www.elsevier.com / locate / bres
Research report
Enhancement of the acoustic startle response by dopamine agonists
after 6-hydroxydopamine lesions of the substantia nigra pars
compacta: corresponding changes in c-Fos expression in the
caudate–putamen
*
Edward G. Meloni , Michael Davis
The Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA 30322, USA Accepted 25 July 2000
Abstract
Rats with 6-hydroxydopamine (6-OHDA) lesions of the nigrostriatal pathway show enhanced locomotor and stereotyped behaviors when challenged with direct and indirect dopamine (DA) agonists due to the development of postsynaptic supersensitivity. To determine if this phenomenon generalizes to other motor behaviors, we have used this rat model of Parkinson’s disease to examine the effects of the direct dopamine D receptor agonist SKF 82958 and the indirect DA agonist1 L-3,4-dihydroxyphenylalanine (L-DOPA) on the acoustic startle response. In addition, we used the expression of c-Fos protein as a marker of neuronal activity to assess any corresponding drug-induced changes in the caudate–putamen (CPu) after L-DOPA administration. Male Sprague–Dawley rats received bilateral injections of 6-OHDA into the substantia nigra pars compacta and 1 week later were tested for startle after systemic administration of SKF 82958 (0.05 mg / kg) or L-DOPA (1, 5, 10 mg / kg). SKF 82958 produced a marked enhancement of startle with a rapid onset in 6-OHDA-lesioned but not SHAM animals.L-DOPA produced a dose- and time-dependent enhancement of startle in 6-OHDA-lesioned rats that had no effect in SHAM animals even at the highest dose (10 mg / kg). Furthermore,L-DOPA produced a dramatic induction of c-Fos in the CPu in 6-OHDA-lesioned animals. Consistent with other literature, these data suggest that neurons in the CPu become supersensitive to the effects of DA agonists after 6-OHDA-induced denervation of the nigrostriatal pathway and that supersensitive dopamine D receptors may mediate the enhancement of startle seen in the present study.1 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Monoamines and behavior
Keywords: SKF 82958;L-DOPA; D1 receptor; Startle; 6-OHDA
1. Introduction tion of drugs that increase dopamine (DA) transmission increase the acoustic startle response [16]. In particular, the
The acoustic startle reflex in rats is a rapid sensorimotor benzazepine derivative SKF 82958, a selective and
high-response elicited by a sudden and intense auditory stimulus efficacy dopamine D1 receptor agonist [53], markedly
[15]. The amplitude of this short-latency response can be enhances the acoustic startle response [43] by activation of
easily quantified, is sensitive to various behavioral and dopamine D receptors in the basal ganglia [42]. Hence,1
pharmacological manipulations, and has been used exten- the enhancement of this reflex by D agonists has been a1
sively to study the neurocircuitry and neurochemistry useful behavior with which to study the neural mechanisms
involved in the modulation of reflex / motor behavior [14]. underlying dopaminergic control of motor responses.
Along these lines, we have found that systemic administra- Over the past 30 years, this field of research has been
aided by the use of the 6-hydroxydopamine (6-OHDA)-lesioned rat model of Parkinson’s disease [71]. In a
*Corresponding author. Fax:11-404-727-3436.
E-mail address: [email protected] (E.G. Meloni). preparation that models the type of neural degeneration
seen in this akinetic disease, DA-containing cells of the River, Raleigh-Durham, NC) housed in group cages of
nigrostriatal pathway are selectively destroyed by in- four rats each until the time of surgery when they were
tracerebral injection of the neurotoxin 6-OHDA. Typically, singly housed. Animals were maintained on a 12-h light /
the lesion is unilateral, leaving a denervated and an intact dark cycle (lights on at 07.00 h) with food and water
side within the same animal. Subsequently, systemic continuously available.
challenge with DA agonists in this ‘hemi-parkinsonian’ rat
produces a circling behavior that is specific to the type of 2.2. Startle apparatus
DA agonist used. Indirect DA agonists such as
amphet-amine induce ipsilateral rotation [73] by releasing DA at Startle testing of animals was conducted using four
the level of the striatum from the intact side. In contrast, identical stabilimeter devices located in separate,
custom-direct DA agonists such as apomorphine [22] and SKF designed 90370370 cm sound-attenuating chambers.
Ven-38393 [48] induce contralateral rotation, presumably due tilation for each chamber was provided by the ventilation
to activation of supersensitized DA receptors in the unit from an Industrial Acoustics Minibooth (Industrial
denervated striatum [72]. Interestingly, systemic adminis- Acoustic Company, Bronx, New York). Each stabilimeter
tration of the anti-Parkinson’s drugL-3,4-dihydroxyphenyl- consisted of a 9315315 cm Plexiglas and wire-mesh cage
alanine (L-DOPA) also induces contralateral rotation suspended between compression springs within a steel
[24,72], despite the fact that 6-OHDA lesions dramatically frame. The floor of each stabilimeter consisted of four 6.0
reduce the activity (.80%) of aromatic L-amino acid mm diameter stainless steel bars spaced 18 mm apart. Cage
decarboxylase (AADC, i.e. the enzyme responsible for the movement resulted in displacement of an accelerometer
conversion ofL-DOPA into DA) [40]. Spared DA neurons (PCB Piezotronics, Depew, NY) where the resultant
[23], serotonergic neurons [3,25], or striatal interneurons voltage was proportional to the velocity of the cage
that contain AADC [40,51] may all be possible sites for displacement. The analog output of the accelerometer was
the conversion of L-DOPA to DA which can act at amplified (PCB Piezotronics, Model 483B21) and digitized
sensitized striatonigral DA receptors to induce contralateral on a scale of 0–2500 units by an InstruNET device (GW
rotation in 6-OHDA-lesioned animals. Instruments, Model 100B; Somerville, MA) interfaced to a
In the present study, we have used the 6-OHDA lesion Macintosh G3 computer. Startle amplitude was defined as
preparation to examine the effects of the dopamine D1 the peak accelerometer voltage that occurred during the
receptor agonist SKF 82958 on startle in animals with first 200 ms after onset of the startle stimulus. A
sur-destruction of the DA-containing cells of the substantia veillance camera (Burle, Model TC651B with a TC9907a
nigra pars compacta (SNc). Because striatonigral neurons lens, Operational Security Systems, Atlanta, GA) was
mediate the enhancement of startle by SKF 82958 [42], positioned behind each stabilimeter and connected to a TV
putative 6-OHDA-induced supersensitivity of D receptors1 monitor located outside the isolation chamber. A red light
located on these neurons should lead to a potentiated bulb (7.5 W) was located on the ceiling of the startle box
startle response by SKF 82958. In addition, we tested the to provide the illumination for the cameras in the otherwise
effects of the indirect DA agonist L-DOPA on startle in completely dark box.
