www.elsevier.com / locate / bres
Research report
Increased ferric iron content and iron-induced oxidative stress in the
brains of scrapie-infected mice
a a a c a
Nam-Ho Kim , Seok-Joo Park , Jae-Kwang Jin , Myung-Sang Kwon , Eun-Kyoung Choi ,
d a,b ,
*
Richard I. Carp , Yong-Sun Kim
a
Institute of Environment and Life Science, Hallym Academy of Sciences, 1 Ockcheon-Dong, Chuncheon, Kangwon-Do 200-702, South Korea
b
Department of Microbiology, College of Medicine, Hallym University, 1 Ockcheon-Dong, Chuncheon, Kangwon-Do 200-702, South Korea
c
Department of Veterinary Medicine, Kangwon National University, 2 Hyoja-Dong, Chuncheon, Kangwon-Do 200-701, South Korea
d
New York State Institute for Basic Research in Development Disabilities, 1050 Forest Hill Road, Staten Island, New York, NY 10314, USA
Accepted 22 August 2000
Abstract
Scrapie is a transmissible neurodegenerative disease of sheep and goats. The neuropathological changes include vacuolation, astrocytosis, the development of amyloid plaques in some instances, and neuronal loss. The mechanisms involved in neuronal cell death in scrapie are not known. Recently, we reported the presence of oxidative stress in the brains of scrapie-infected animals and suggested that this is the main mechanism that induces neuronal cell loss. It is known that oxidative stress induced by free radicals is associated with iron
21 31
accumulation; this association led to an examination of the levels of iron (total iron, Fe and Fe ) in the brains of control and 31
scrapie-infected mice by biochemical methods. In the scrapie-infected group, both the level of total iron and the Fe level were significantly increased in cerebral cortex, striatum, and brainstem as compared to the values in the control group. A shift in the ratio of
21 31
Fe / Fe was observed in the same regions of infected mice. Additionally, in this scrapie model, we confirmed the presence of oxidative stress, as evidenced by the increase of free malondialdehyde. These results suggest that iron metabolism is changed and that iron-induced oxidative stress partly contributes to neurodegeneration in scrapie infection. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Degenerative disease: other
Keywords: Scrapie; Iron; Oxidative stress; Neurodegeneration
1. Introduction successfully transmitted to mice which led to the develop-ment of experidevelop-mental animal models that show different Prion diseases are transmissible neurodegenerative dis- scrapie-strain specific pathological findings and incubation eases in humans and other animals [9,11]. Scrapie, the periods [5–7]. The pathogenesis of neuronal cell death in archetype prion disease, is a natural disease of sheep and neurodegenerative diseases, including prion diseases, re-goats, and its characteristics include a long preclinical mains unclear.
period, progressive ataxia, tremor, and death [3,19]. Histo- Recently, it was proposed that oxidative stress induced pathological changes are primarily in the brain and include by free radicals causes neuronal cell loss in neurodegenera-vacuolation, astrocytosis, neuronal loss and, in some cases, tive diseases [8,17,25]. The basis of the claim is that amyloid plaque formation [3,9,11]. Scrapie has been various oxidants are produced continuously in the diseases and that antioxidants and antioxidant enzymes are de-pleted. Thus, excessive formation of hydrogen peroxide
*Corresponding author. Tel.: 182-33-240-1951; fax: 1
82-33-241-and oxygen-derived free radicals can cause lipid
peroxida-3422.
E-mail address: [email protected] (Y.-S. Kim). tion, protein oxidation, DNA damage and cell death. The
N.-H. Kim et al. / Brain Research 884 (2000) 98 –103 99
brain and nervous system are especially vulnerable to 2.3. Determination of iron level in brain and blood free-radical damage for a number of reasons: the brain is
poor in endogenous antioxidants, consumes high levels of Individual brain samples stored at2708C were dried for oxygen and contains large amounts of lipid and iron which 48 h by using a freeze-dryer. Dried brain and blood are involved in the formation of free radicals [14,15,20]. samples were weighed. Each sample was wet-acid digested Iron is particularly important because it is involved in a with concentrated nitric–perchloric acid mixture (4 to 1 number of critical biological functions including protein ratio). An aliquot of each sample was analyzed by using synthesis, DNA replication, and membrane receptor forma- iron flame or graphite atomic absorption spectrophotometer tion [13]. In the normal brain, iron is not reactive despite (Z-8100, Hitachi, Japan) [23].
