252 (2000) 97–107
www.elsevier.nl / locate / jembe
Effect of juvenile hormone and serotonin (5-HT) on mixis
induction of the rotifer Brachionus plicatilis Muller
a,b ,* c d
Wenresti G. Gallardo , Atsushi Hagiwara , Terry W. Snell
a
Graduate School of Marine Science and Engineering, Nagasaki University, Bunkyo 1-14, Nagasaki852-8131, Japan
b
Aquaculture Department, Southeast Asian Fisheries Development Center (SEAFDEC), Tigbauan, Iloilo5021, Philippines
c
Faculty of Fisheries, Nagasaki University, Bunkyo 1-14, Nagasaki 852-8521, Japan
d
School of Biology, Georgia Institute of Technology, Atlanta, GA 30332-0230, USA
Received 7 December 1999; received in revised form 18 April 2000; accepted 12 June 2000
Abstract
Juvenile hormone (JH) and serotonin (5-HT) were previously shown to enhance mictic (sexual) female production of the rotifer Brachionus plicatilis in batch cultures. To explore the basis of these effects, experiments were conducted on isolated individuals. JH treatment of maternal
21
rotifers with 5 and 50mg ml (18.8 and 187.7mM) resulted in significantly higher (P,0.05) mictic female production in the second (F ) and third (F ) generations. JH treatment was effective2 3
5 21
even at a lower food concentration of 7310 cells ml , but it was not effective when free
21
ammonia was added at 2.4 and 3.1mg ml . Mictic female production was not increased with
21
exposure to 5-HT up to 50mg ml (129.1mM) concentrations. When food level was reduced to
5 21
7310 cells ml , however, 5-HT-treated rotifers produced significantly (P,0.05) more mictic females than the control, particularly in F generation. Mictic female production of 5-HT-treated3
rotifers did not differ from that of the control with or without free ammonia, but the intrinsic rate
21
of natural increase (r) of 5-HT-treated rotifers at 3.1 mg ml free ammonia was significantly higher than the control. These results show that juvenile hormone increases mictic female production under optimum and sub-optimum food levels, whereas 5-HT increases both mictic female production at low food level and population growth rate at high free ammonia concentrations. These compounds could be used to manage rotifer cultures and probe the mechanisms controlling the rotifer life cycle as it switches to mictic reproduction. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Rotifera; Brachionus plicatilis; Sexual and asexual reproduction; Mictic female; Juvenile hormone; 5-Hydroxytryptamine; Free ammonia; Food level
*Corresponding author. Fax: 163-33-335-1008.
E-mail address: [email protected] (W.G. Gallardo).
1. Introduction
The monogonont rotifer Brachionus plicatilis is capable of reproducing both asexually and sexually. In asexual or amictic reproduction, an amictic female rotifer produces clones of herself via ameiotic parthenogenesis, whereas in sexual or mictic reproduction, females produce mictic daughters whose meiotically produced eggs, if not fertilized, will become haploid males, or if fertilized will become resting eggs (cysts). Resting eggs undergo dormancy thereby ensuring survival of the species during adverse environmen-tal conditions. Initiation of mixis reproduction therefore is a critical phase of the life cycle that has important ecological and evolutionary consequences for the population (Pourriot and Snell, 1983; Snell and Boyer, 1988).
The occurrence of sexual or mictic reproduction depends on external and internal factors. Several external factors including population density, temperature, salinity, and food have already been investigated, whereas only few internal factors have been studied (Hagiwara and Hirayama, 1993). These internal factors include genetic variation among clones (Hino and Hirano, 1976; Snell and Hoff, 1985), culture history (Hino and Hirano, 1985, 1988), cumulative generation (Hino and Hirano, 1977), and aging (Snell and Childress, 1987). Hagiwara et al. (1994) indicated that a water soluble substance extracted from rotifer biomass increased sexual reproduction but this substance has not been characterized.
Our initial survey of several vertebrate and invertebrate hormones for effects on B.