6-OHDA-lesioned animals. Previous studies have reported Constant wide band background noise (60 dB) was
either no effect [19] or an enhanced startle response only produced by a General Radio noise generator (Type
1390-after high doses of L-DOPA [29] in normal animals. Like B) and delivered through high frequency speakers (Radio
SKF 82958, we predict that L-DOPA should potentiate the Shack Supertweeters, range 5–40 kHz) located 5 cm from
startle response in 6-OHDA-lesioned animals via superse- the front of each cage. Startle responses were evoked by 50
nsitive striatonigral DA receptors. An additional goal of ms bursts of white-noise (5 ms rise-decay) generated by
the present study was to measure any L-DOPA-induced the Macintosh G3 computer (0–22 kHz), amplified by a
changes in the activity of caudate–putamen (CPu) cells Radio Shack amplifier (100 W; Model MPA-200), and
using c-Fos immunohistochemistry, a well-established delivered through the same speakers. Sound level
measure-¨
marker for neuronal activation [64]. Several studies have ments (SPL) were made with a Bruel and Kjaer model
reported a dramatic increase in c-Fos expression in the 2235 sound-level meter (A scale; random input) with the
CPu of unilateral 6-OHDA-lesioned animals after L-DOPA microphone (Type 4176) located 10 cm from the center of
treatment [11,61] that may reflect a supersensitive response the speaker, which approximates the distance of the rat’s
by striatal receptors to DA converted from exogenous ear from the speaker during testing. The presentation and
L-DOPA. sequencing of all stimuli were under the control of the
Macintosh G3 computer using specially designed software (The Experimenter, Glassbeads Inc., Newton, CT).
2. Materials and methods
2.3. Bilateral 6-OHDA lesions 2.1. Animals
Rats were anesthetized with Nembutal (50 mg / kg,
instrument (Model 900) with blunt ear bars. The skin was measurements were made by sampling cage movement
retracted and bilateral holes were drilled in the skull above (200 ms duration) 15 s after the onset of each startle
the SNc. A 28 g stainless steel needle attached to a stimulus.
Hamilton microsyringe (10ml) by polyethylene tubing was
lowered into the brain using the following coordinates: 2.4.3. SKF 82958 and startle
25.3 and 26.5 mm posterior to bregma (two injections / One week after surgery, half of the animals in the
side),62.1 mm lateral to the midline,27.3 mm ventral to 6-OHDA and SHAM groups (n54 / group) received either
dura. A Harvard Apparatus (Model 22) infusion pump was SKF 82958 ([6]-chloro-APB HBr, 0.05 mg / kg) or saline
used to deliver 0.5 ml / injection of 6-OHDA (2.5 mg in (0.9%) and were immediately placed in the startle cages
0.9% saline containing 0.1% ascorbic acid; Sigma, St and received a test session as described above. The dose of
Louis, MO) directly into the SNc at a rate of 0.25ml / min SKF 82958 was chosen based on its weak startle
enhanc-for 2 min followed by a 1 min diffusion wait. SHAM ing effects determined in previous studies [43]. All
in-animals received injections of vehicle into the SNc using jections were subcutaneous (s.c.) and SKF 82958
(Re-the procedure described above. After (Re-the injection, (Re-the search Biochemical International; Natick, MA) was
dis-skull holes were packed with sterile Gelfoam and the solved in 0.9% saline. Three days later, the drug order was
wound was closed with surgical clips. reversed so that systemic treatment with SKF 82958 was a
Because 6-OHDA-lesioned animals showed consummat- within-subjects measure.
ory deficits after recovery from surgery, in addition to the normal availability of food and water, these rats were
2.4.4. L-DOPA and startle
given highly palatable foods such as cookies (Chips Ahoy)
One week after surgery, 6-OHDA animals received a and ground rat chow mixed with water and sucrose. A
matching test as described above and were divided into liquid supplement (PediaLyte) was also placed in a small
four groups (n55 per group) with equivalent levels of
container on the floor of the cage. These methods for
startle. SHAM animals also received a matching test and maintaining the health of aphagic and adipsic
6-OHDA-were divided into two groups (n55 per group) with
lesioned animals, without the necessity for intragastric
equivalent levels of startle. Three days later, animals in feeding tubes, have been previously described [7,13,52]. In
each group were pretreated (i.p.) with the peripheral amino addition, because pilot studies have shown that
6-OHDA-acid decarboxylase inhibitor benserazide (25 mg / kg; lesioned rats tend to lose between 100 and 150 g in the few
Sigma) followed after 20 min by injections (i.p.) of L
-days after surgery, heavier rats (500–600 g) were used as
DOPA (Sigma): 6-OHDA animals (0, 1, 5 or 10 mg / kg), these animals were more resilient to the weight-loss effects
SHAM animals (0 or 10 mg / kg). Ten minutes later, all of bilateral 6-OHDA lesions. SHAM animals weighing
animals received a startle test as described above. Ben-between 400 and 450 g were used such that both groups of
serazide was dissolved in water and given in a volume of 1 rats had roughly equivalent weights at the time of testing.
ml / kg and L-DOPA was dissolved in 1 N HCL, pH
balanced to 7.0 and given in a volume of 2 ml / kg. 2.4. Behavioral procedure
2.4.1. Matching 2.5. Statistical analysis
Rats were placed in the startle cages and received a 5
min acclimation period followed by presentation of five Startle amplitude data were expressed as the
habituating startle stimuli (95 dB, 30 s interstimulus mean6S.E.M. across startle stimuli at each of the five test
interval; ISI). Rats were then presented with 50 startle intensities or as data points collapsed across intensity for
stimuli, 10 at each of five different intensities (80, 85, 90, an analysis of drug effects across time. For SKF 82958
95 and 100 dB) in a semirandom order with a 30 s ISI. effects on startle, significant differences were evaluated
Rats were assigned to the various treatment conditions using analysis of variance (ANOVA) followed by
indi-based on their startle responses such that the mean startle vidual comparisons using Student’s t-tests. Lesion group
level for each group was comparable. In addition to startle (6-OHDA and SHAM) was a between-groups factor and
measurements, baseline activity measurements were made drug treatment (SKF 82958 and saline), intensity (80, 85,
by sampling cage movement (200 ms duration) 15 s after 90, 95, 100 dB) and time were within-groups factors.
the onset of each startle stimulus. Activity level differences were evaluated separately from
the startle amplitude data with an ANOVA.
2.4.2. Startle testing For L-DOPA effects on startle and c-Fos cell counts,
Rats were placed in the startle cages and received a 5 significant differences were evaluated using analysis of
min acclimation period followed by presentation of five variance (ANOVA) followed by individual comparisons
habituating startle stimuli (95 dB, 30 s ISI). Rats were then using Student’s t-tests. Lesion group (6-OHDA and
presented with 100 startle stimuli, 20 at each of five SHAM) and L-DOPA dose (0, 1, 5, and 10 mg / kg) were
different intensities (80, 85, 90, 95 and 100 dB) in a between-groups factors whereas intensity (80, 85, 90,95,
Activity level differences were evaluated separately from Cruz Biotechnology, Inc., Santa Cruz, CA) diluted in
the startle amplitude data with an ANOVA. antibody medium (1:5000). The sections were washed with
PBS and incubated (1 h at room temperature) with a
2.6. Tyrosine hydroxylase (TH) immunohistochemistry biotinylated secondary antibody against rabbit
immuno-globulin G (Jackson Laboratories) in the antibody medium
At the end of the experiment, the animals were over- (1:400). The sections were washed and incubated for 30
dosed with chloral hydrate and perfused intracardially with min with the avidin–biotin complex (Vector Laboratories).
