995. Acetate uptake test; the basis of a rapid method for determining potencies for structure–activity studies. J. Insect Physiol. 41, 163–170.
Mayer, R.J., Candy, D.J., 1969. Control of haemolymph lipid concen-tration during locust flight: an adipokinetic hormone from the corpora cardiaca. J. Insect Physiol. 15, 611–620.
Orchard, I., Lange, A.B., 1983. Release of identified adipokinetic hor-mones during flight and following neural stimulation in Locusta
Oudejans, R.C.B.M., Vroemen, S.F., Jansen, R.F.R., Van der Horst, D.J., 1996. Locust adipokinetic hormones: carrier-independent transport and differential inactivation at physiological concen-trations during rest and flight. Proc. Natl. Acad. Sci. USA 93, 8654–8659.
Oudejans, R.C.H.M., Kooiman, F.P., Heerma, W., Versluis, C., Slot-boom, A.J., Beenakkers, A.M.T., 1991. Isolation and structure elucidation of a novel adipokinetic hormone (Lom–AKH-III) from the glandular lobes of the corpus cardiacum of the migratory locust,
Locusta migratoria. Eur. J. Biochem. 195, 351–359.
Oudejans, R.C.H.M., Mes, T.H.M., Kooiman, F.P., Van der Horst, D.J., 1993. Adipokinetic peptide hormone content and biosynthesis during locust development. Peptides 14, 877–881.
Scarborough, R.M., Jamieson, G.C., Kalish, F., Kramer, S.J., McEnroe, G.A., Miller, C.A., Schooley, D.A., 1984. Isolation and primary structure of two peptides with cardioacceleratory and hypergly-cemic activity from corpora cardiaca of Periplaneta americana. Proc. Natl. Acad. Sci. USA 81, 5575–5579.
Schaffer, M., Noyes, B.E., Slaughter, C.A., Thorne, C.A., Thorne, G.C., Gaskell, S.J., 1990. The fruitfly Drosophila melanogaster contains a novel charged adipokinetic-hormone-family peptide. Biochem. J. 269, 315–320.
Schoofs, L., Holman, G.M., Hayes, T.K., Nachman, R.J., De Loof, A., 1991. Isolation, identification and synthesis of locustamyoinhibit-ing peptide (Lom-MIP), a novel biologically active neuropeptide from Locusta migratoria. Regul. Pept. 36, 111–119.
Siegert, K.J., Morgan, P.J., Mordue, W., 1985. Primary structures of locust adipokinetic hormones II. Biol. Chem. Hoppe-Seyler 366, 723–727.
Spik, G., Montreuil, J., 1964. Deux causes d’erreur dans les dosages colorimetriques des oses neutres totaux. Bull. Soc. Chim. Biol. 46, 739–749.
Steele, J.E., 1961. Occurrence of a hyperglycaemic factor in the corpus cardiacum of an insect. Nature 192, 680–681.
Stone, J.V., Mordue, W., Batley, K., Morris, H.R., 1976. Structure of the locust adipokinetic hormone, a neurohormone that regulates lipid utilisation during flight. Nature 263, 207–211.