21
plicatilis, found that porcine growth hormone (GH, 0.0025 IU ml ) and gamma-amino
21
butyric acid (GABA, 50 mg ml ) increased population growth, whereas juvenile
21
hormone (JH, 0.5 mg ml ) enhanced mictic female production and serotonin
(5-21
hydroxytryptamine, 5-HT; 5 mg ml ) enhanced both population growth and mictic female production in batch cultures (Gallardo et al., 1997). However, since these experiments were in batch cultures, the life history of individual rotifers was not known. We therefore conducted individual culture experiments to determine the intrinsic rate of natural increase (r), net reproduction rate (Ro), and mixis reproduction of hormone-treated rotifers. Subsequent experiments with individually cultured rotifers exposed to GH and GABA showed that GH enhances reproduction when culture conditions are optimum, whereas GABA enhances reproduction when rotifers are stressed (Gallardo et al., 1999). Following this same protocol, we conducted individual culture experiments with JH and 5-HT to confirm their effects on mixis induction. Individual culture reduces the effect of population density on mictic female production (Hino and Hirano, 1976, 1977; Snell and Boyer, 1988; Carmona et al., 1994), thus, we could investigate the effect of hormones on mictic female production of isolated females. Further, individual culture experiments were conducted to determine if JH or 5-HT were still effective in a stressful environment, as was the case of GABA which enhanced population growth through amictic reproduction even at low food or high free ammonia (Gallardo et al., 1999). Snell and Boyer (1988) reported that low food level and high free ammonia con-centration suppress mictic female production.
present study will provide insights into the regulation of the rotifer life cycle by hormonal signals and provide means for manipulating mictic reproduction of B. plicatilis cultures.
2. Materials and methods
2.1. Effective hormone concentration
JH and 5-HT were purchased from Sigma Chemical Company (product numbers J2000 and H7752, respectively). Individual culture experiments were done in 24-well microplates (Iwaki Co., Japan) in volumes of 1 ml of Nannochloropsis oculata
6 21
suspensions of 7310 cells ml . Concentrations of JH and 5-HT tested were 0.05, 0.5,
21
5, and 50mg ml (same concentrations as in Gallardo et al., 1997). For each hormone concentration and the control (no hormone) eight replicate wells were prepared, each with one rotifer.
Amictic eggs of the rotifer B. plicatilis (NH3L strain, Balompapueng et al., 1997) were collected by shaking egg-bearing females vigorously in a screw-capped bottle. Detached eggs were placed in a Petri dish with algal suspension until hatching. When the newly hatched amictic females extruded their first egg, they were pipetted individually
6 21
into the well plates with 1 ml of culture medium (7310 N. oculata cells ml ) at the designated hormone concentration. The experimental conditions were 258C, 22‰ salinity, pH 8 and darkness. These isolated rotifers (eight per treatment) were exposed to JH or 5-HT for 48 h, the same time with our batch culture experiments (Gallardo et al., 1997). After 24 h, offspring were removed to avoid confusing them with the mother during subsequent observations. After 48 h, the mother was transferred daily to a new well containing food suspension without hormone. The first offspring which were exposed to the hormone together with the mother in the first well was not used for F1
culture. The offspring produced in the second well after 48 h were not exposed to the hormone. They were transferred to a new set of multi-well plates for F culture. For F ,1 2
the first offspring of F were transferred and cultured in another set of multi-well plates.1
For each generation, the number of offspring was counted daily and reared until the mother died. When the offspring reached maturity (egg bearing), they were classified as amictic or mictic based on the type of egg they carried (Hagiwara et al., 1988). The intrinsic rate of natural increase (r) was computed based on Birch (1948). From the raw data, fecundity or net reproduction rate (Ro), lifespan and reproductive period were calculated.
2.2. Effect of hormone at low food or high free ammonia
4 5 6
Rotifers were cultured in three food levels (7310 , 7310 , and 7310 N. oculata
21 21 21
cells ml ) with or without hormone (0.5 mg ml JH or 5mg ml 5-HT). Addition of
21
21 21
mg ml JH or 5 mg ml 5-HT). The procedure in the first experiment (effective hormone concentration) was followed in this experiment.
2.3. Statistical analysis
Paired t-tests (Systat, 1997) were performed to identify significant differences between treatments and controls of the r, Ro, reproductive period, lifespan, and the arcsine-transformed mictic female percentage data (Zar, 1999).
3. Results
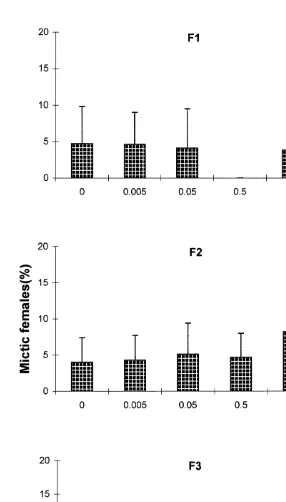
3.1. Effective hormone concentration
In the first generation of JH-treated rotifers, the percentage of mictic females did not differ significantly from that of the control. In the F and F , mictic female production of2 3
21
rotifers treated with 5 and 50 mg ml JH was significantly higher than the control by
21
4.2 and 4.3%, respectively (Fig. 1). In the F , JH at 0.053 mg ml also resulted in a significantly higher percentage of mictic females. Mictic female production of rotifers
21
treated with 5-HT at concentrations ranging from 0.05 to 50mg ml did not differ from the control. There also was no significant difference in intrinsic rate of natural increase (r), net reproduction rate (Ro), lifespan, or reproductive period with any of the JH or 5-HT treatments and controls.