0.9% saline followed by 10% formalin. The brains were The sections were then incubated (10 min at room
removed from the skull and immersed in the fixative for 2 temperature) in 3,39-diaminobenzidine / H O (Sigma) as a2 2
h at 48C. After fixation, the brains were stored for 4 days chromagen for visualization of c-Fos reaction product.
in a 30% sucrose / 0.1 M PBS (pH 7.4) solution at 48C and Sections were mounted on gelatin-coated slides and
cover-subsequently, 30 mm frozen coronal sections were cut slipped with Permount for immunohistological
examina-through the SNc and striatum. Every third section was tion.
collected in a glass vial for a total of 20 sections / vial. The
sections were immunostained using the avidin–biotin– 2.8. Microscopy and cell count
peroxidase (ABC) method [26]. Briefly, the sections were
incubated in 0.6% hydrogen peroxide in 0.1 M PBS (pH For each animal, a c-Fos immunostained section of the
7.4) for 30 min to eliminate endogenous peroxidases. To CPu corresponding to Plate 18 (20.26 mm caudal to
reduce non-specific binding, the sections were preincu- bregma; Fig. 1) from the atlas of Paxinos and Watson [56]
bated in antibody medium (2% normal goat serum, 1% was visualized under bright-field microscopy with a Zeiss
bovine serum albumin, 0.3% Triton X-100 in 0.1 M PBS, Axioscope (Oberkochen, Germany). Still frame, 310
pH 7.4) for 1 h followed by incubation (24 h at room images of the CPu were captured with a Sony DXC5000
temperature) with a primary antibody; mouse monoclonal digital camera interfaced with a Macintosh G3 computer.
antibody against TH (MAB 318, Chemicon, Temecula, To quantify the number of c-Fos-positive cells within the
2
CA) diluted in antibody medium (1:1000). The sections CPu, 1 mm squares in the dorsolateral (DL), dorsomedial
were washed with PBS and incubated (1 h at room (DM), ventrolateral (VL) and ventromedial (VM) CPu (see
temperature) with a biotinylated secondary antibody Fig. 1) were affixed to each of the captured images using
against mouse immunoglobulin G (Jackson Laboratories, Adobe Photoshop software (Adobe System Incorporated,
West Grove, PA) in the antibody medium (1:400). The Mountain View, CA). For each animal, neurons with
sections were washed and incubated for 30 min with the round / oval nuclei clearly stained dark brown were counted
avidin–biotin complex (Vector Laboratories, Burlingame, in each of the four CPu areas and averaged across each
CA). The sections were then incubated (10 min at room treatment group.
temperature) in 3,39-diaminobenzidine / H O (Sigma Fast;2 2
Sigma, St Louis, MO) as a chromagen for visualization of
TH reaction-product. Sections were mounted on gelatin- 3. Results
coated slides and coverslipped with Permount (Fisher
Scientific, Unionville, Ontario) for immunohistological 3.1. 6-OHDA lesions
examination of the extent of the 6-OHDA-induced lesion.
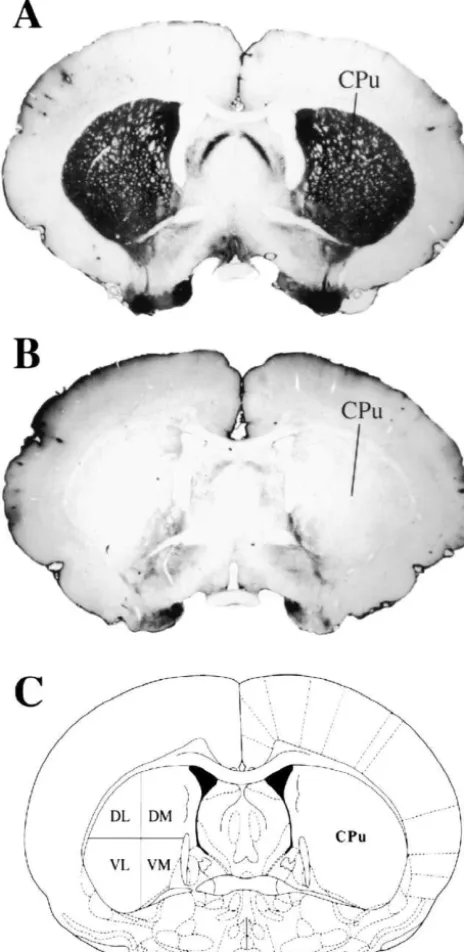
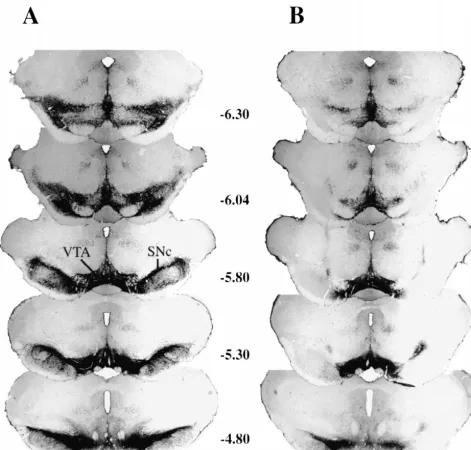
Fig. 2 shows the type of lesion produced after bilateral
2.7. c-Fos immunohistochemistry 6-OHDA injections into the SNc. By using two injections
per side, severe loss of TH-containing cells and processes
Ninety minutes after L-DOPA injections, animals were (TH-containing dendrites in the substantia nigra pars
sacrificed by an overdose of chloral hydrate and perfused reticulata; SNr) was seen throughout the rostrocaudal
intracardially with 0.9% saline followed by 10% formalin extent of the SNc. In addition, most animals had nearly
for 20 min. The brains were removed from the skull and complete sparing of the ventral tegmental area (VTA). Fig.
immersed in the fixative for 2 h at 48C. After fixation, the 1 shows intense TH-staining in the CPu in SHAM animals
brains were stored for 4 days in a 30% sucrose / 0.1 M PBS (Fig. 1A) versus a dramatic loss of TH-staining in the CPu
(pH 7.4) solution at 48C and subsequently, 30 mm frozen in animals that received bilateral 6-OHDA lesions of the
coronal sections were cut through the striatum and DR. SNc (Fig. 1B). Taken together, these data suggest that the
Adjacent sections through each brain region were collected DA-containing cells of the SNc, and subsequently the DA
in a glass vials for a total of 20 sections / vial. For c-Fos innervation of the CPu, were destroyed by 6-OHDA.
immunohistochemistry of the CPu, sections were immuno- Despite being adipsic and aphagic for the first couple
stained using the avidin–biotin–peroxidase (ABC) method days after 6-OHDA lesions, rats responded well to the
described above. Briefly, the sections were incubated (24 h highly palatable foods placed in their cages to stave weight
at room temperature) with a rabbit polyclonal antibody loss and help restore their health. In general,
recovery as these animals were more resilient to the few days of ‘food-deprivation’ as a result of the 6-OHDA lesions.
3.2. Systemic SKF 82958 and startle
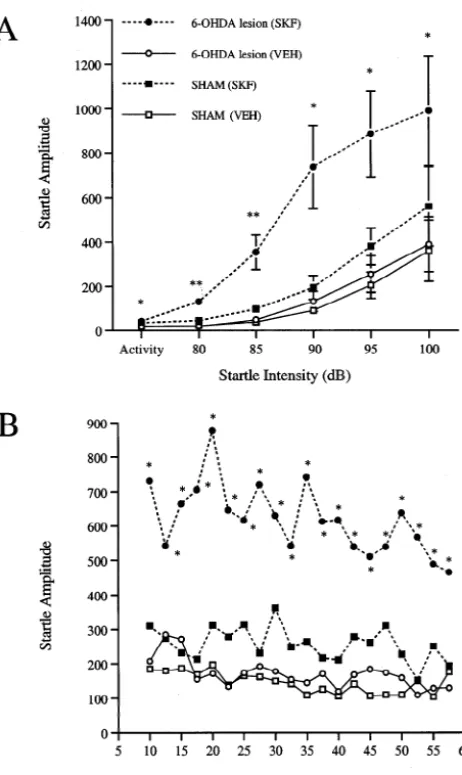
Fig. 3 shows the effect of systemic administration of SKF 82958 (0.05 mg / kg) on startle in SHAM and 6-OHDA-lesioned rats. A two-way ANOVA of the effects of SKF 82958 across intensity (Fig. 3A) revealed a significant
main effect of group [F3,1259.5, P,0.005], intensity
[F4,12540.1, P,0.0001] and a group by intensity
inter-action [F12,4853.07, P,0.005]. Individual comparisons
revealed that this low dose of SKF 82958 produced a significant enhancement of startle at each intensity in 6-OHDA-lesioned animals. SHAM animals showed a trend for a higher startle response by SKF 82958, but this effect was not significant at any intensity. An analysis of activity levels (one-way ANOVA) revealed a significant elevation in activity by SKF 82958 in 6-OHDA-lesioned, but not SHAM, animals.
A two-way ANOVA of the effects of SKF 82958 on startle over time (collapsed across intensity for the duration of the test session; Fig. 3B), revealed a significant main effect of group [F3,1259.5, P,0.005] and time [F19,125
2.0, P,0.01]. The group by time interaction was not
significant. Linear contrast analyses showed that 6-OHDA-lesioned animals that received SKF 82958 had a significant elevation in startle across time compared to those that
received vehicle [F1,12519.8, P,0.001]. SHAM animals
that received SKF 82958 were not significantly different from those that received vehicle.
3.3. Systemic L-DOPA and startle
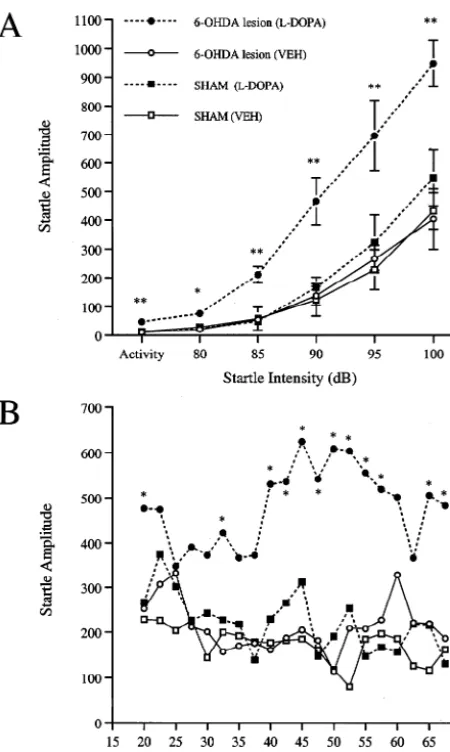
Fig. 4 shows that systemic administration ofL-DOPA (0,
1, 5, and 10 mg / kg) produced a dose-dependent enhance-ment of startle in 6-OHDA-lesioned rats. A two-way ANOVA revealed a significant main effect of dose [F3,165 4.2, P,0.05], intensity [F4,16547.6, P,0.0001] and a group by intensity interaction [F12,6452.7, P,0.005].
Fig. 1. Photomicrographs of coronal sections through the mid-striatum Individual comparisons revealed that compared to
vehicle-stained for tyrosine hydroxylase (black areas) from representative SHAM treated rats, the high dose of L-DOPA (10 mg / kg) pro-(A) and 6-OHDA-lesioned (B) rats corresponding to Plate 18 (20.26 mm
duced a significant enhancement of startle at each
intensi-posterior to bregma, C) from the atlas of Paxinos and Watson [56]. The
ty. Because of large variances in the amplitude of their
caudate–putamen (CPu) was divided into four regions at this level:
startle responses, animals treated with 5 mg / kg ofL-DOPA
dorsolateral (DL), dorsomedial (DM), ventrolateral (VL) and
ventrome-2
dial (VM) and c-Fos positive cells were counted in 1 mm areas within showed a significant elevation at the 80 dB intensity only.
each subdivision. There was no significant effect on startle at any intensity in
animals treated with the low dose ofL-DOPA (1 mg / kg).
the first three to 4 days after surgery before reaching a An analysis of activity levels (one-way ANOVA) revealed
steady body weight. This observation is consistent with a significant elevation in activity by L-DOPA (5 and 10
other reports that rats are able to recover from consummat- mg / kg doses) in these 6-OHDA-lesioned rats.
ory deficits caused by bilateral 6-OHDA lesions and Fig. 5 shows the effect of the high dose ofL-DOPA (10
increase their body weight in a regular fashion when cared mg / kg) on startle in SHAM and 6-OHDA-lesioned
ani-for in this manner [13]. In addition, the use of heavier rats mals. A two-way ANOVA of the effects ofL-DOPA across
Fig. 2. Photomicrographs of coronal sections (in millimeters posterior to bregma) through the substantia nigra pars compacta (SNc) from representative SHAM (A) and 6-OHDA-lesioned (B) rats. Extensive loss of tyrosine hydroxylase (TH)-containing cells and processes (black areas) of the SNc, but not the ventral tegmental area (VTA), was seen after bilateral 6-OHDA-lesions of the SNc.
group [F3,16512.5, P,0.0005], intensity [F4,165109.1, main effect of time, and the group by time interaction, was
P,0.0001] and a group by intensity interaction [F12,645 not significant. Linear contrast analyses showed that L
-5.1, P,0.0001]. Individual comparisons revealed that this DOPA produced a significant elevation in startle across
dose of L-DOPA produced a significant enhancement of time in 6-OHDA-lesioned animals compared to 6-OHDA
startle at each intensity in 6-OHDA-lesioned animals. animals that received vehicle [F1,16517.4, P,0.001].
Startle was not significantly affected byL-DOPA in SHAM SHAM animals that received L-DOPA were not
sig-animals at any intensity. An analysis of activity levels nificantly different from those that received vehicle.
(one-way ANOVA) revealed a significant elevation in
activity byL-DOPA in 6-OHDA-lesioned, but not SHAM, 3.4. c-Fos expression in the CPu
animals.
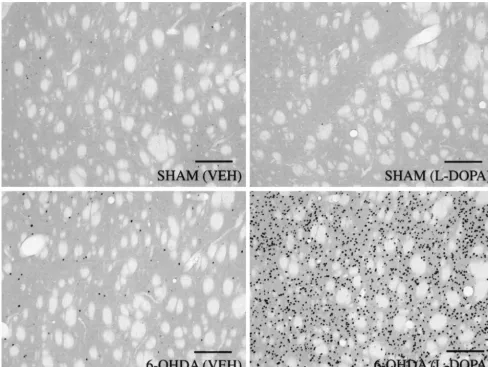
A two-way ANOVA of the effects of L-DOPA (10 Fig. 6 shows representative digitized photomicrographs
mg / kg) on startle over time (collapsed across intensity for of the CPu (DL division) from SHAM and
6-OHDA-the duration of 6-OHDA-the test session; Fig. 5B), revealed a lesioned rats that received either vehicle or L-DOPA
Fig. 4. Dose-dependent enhancement of startle byL-DOPA in
6-OHDA-lesioned rats. Significant differences from vehicle response: *P,0.05, **P,0.005.
a practical application to the study of Parkinson’s disease [72]. Animals with unilateral 6-OHDA lesions of the nigrostriatal dopamine system show a gain of motor function expressed as a contralateral (away from the lesioned side) turning behavior when challenged with
direct DA agonists such as apomorphine (mixed D / D1 2
agonist) [22,79], SKF 38393 (D agonist) [49,63], or the1
Fig. 3. Enhancement of startle by the dopamine D receptor agonist SKF1 indirect DA agonist L-DOPA [72]. These effects are
82958 (SKF; 0.05 mg / kg) in 6-OHDA-lesioned versus SHAM rats across
believed to represent an enhanced sensitivity
(supersen-intensity (A) and time (B). Significant differences from vehicle (VEH)
sitivity) by DA receptors in the denervated striatum to DA
response: *P,0.05, **P,0.005.
agonists which subsequently leads to an enhanced be-havioral response [72].