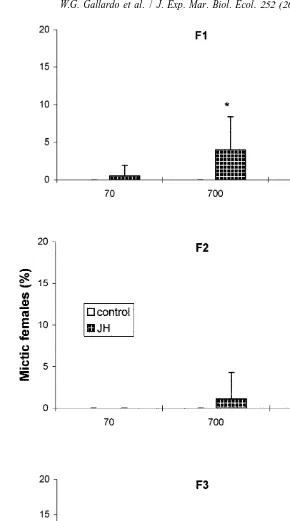
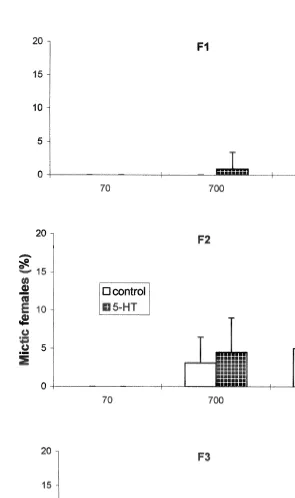
3.2. Effect of hormone at low food or high free ammonia level
4 21
At a low food concentration of 7310 cells ml , there was no mictic female production in either JH or 5-HT experiment (Figs. 2 and 3), except in the F1 of JH-treated rotifers which produced a very low percentage (0.5%) of mictic females. At
5 21
7310 cells ml , mictic females appeared only in JH-treated rotifers (Fig. 2). In the 5-HT experiment, untreated rotifers also had mictic female production, but 5-HT-treated rotifers had more mictic females particularly in the F (P3 ,0.05, Fig. 3). Intrinsic rate of natural increase (r), net reproduction rate (Ro), lifespan, or reproductive period of rotifers with or without hormonal treatment did not differ from that of the control.
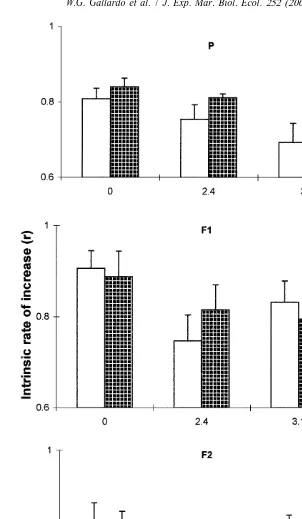
21
With the addition of free ammonia (2.4 and 3.1 mg ml ) to the culture medium, neither JH nor 5-HT treatment resulted in increased mictic female production, but the intrinsic rate of natural increase (r) of 5-HT-treated rotifers (P) was significantly higher than that of the control (P,0.01, Fig. 4).
4. Discussion
Fig. 1. Percent mictic females (mean and S.D.) among F , F and F offspring of B. plicatilis treated with1 2 3
21
21
21
21
because most rotifer mechano- and chemoreceptors sensitive to environmental stimula-tion are in direct contact with external medium (Clement et al. (1983).
21
The effective JH concentrations of 5 and 50mg ml were much higher in individual
21
culture experiments than the 0.05 and 0.5 mg ml in our batch culture experiment (Gallardo et al., 1997). This suggests that a stronger stimulus is needed for mictic female production by isolated rotifers than those in batch cultures where other factors such as population density can contribute to mictic female production (Hino and Hirano, 1976, 1977). The daily renewal of the medium in the individual culture experiment as compared to every 2 days in the batch culture may have diminished the stimulatory effect of conditioned medium as suggested by Hino and Hirano (1976) and Carmona et al. (1994). The positive effect on mictic female production in the F and F even if these2 3
were not directly treated with JH implies that JH has a long lasting effect. This is in agreement with our batch culture results where a significantly higher percentage of mictic females was produced on day 6 and 8 (Gallardo et al., 1997). The low percentages of mictic females in this study are similar to the mictic levels in other studies (Snell and Boyer, 1988; Carmona et al., 1994; Hagiwara et al., 1994).