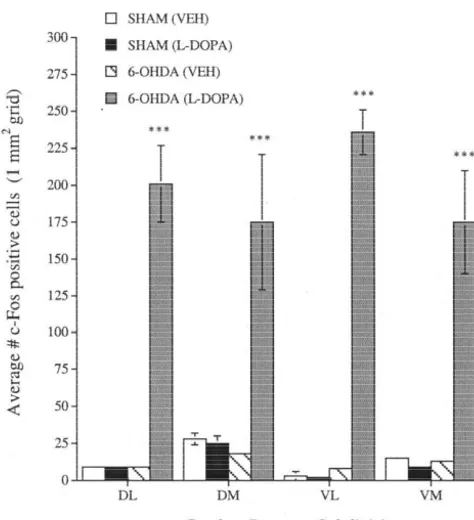
expression of c-Fos throughout the CPu, in all divisions, in In the present study, we have found that the acoustic
6-OHDA-lesioned but not SHAM rats that received L- startle reflex is also sensitive to the effects of specific DA
DOPA (10 mg / kg). Quantification of c-Fos-positive cells agonists in animals with 6-OHDA lesions of the
nigros-2
in each of the CPu subdivisions (1 mm grids) for 6- triatal pathway. Systemic administration of a threshold
OHDA-lesioned and SHAM animals that received either dose of the dopamine D agonist SKF 82958 (0.05 mg / kg)1
vehicle orL-DOPA is shown in Fig. 7. A two-way ANOVA produced a marked enhancement of startle in
6-OHDA-revealed a significant main effect of group [F3,16562.6, lesioned, but not SHAM animals. In fact, this enhancement
P,0.0001] and a group by intensity interaction [F9,485 was roughly equal to that seen in normal animals using a
3.1, P,0.01]. The main effect of CPu subdivision was not 20-fold higher dose of SKF 82958 [43] and thus reflects a
significant. Individual comparisons showed that c-Fos shift-to-the-left in the dose–response curve. Because we
expression was significantly elevated by L-DOPA in each have shown that D receptors mediate SKF 82958-induced1
CPu subdivision in 6-OHDA-lesioned animals. effects on startle [42], the enhanced behavioral response to
SKF 82958 in 6-OHDA-lesioned animals in the present study is consistent with the hypothesis that DA receptors,
4. Discussion specifically D receptors, are supersensitized as a result of1
DA denervation. However, because many DA
agonist-Since the initial report by Ungerstedt [71] over 30 years induced behaviors rely on a D / D1 2 receptor interaction
ago, the 6-OHDA-lesion preparation has been a useful tool [75], includingL-DOPA-induced contralateral turning [69],
attempted to address this concern at the receptor level
3
using [ H]SCH 23390 have produced varied results rang-ing from elevated [10,58], unchanged [34,70], or
dimin-ished [36] D1 receptor binding levels (Bmax) in the
striatum after 6-OHDA lesions. Likewise, an analysis of post-mortem brain tissue from Parkinson’s disease patients has yielded discrepancies with reports of elevated [60] or
unchanged [57] D1 receptor binding levels. Postreceptor
mechanisms such as an increase in the activity of adenylate cyclase [44,45,55] or downstream signal transduction molecules [12] may represent potential sites of 6-OHDA-induced supersensitivity. We propose that the enhancement of startle by SKF 82958 in the 6-OHDA lesion preparation may provide a useful behavior with which to study the biochemical mechanisms involved in the development of neural supersensitivity.
Systemic administration of L-DOPA produces a
dose-dependent enhancement of startle in 6-OHDA-lesioned animals. These results are consistent with a body of work showing a gain of motor function in 6-OHDA-lesioned rats
after systemic L-DOPA treatment (after Ref. 72). The dose
that produced the greatest enhancement of startle in the present study (10 mg / kg) is equivalent to that used in an initial report by Ungerstedt [72] who found an increase in the number of contralateral turns when given to rats with
unilateral 6-OHDA lesions. In addition,L-DOPA-enhanced
startle shows a similar time-course to L-DOPA-induced
rotation [50,65,72] and stereotypy [13] with behaviors
peaking between 30 and 40 min afterL-DOPA
administra-tion. As opposed to the rapid onset of startle enhancement seen with the direct DA agonist SKF 82958, the delay in
the peak enhancement of startle by L-DOPA most likely
reflects the time required for conversion of exogenous
Fig. 5. Enhancement of startle by L-DOPA (10 mg / kg) in
6-OHDA-lesioned versus SHAM rats across intensity (A) and time (B). Significant L-DOPA into DA. This is consistent with microdialysis
differences from vehicle (VEH) response: *P,0.05, **P,0.005. studies showing a rise in striatal DA levels (peaking
between 30 and 40 min) after systemicL-DOPA
adminis-studies are needed to test the contribution of different DA tration (plus a peripheral decarboxylase inhibitor) in
non-receptor subtypes in the enhancement of startle by SKF lesioned rats [17,30,46]. Such an increase in striatal DA
82958 in 6-OHDA-lesioned animals. These results also levels alone, however, is not sufficient to explain the
extend the findings of earlier studies demonstrating an increase in startle as SHAM animals showed no
enhance-enhancement of stereotyped behaviors by direct DA agon- ment of startle afterL-DOPA administration. The combined
ists in bilateral 6-OHDA-lesioned animals [8,13,59]. 6- effect of an increase in striatal DA levels plus
6-OHDA-OHDA-lesioned animals that received SKF 82958 showed induced supersensitivity of DA receptors would explain the
an increase in such motor activities as grooming, sniffing enhanced startle response seen in the present study. This
and licking but very little circling behavior, perhaps owing hypothesis is supported by the observation that animals
to the confines of the startle cage which makes it more with unilateral 6-OHDA lesions also show an increase in
difficult for the animals to turn. Because DA-induced DA levels in the denervated striatum after L-DOPA
treat-stereotypy is believed to be mediated in the CPu [18,28], ment [1,6,54], including a concomitant induction of
con-these data indicate that neurons in this region of the tralateral rotation (30–40 min afterL-DOPA) [78].
striatum may be hyper-responsive to DA agonists in An important question that is raised by these findings
animals with 6-OHDA lesions of the nigrostriatal pathway concerns the locus of conversion ofL-DOPA into DA after
[8]. 6-OHDA lesions. Although not specifically quantified in
Of considerable interest to the phenomenon of DA- the present study, rats with bilateral lesions of the SNc
induced motor responses in the 6-OHDA-lesion model showed extensive loss of DA terminals (estimate .95%
concerns the mechanism by which DA-denervation leads to assessed with TH-immunohistochemistry) in the CPu.
Fig. 6. Digitized photomicrographs (310) of c-Fos expression in the caudate–putamen (CPu, dorsolateral division) from representative SHAM and 6-OHDA-lesioned rats after vehicle (VEH) orL-DOPA (10 mg / kg) treatment. The expression of c-Fos was quantified by counting the darkly stained nuclei (seen as dark dots) of cells in each of the CPu subdivisions. Systemic administration ofL-DOPA produced a dramatic induction of c-Fos throughout the CPu in 6-OHDA-lesioned versus SHAM rats. Scale bars5200mm.
amount of AADC in the striatum by.80% [23,40] leading The expression of the c-Fos protein, the product of the
to the suggestion that other compartments of AADC within immediate early gene c-fos as a marker of neuronal
the striatum may handle the conversion of exogenous activity [64] has been a useful tool with which to study the
L-DOPA into DA [38]. The heavy innervation of the involvement of various brain structures in response to
striatum by 5-HT-containing neurons [74], which have pharmacological challenges. Previous studies have shown
been shown to contain AADC [3,4], represents one po- a marked induction of c-Fos in the CPu of
6-OHDA-tential site of conversion of L-DOPA into DA [72]. That lesioned rats after L-DOPA administration [11,27,47,61],
5-HT neurons are involved in the production of DA is an effect that we have replicated in the present study. The
supported by the observation thatL-DOPA-induced circling direct D1 agonist SKF 38393 also induces c-Fos in the
in 6-OHDA-lesioned rats is reduced after destruction of denervated striatum [66] expressed in the medium spiny
5-HT fibers [20,25]. This finding, however, has not been neurons of the striatonigral pathway [62]. We found the
replicated by others [39]. A more likely site for DA pattern of c-Fos distribution to be uniform throughout the
conversion from L-DOPA after 6-OHDA lesions may be rostrocaudal extent of the CPu (data not shown) as well as
striatal neurons that contain AADC [40,51,68]. While post- the four quadrants (DL, DM, VL, and VM) of the
mid-mortem brain tissue from the CPu of Parkinson’s patients striatum (Fig. 7). Taken together, these data provide a
shows a reduction in AADC (.85%) [35], the existence of locus (the CPu) for the denervation supersensitivity
pro-AADC-containing striatal neurons that could assume the duced by 6-OHDA lesions of the SNc and suggest that
role of converting L-DOPA into DA remains to be de- striatonigral neurons are the most likely neural substrate to
adminis-References
[1] E.D. Abercrombie, A.E. Bonatz, M.J. Zigmond, Effects ofL-DOPA
on extracellular dopamine in striatum of normal and 6-hydroxy-dopamine-treated rats, Brain Res. 525 (1990) 36–44.
[2] T. Akai, M. Ozawa, M. Yamaguchi, E. Mizuta, S. Kuno, Behavioral involvement of central dopamine D1 and D2 receptors in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned parkinsonian monkeys, Jap. J. Pharmacol. 67 (1995) 117–124.
[3] R. Arai, N. Karasawa, M. Geffard, T. Nagatsu, I. Nagatsu, Immuno-histochemical evidence that central serotonin neurons produce dopamine from exogenous l-dopa in the rat, with reference to the involvement of aromatic l-amino acid decarboxylase, Brain Res. 667 (1994) 295–299.
[4] R. Arai, N. Karasawa, I. Nagatsu, Aromatic L-amino acid decarbox-ylase is present in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study, Brain Res. 706 (1996) 177–179.
[5] P.J. Blanchet, R. Grondin, P.J. Bedard, K. Shiosaki, D.R. Britton, Dopamine D1 receptor desensitization in MPTP lesioned primates, Eur. J. Pharmacol. 309 (1996) 113–120.
[6] T. Brannan, P. Knott, H. Kaufmann, M. Yahr, In vivo studies of striatal dopamine release and metabolism following administration ofL-DOPA, Adv. Neurol. 53 (1990) 75–81.
[7] G.R. Breese, B.R. Cooper, Chemical lesioning: catecholamine pathways, in: R.D. Myers (Ed.), Methods in Psychobiology, Vol. 3, Fig. 7. Quantification of c-Fos expression in each of the caudate– Academic Press, New York, 1977, pp. 27–46.
putamen subdivisions (see Fig. 1 for abbreviations) for SHAM and [8] G.R. Breese, G.E. Duncan, C. Napier, S.C. Bondy, L.C. Iorio, R.A. 6-OHDA-lesioned animals that received vehicle (VEH) orL-DOPA (10
Mueller, 6-hydroxydopamine treatments enhance behavioral re-mg / kg) and were tested for startle (behavioral data presented in Fig. 5). sponses to intracerebral microinjections of D1- and D2-dopamine ***P,0.0005 compared to vehicle response. agonists into nucleus accumbens and striatum without changing dopamine antagonist binding, J. Pharmacol. Exp. Ther. 240 (1987) 167–176.
[9] J.M. Brotchie, Adjuncts to dopamine replacement: a pragmatic approach to reducing the problem of dyskinesia in Parkinson’s
tration of DA agonists. However, because DA receptors are disease, Movement Disord. 13 (1998) 871–876.
[10] M. Buonamici, C. Caccia, M. Carpentieri, L. Pegrassi, A.C. Rossi,
located on both the cell bodies and terminals of
striatonigr-G. Di Chiara, D-1 receptor supersensitivity in the rat striatum after
al neurons (in the SNr) [21,76], DA denervation would
unilateral 6-hydroxydopamine lesions, Eur. J. Pharmacol. 126
most likely affect DA receptors in both areas. This is (1986) 347–348.
consistent with reports that infusion of dopamine agonists [11] D.G. Cole, J.H. Growdon, M. DiFiglia, Levodopa induction of Fos
into the SNr produces a locomotor response in 6-OHDA- immunoreactivity in rat brain following partial and complete lesions
of the substantia nigra, Exp. Neurol. 120 (1993) 223–232.
lesioned, but not intact, animals [31,67]. Furthermore, this
[12] D.G. Cole, L.A. Kobierski, C. Konradi, S. Hyman,
6-hydroxy-hypothesis is supported by our previous report that
activa-dopamine lesions of rat substantia nigra up-regulate activa-
dopamine-tion of D1 receptors in the SNr is necessary for the induced phosphorylation of the cAMP-response element-binding
enhancement of startle by SKF 82958 [42] as well as other protein in striatal neurons, Proc. Natl. Acad. Sci. USA 91 (1994)
DA agonist-induced motor behaviors [33,37,77]. Thus, the 9631–9635.
[13] I. Creese, S.D. Iversen, The pharmacological and anatomical
sub-SNr may be an additional site at which DA agonists can
strates of the amphetamine response in rats, Brain Res. 83 (1975)
affect motor behaviors in 6-OHDA lesioned animals
419–436.
[50,54]. [14] M. Davis, Neurochemical modulation of sensory-motor reactivity:
In conclusion, the results of the present study demon- acoustic and tactile startle reflexes, Neurosci. Biobehav. Rev. 4
strate that the acoustic startle response is sensitive to the (1980) 241–263.
[15] M. Davis, The mammalian startle response, in: R.C. Eaton (Ed.),
effects of DA agonists in animals with 6-OHDA lesions of
Neural Mechanisms of Startle Behavior, Plenum Publishing
Corpo-the nigrostriatal DA system and may be a useful behavior
ration, New York, 1984, pp. 287–351.
with which to study the neural circuitry and mechanisms [16] M. Davis, R.L. Commissaris, J.V. Cassella, S. Yang, L. Dember, T.P. underlying the regulation and generation of movement. In Harty, Differential effects of dopamine agonists on acoustically and
addition, the modulation of startle by DA agonists in the electrically elicited startle responses: comparison to effects of strychnine, Brain Res. 371 (1986) 58–69.