The absence of mictic female production in untreated rotifers at very low food levels and high free ammonia concentration is in agreement with previous reports that mictic reproduction does not occur during times of environmental stress (Snell, 1986; Snell and Boyer, 1988; Hagiwara et al., 1988). Under limited food conditions, energy is utilized for amictic reproduction, and mictic reproduction is suppressed due to its higher energy requirement (Snell and Boyer, 1988). However, the ability of JH and 5-HT to stimulate
5 21
mictic female production at low food levels of 7310 cells ml suggests that compounds similar to these hormones may have a function in regulating mictic female production of B. plicatilis. The JH effect on F and 5-HT effect on F may indicate that1 3
JH has an immediate effect in contrast to 5-HT.
5
The positive effects of 5-HT on mictic female production at low food level (7310
21
cells ml ) and on intrinsic rate of natural increase at high free ammonia concentration
21
(3.1 mg ml ) confirm our results in batch cultures where 5-HT enhanced both population growth and mictic female production (Gallardo et al., 1997). The negative effect of 5-HT on mictic female production at optimum food density in individual
5
culture and its positive effects on mictic female production at low food (7310
21 21
cells ml ) and on intrinsic rate of natural increase at high free ammonia (3.1 mg ml ) suggest that 5-HT is effective only when there is environmental stress. This is similar to the effect of GABA on asexual reproduction (Gallardo et al., 1999). Both GABA and 5-HT are neurotransmitters in vertebrates and have also been found in crustaceans (Fingerman et al., 1994; Foa and Cooke, 1998). Recently, we also found that GABA and 5-HT are present in the rotifers B. plicatilis and B. rotundiformis (Gallardo et al., 2000). The presence of JH in rotifers is yet unknown.
Gilbert (1963) stated that mictic female production in the freshwater rotifer B.
calyciflorus is controlled by environmental stimuli and is not part of some internal,
stimuli like the compounds produced during conditioning of the medium at high population density. Hagiwara et al. (1994) reported that water soluble extracts from 50 g wet weight rotifer biomass increased sexual reproduction in 5-ml B. plicatilis culture. In
B. plicatilis, JH and 5-HT or similar compounds may have such a stimulatory role, but more work is required to define their mode of action. The endogenous presence of 5-HT in rotifers (Gallardo et al., 2000) helps explain the mechanism of hormone effect in rotifers. Although the presence of JH in rotifers is yet unknown, there is a good possibility that it is endogenously present because of the positive effect of adding JH into the culture medium. The positive effect implies the presence of a receptor for the endogenous hormone.
One practical application of our research findings is on resting egg production. Resting eggs are important in aquaculture (Lubzens, 1989; Hagiwara, 1994) and in toxicity tests using rotifers (Snell and Janssen, 1995). Resting egg production may be enhanced by JH or 5-HT treatment of rotifer mass cultures. The very low dosage
21
(0.05–5 mg ml ) and short exposure (48 h) would not be costly to apply.
This study showed that exogenous presence of JH and 5-HT affects rotifer life history including sexual and asexual reproduction. From the viewpoint of chemical ecology, it is of interest to know whether these substances occur in nature in biologically active concentrations. Such as study may provide new insights on the mixis strategy of rotifer population. Our results also imply that since exogenous hormones such as JH and 5-HT affect rotifer reproduction, some environmental hormones may also modify rotifer reproduction. Endocrine disruptors mimicking such hormones may also affect rotifers (Snell, 1998), thus, rotifers may be used as biomarkers for the presence of endocrine-disrupting chemicals in the environment.
Acknowledgements
This study was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (No. 10660187) and by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (ED-99-II-3-2). [SS]
References
Balompapueng, M.D., Hagiwara, A., Nishi, A., Imaizumi, K., Hirayama, K., 1997. Resting egg formation of the rotifer Brachionus plicatilis using a semi-continuous culture method. Fish. Sci. 63, 236–241. Birch, L.C., 1948. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 17, 15–26. Carmona, M.J., Serra, M., Miracle, M.R., 1994. Relationship between mixis in Brachionus plicatilis and
preconditioning of culture medium by crowding. Hydrobiologia 255–256, 145–256.
Clement, P., Wurdak, E., Amsellem, J., 1983. Behavior and ultrastructure of sensory organs in rotifers. Hydrobiologia 104, 89–130.
Fingerman, M., Nagabushanam, R., Sarojini, R., Reddy, P.S., 1994. Biogenic amines in crustaceans: identification, localization, and roles. J. Crust. Biol. 14 (3), 413–437.