6-OHDA-lesioned rat may serve as a practical research
¨
[17] M.A. De Souza Silva, C. Mattern, R. Hacker, C. Tomaz, J.P.
tool in the identification of better drugs in the treatment of
Huston, R.K.W. Schwarting, Increased neostriatal dopamine activity
movement disorders such as Parkinson’s disease, as well after intraperitoneal or intranasal administration ofL-DOPA: on the
as the development of adjuncts to dopamine replacement role of benserazide pretreatment, Synapse 27 (1997) 294–302.
induced by amphetamine microinjected into striatum: an anatomical [39] E. Melamed, F. Hefti, J. Liebman, A.J. Schlosberg, R.J. Wurtman, mapping study, Neuroscience 61 (1994) 81–91. Serotonergic neurons are not involved in action of L-dopa in
Parkinson’s disease, Nature 283 (1980) 772–774. [19] L.D. Fetcher, The effect of L-dopa, clonidine and apomorphine on
the acoustic startle reaction in rats, Pharmacol. Biochem. Behav. 2 [40] E. Melamed, F. Hefti, D.J. Pettibone, J. Liebman, R.J. Wurtman, (1974) 161–172. Aromatic L-amino acid decarboxylase in rat corpus striatum: implications for action of L-dopa in parkinsonism, Neurology 31 [20] O.S. Gershanik, R.E. Heikkila, R.C. Duvoisin, The role of serotonin
(1981) 651–655. neurons in the action of L-Dopa in an animal model of
Parkinson-ism, Neurology 29 (1979) 553. [41] E. Melamed, J. Zoldan, G. Friedberg, I. Ziv, A. Weizmann, Involvement of serotonin in clinical features of Parkinson’s disease [21] M.B. Harrison, R.G. Wiley, G.F. Wooten, Selective localization of
and complications of L-DOPA therapy, Adv. Neurol. 69 (1996) striatal D receptors to striatonigral neurons, Brain Res. 528 (1990)1
545–550. 317–322.
[42] E. Meloni, M. Davis, Involvement of the substantia nigra pars [22] F. Hefti, E. Melamed, B.J. Sahakian, R.J. Wurtman, Circling
reticulata in D dopamine receptor agonist facilitation of the behavior in rats with partial, unilateral nigro-striatal lesions: effect 1
acoustic startle response in rats, Soc. Neurosci. Abstr. 23 (1997) of amphetamine, apomorphine, and DOPA, Pharmacol. Biochem.
1856. Behav. 12 (1980) 185–188.
[43] E.G. Meloni, M. Davis, Enhancement of the acoustic startle [23] F. Hefti, E. Melamed, R.J. Wurtman, The site of dopamine formation
response in rats by the dopamine D receptor agonist SKF 82958, in rat striatum after l-dopa administration, J. Pharmacol. Exp. Ther. 1
Psychopharmacology 144 (1999) 373–380. 217 (1981) 189–197.
[44] R.K. Mishra, E.L. Gardner, R. Katzman, M.H. Makman, Enhance-[24] B. Henry, A.R. Crossman, J.M. Brotchie, Characterization of
ment of dopamine-stimulated adenylate cyclase activity in rat enhanced behavioral responses to L-DOPA following repeated
caudate after lesions in substantia nigra: evidence for denervation administration in the 6-hydroxydopamine-lesioned rat model of
supersensitivity, Proc. Natl. Acad. Sci. USA 71 (1974) 3883–3887. Parkinson’s disease, Exp. Neurol. 151 (1998) 334–342.
[45] R.M. Mishra, A.M. Marshall, S.L. Varmuza, Supersensitivity in rat [25] A.S. Hollister, G.R. Breese, R.A. Mueller, Role of monoamine
caudate nucleus: effects of 6-hydroxydopamine on the time course neural systems in L-dihydroxyphenylalanine-stimulated activity, J.
of dopamine receptor and cyclic AMP changes, Brain Res. 200 Pharmacol. Exp. Ther. 208 (1979) 37–43.
(1980) 47–57. [26] S.M. Hsu, L. Raine, H. Fanger, Use of the avidin-biotin-peroxidase
[46] S. Miwa, P.-G. Gillberg, P. Bjurling, N. Yumoto, I. Odano, Y. complex (ABC) in immunoperoxidase techniques, J. Histochem.
Watanabe, B. Langstrom, Assessment of dopamine and its metabo-Cytochem. 29 (1981) 577–585.
lites in the intracellular and extracellular compartments of the rat [27] Y. Ishida, I. Kuwahara, K. Todaka, H. Hashiguchi, T. Nishimori, Y.
11
striatum after peripheral administration of L-[ C]DOPA, Brain Res. Mitsuyama, Dopaminergic transplants suppress L-DOPA-induced
578 (1992) 122–128. Fos expression in the dopamine-depleted striatum in a rat model of
Parkinson’s disease, Brain Res. 727 (1996) 205–211. [47] M. Morelli, A. Cozzolino, A. Pinna, S. Fenu, A. Carta, G. Di [28] P.H. Kelly, Drug-induced motor behavior, in: L.L. Iversen, S.D. Chiara, L-Dopa stimulates c-fos expression in dopamine denervated Iversen, S.H. Snyder (Eds.), Handbook of Psychopharmacology, Vol. striatum by combined activation of D-1 and D-2 receptors, Brain 8, Plenum Press, New York, 1977, pp. 295–331. Res. 623 (1993) 334–336.
[29] L. Kokkinidis, E.P. MacNeill, Potentiation of d-amphetamine and [48] M. Morelli, S. Fenu, A. Carta, G. Di Chiara, Effect of MK 801 on L-Dopa-induced acoustic startle activity after long-term exposure, priming of D1-dependent contralateral turning and its relationship to Psychopharmacology 78 (1982) 331–335. c-fos expression in the rat caudate-putamen, Behav. Brain Res. 79 [30] K. Koshimura, T. Ohue, Y. Akiyama, A. Itoh, S. Miwa, L-DOPA (1996) 93–100.
administration enhances exocytotic dopamine release in vivo in the [49] M. Morelli, S. Fenu, A. Pinna, G. Di Chiara, Opposite effects of rat striatum, Life Sci. 51 (1992) 747–755. NMDA receptor blockade on dopaminergic D1- and D2-mediated [31] M.R. Kozlowski, S. Sawyer, J.F. Marshall, Behavioural effects and behavior in the 6-hydroxydopamine model of turning: relationship supersensitivity following nigral dopamine receptor stimulation, with c-fos expression, J. Pharmacol. Exp. Ther. 260 (1992) 402–
Nature 287 (1980) 51–53. 408.
[32] S. Kuno, Differential therapeutic effects of dopamine D1 and D2 [50] A. Mura, J. Feldon, M. Mintz, Re-evaluation of the striatal role in agonists in MPTP induced parkinsonian monkeys: clinical implica- the expression of turning behavior in the rat model of Parkinson’s tions, Eur. Neurol. 38 (Suppl.) (1997) 18–22. disease, Brain Res. 808 (1998) 48–55.
[33] G.J. LaHoste, J.F. Marshall, Nigral D and D receptors mediate the1 2 [51] A. Mura, D. Jackson, M.S. Manley, S.J. Young, P.M. Groves, behavioral effects of dopamine agonists, Behav. Brain Res. 38 Aromatic L-amino acid decarboxylase immunoreactive cells in the (1990) 233–242. rat striatum: a possible site for the conversion of exogenousL-DOPA [34] S.Z. Langer, C. Pimoule, G.P. Reynolds, H. Schoemaker, Dopa- to dopamine, Brain Res. 704 (1995) 51–60.
3
minergic denervation does not affect [ H]-SCH 23390 binding in the [52] R.D. Myers, G.E. Martin, 6-OHDA lesions of the hypothalamus: rat striatum: similarities to Parkinson’s disease, Br. J. Pharmacol. 87 interaction of aphagia, food palatability, set-point for weight regula-(1986) 161. tion, and recovery of feeding, Pharmacol. Biochem. Behav. 1 (1973) [35] K.G. Lloyd, L. Davidson, O. Hornykiewicz, The neurochemistry of 329–345.
Parkinson’s disease: effect of L-DOPA therapy, J. Pharmacol. Exp. [53] K.M. O’Boyle, D.E. Gaitanopolis, D.E. Brenner, J.L. Waddington, Ther. 195 (1975) 453–464. Agonist and antagonist properties of benzazepine and thienopyridine [36] J.F. Marshall, R. Navarrete, J.N. Joyce, Decrease striatal D1 binding derivatives at the D1 dopamine receptor, Neuropharmacology 28
density following mesotelencephalic 6-hydroxydopamine injections: (1989) 401–405.
an autoradiographic analysis, Brain Res. 1989 (1989) 247–257. [54] D. Orosz, J.P. Bennett, Simultaneous microdialysis in striatum and [37] A.J. Mayorga, J.T. Trevitt, A. Conlan, G. Gianutsos, J.D. Salamone, substantia nigra suggests that the nigra is a major site of action of Striatal and nigral D1 mechanisms involved in the antiparkinsonian L-dihydroxyphenylalanine in the ‘hemiparkinsonian’ rat, Exp. effects of SKF 82958 (APB); studies of tremulous jaw movements Neurol. 115 (1992) 388–393.
in rats, Psychopharmacology 143 (1999) 72–81. [55] M. Parenti, S. Gentleman, M.C. Olianas, N.H. Neff, The dopamine [38] E. Melamed, Interactions of exogenous L-Dopa with nigrostriatal receptor adenylate cyclase complex: evidence for post recognition dopaminergic neurons in Parkinson’s disease, Adv. Neurol. 53 site involvement for the development of supersensitivity,
[56] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, 6-hydroxydopamine-lesioned rat, Neurosci. Lett. 34 (1982) 277– 3rd ed, Academic Press, New York, 1997. 282.
3
[57] C. Pimoule, H. Schoemaker, G.P. Reynolds, S.Z. Langer, [ H]SCH [68] F. Tison, N. Mons, M. Geffard, P. Henry, The metabolism of 23390 labeled D1 dopamine receptors are unchanged in schizophre- exogenous L-Dopa in the brain: an immunohistochemical study of nia and Parkinson’s disease, Eur. J. Pharmacol. 114 (1985) 235– its conversion to dopamine in non-catecholaminergic cells of the rat
237. brain, J. Neural Transm. 3 (1991) 27–39.
[58] M.L. Porceddu, O. Giorgi, G. De Montis, S. Mele, L. Cocco, E. [69] J.M. Trugman, C.L. James, G.F. Wooten, D1 / D2 dopamine receptor Ongini, G. Biggio, 6-hydroxydopamine-induced degeneration of stimulation byL-DOPA, Brain 114 (1991) 1429–1440.
nigral dopamine neurons: differential effect on nigral and striatal [70] J.M. Trugman, C.J. Pronsky, G.F. Wooten, Basal ganglia dopamine D-1 dopamine receptors, Life Sci. 41 (1987) 697–706. depletion does not alter D1 dopamine receptor binding properties, [59] T.C. Price, H.C. Fibiger, Apomorphine and amphetamine stereotypy Adv. Neurol. 53 (1990) 107–110.
after 6-hydroxydopamine lesions of the substantia nigra, Eur. J. [71] U. Ungerstedt, 6-hydroxy-dopamine induced degeneration of central Pharmacol. 29 (1974) 249–252. monoamine neurons, Eur. J. Pharmacol. 5 (1968) 107–110. [60] R. Raisman, R. Cash, M. Ruberg, F. Javoy-Agid, Y. Agid, Binding [72] U. Ungerstedt, Postsynaptic supersensitivity after
6-hydroxy-3
of [ H]SCH 23390 to D-1 receptors in the putamen of control and dopamine induced degeneration of the nigro-striatal dopamine Parkinsonian subjects, Eur. J. Pharmacol. 113 (1985) 467–468. system, Acta Physiol. Scand. 367 (Suppl.) (1971) 89–93. [61] G.S. Robertson, D.G. Herrera, M. Dragunow, H.A. Robertson, [73] U. Ungerstedt, G.W. Arbuthnott, Quantitative recording of rotational
L-DOPA activates c-fos in the striatum ipsilateral to a 6-hydroxy- behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriat-dopamine lesion of the substantia nigra, Eur. J. Pharmacol. 159 al dopamine system, Brain Res. 24 (1970) 485–493.
(1989) 99–100. [74] R.P. Vertes, A PHA-L analysis of ascending projections of the dorsal [62] G.S. Robertson, S.R. Vincent, H.C. Fibiger, Striatonigral projection raphe nucleus in the rat, J. Comp. Neurol. 313 (1991) 643–668.
neurons contain D1 dopamine receptor-activated c-fos, Brain Res. [75] J.L. Waddington, S.E. Daly, P.G. McCauley, K.M. O’Boyle, Levels 523 (1990) 288–290. of functional interaction between D1-like and D2-like dopamine [63] H.A. Robertson, M.R. Peterson, K. Murphy, G.S. Robertson, D1- receptor system, in: H.B. Niznik (Ed.), Dopamine Receptors and
dopamine receptor agonists selectively activate striatal c-fos in- Transporters, New York, 1994, pp. 511–537.
dependent of rotational behavior, Brain Res. 503 (1989) 346–349. [76] K.K.L. Yung, J.P. Bolam, A.D. Smith, S.M. Hersch, B.J. Ciliax, A.I. [64] S.M. Sagar, F.R. Sharp, T. Curran, Expression of c-fos protein in Levey, Immunocytochemical localization of D and D dopamine1 2
brain: metabolic mapping at the cellular level, Science 240 (1988) receptors in the basal ganglia of the rat: light and electron
micro-1328–1331. scopy, Neuroscience 65 (1995) 709–730.
[65] R.I. Schoenfeld, N.J. Uretsky, Enhancement by 6-hydroxydopamine [77] D.M. Yurek, S.B. Hipkens, Intranigral injections of SCH 23390 of the effects of dopa upon the motor activity of rats, J. Pharmacol. inhibit SKF 82958-induced rotational behavior, Brain Res. 639
Exp. Ther. 186 (1973) 616–624. (1994) 329–332.
¨
[66] P.E. Simson, K.B. Johnson, H.A. Jurevics, H.E. Criswell, T.C. [78] T. Zetterstrom, M. Herrera-Marschitz, U. Ungerstedt, Simultaneous Napier, G.E. Duncan, R.A. Mueller, G.R. Breese, Augmented measurement of dopamine release and rotational behavior in 6-sensitivity of D1-dopamine receptors in lateral but not medial hydroxydopamine denervated rats using intracerebral dialysis, Brain striatum after 6-hydroxydopamine-induced lesions in the neonatal Res. 376 (1986) 1–7.
rat, J. Phar. Exp. Ther. 263 (1992) 1454–1463. [79] M.J. Zigmond, E.M. Stricker, Supersensitivity after intraventricular [67] M.S. Starr, M. Summerhayes, Multifocal brain sites for apomor- 6-hydroxydopamine: relation to dopamine depletion, Experientia 36