Gallardo, W.G., Hagiwara, A., Tomita, Y., Soyano, K., Snell, T.W., 1997. Effect of some vertebrate and invertebrate hormones on the population growth, mictic female production, and body size of the marine rotifer Brachionus plicatilis Muller. Hydrobiologia 358, 113–120.
Gallardo, W.G., Hagiwara, A., Tomita, Y., Snell, T.W., 1999. Effect of growth hormone and gamma-aminobutyric acid on Brachionus plicatilis (Rotifera) reproduction at low food or high ammonia levels. J. Exp. Mar. Biol. Ecol. 240, 179–191.
Gallardo, W.G., Hagiwara, A., Hara, K.,, Soyano, K., Snell, T.W., (2000). GABA, 5-HT and other amino acids in the rotifers Brachionus plicatilis and B. rotundiformis. Comp. Biochem. Physiol. A. (submitted). Gilbert, J.J., 1963. Mictic female production in the rotifer Brachionus calyciflorus. J. Exp. Zool. 53, 113–124. Hagiwara, A., 1994. Practical use of rotifer cysts. Israeli J. Aquacult. Bamidgeh 46, 13–21.
Hagiwara, A., Hirayama, K., 1993. Preservation of rotifers and its application in the finfish hatchery. TML Conf. Proc. 3, 61–71.
Hagiwara, A., Hino, A., Hirano, R., 1988. Effects of temperature and chlorinity on resting egg formation in the rotifer Brachionus plicatilis. Nippon Suisan Gakkaishi 54, 569–575.
Hagiwara, A., Hamada, K., Hori, S., Hirayama, K., 1994. Increased sexual reproduction in Brachionus plicatilis (Rotifera) with the addition of bacteria and rotifer extracts. J. Exp. Mar. Biol. Ecol. 181, 1–8. Hino, A., Hirano, R., 1976. Ecological studies on the mechanism of bisexual reproduction in the rotifer
Brachionus plicatilis. I. General aspects of bisexual reproduction inducing factors. Bull. Jap. Soc. Sci. Fish. 42, 1093–1099.
Hino, A., Hirano, R., 1977. Ecological studies on the mechanism of bisexual reproduction in the rotifer Brachionus plicatilis. II. Effects of cumulative parthenogenetic generation on the frequency of bisexual reproduction. Bull. Jap. Soc. Sci. Fish. 43, 1147–1155.
Hino, A., Hirano, R., 1985. Relationship between the temperature given at the time of fertilized egg formation and bisexual reproduction pattern in the deriving strain of the rotifer Brachionus plicatilis. Bull. Jap. Soc. Sci. Fish. 51, 511–514.
Hino, A., Hirano, R., 1988. Relationship between water chlorinity and bisexual reproduction rate in the rotifer Brachionus plicatilis. Bull. Jap. Soc. Sci. Fish. 54, 1329–1332.
Lubzens, E., 1989. Possible use of rotifer resting eggs and preserved live rotifers (Brachionus plicatilis) in aquaculture. In: De Pauw, N., Jaspers, E., Ackefors, H., Wilkins, N. (Eds.), Aquaculture: A Biotechnology in Progress. European Aquaculture Society, Bredene, Belgium, pp. 741–750.
Pourriot, R., Snell, T.W., 1983. Resting eggs in rotifers. Hydrobiologia 104, 213–224.
Snell, T.W., 1986. Effect of temperature, salinity and food level on sexual and asexual reproduction in Brachionus plicatilis (Rotifera). Mar. Biol. 92, 157–162.
Snell, T.W., 1998. Chemical ecology of rotifers. Hydrobiologia 387–388, 267–276.
Snell, T.W., Boyer, E.M., 1988. Thresholds for mictic female production in the rotifer Brachionus plicatilis (Muller). J. Exp. Mar. Biol. Ecol. 124, 73–85.
Snell, T.W., Childress, M.J., 1987. Aging and loss of fertility in male and female Brachionus plicatilis (Rotifera). Int. J. Invertebrate Reprod. Develop. 12, 103–110.
Snell, T.W., Hoff, F.H., 1985. The effect of environmental factors on fresting egg production in the rotifer Brachionus plicatilis. J. World Maricult. Soc. 16, 484–497.
Snell, T.W., Janssen, C.R., 1995. Rotifers in ecotoxicology: a review. Hydrobiologia 313–314, 231–247. Systat, 1997. Systat version 7.0. SPSS, USA.