its high level, probably because its absorption, transport and storage are tightly regulated. If there is an excess of
ionic iron, iron-induced oxidative damage can occur [10]. 2.4. Measurement of ferrous, ferric and total iron in Redox reactions that generate oxygen-free radicals are brain
catalyzed by transition metals such as iron, copper and
manganese [1]. Iron is able to convert H O2 2 into toxic Ferrous iron was determined using the ferrous iron OHE(Fenton reaction), and redox cycling of iron between chelator Ferrozine kit (Hoffmann-La Roche). Brain
sam-21 31
ples were homogenized in 1.0 ml of buffer (sodium its Fe (ferrous) and Fe (ferric) valence states can lead
acetate, pH 4.5, 38% guanidine hydrochloride) and 50:1 of to the formation of a cascade of free radicals and
con-39 mM Ferrozine. Homogenates were divided into two sequent tissue damage [14,26]. Thus, although iron is
21
portions. In the first portion, Fe was determined, and 10 physiologically essential, iron can also be a toxin, and
mg of granulated ascorbic acid was added to the next abnormal iron metabolism is implicated in human
neuro-31 21
portion to reduce Fe to Fe . These homogenates were logical disorders such as Alzheimer’s disease, Parkinson’s
incubated for 20 min at 378C and centrifuged at 15 000 disease and Huntington’s disease [13].
rpm for 30 min, and then the absorbances of the super-Recently, we reported that changes in the levels of
natants and iron standards were read at 578 nm [27]. antioxidant enzymes and the occurrence of free
radical-induced oxidative mitochondrial dysfunction are observed in neurodegeneration in scrapie [8,20]. For this reason, we
2.5. Analysis of lipid peroxidation measured iron levels in the brains and blood in
scrapie-infected mice and compared these values to those in
21 31
Malondialdehyde (MDA) formation level was measured controls. In addition, Fe and Fe valence states and
using the thiobarbituric acid (TBA) colored reaction and lipid peroxidation were examined.
HPLC [20,24]. Briefly, 14 mM SDS, 1.25 M acetic acid (pH 3.5) and 21 mM TBA were added to 0.2 ml of 10% brain homogenate (w / v). The mixture was then heated at
2. Material and methods
1008C for 30 min. After cooling, the mixture was cen-trifuged at 3000 g for 30 min, and absorption of super-2.1. Mouse and Scrapie strain
natants was measured at 535 nm by using a spec-trophotometer. The content of free MDA in brain homoge-Male C57BL / 6J mice, 4–6 weeks of age, were obtained
nates was determined at 270 nm by HPLC on an amino-from the Experimental Animal Center of Hallym
Universi-phase (Shimadzu, 25034 mm) column with an
ty. ME7 scrapie strain was provided by Dr. Alan
Dickin-acetonitrile–0.03 M Tris buffer, pH 7.4 (1:9, v / v). Results son (Neuropathogenesis Unit, Edinburgh, UK). For the
are expressed as nmol / mg protein. scrapie-infected group, mice were inoculated
intracerebral-ly with 30 ml of 1% (w / v) brain homogenate in 0.01 M PBS (phosphate-buffered saline, pH 7.4) prepared from
2.6. Statistical analysis C57BL mice infected with the ME7 scrapie strain. Control
mice were inoculated with 1% brain homogenate of
Statistical significance was evaluated using the Student’s uninfected C57BL mice.
t-test. Values of P,0.05 and P,0.01 were considered statistically significant.
2.2. Sample collection
After 15065 days, mice developed clinical symptom
3. Results
(tremor, ataxia) and were anesthetized with sodium pen-tobarbital. Blood was collected from the vena cava
3.1. Level of total iron in the brain and blood caudalis with a syringe containing heparin and stored at
2208C. Brains were removed from anesthetized mice and
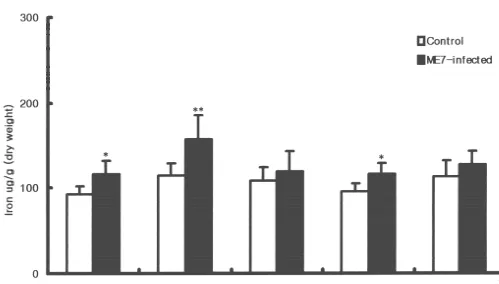
Fig. 1. Iron (Fe) concentration in control and ME7-infected C57BL mice. Iron concentration in (A) whole brain and (B) blood. Iron measurement was done by using atomic absorption spectrophotometry. Data are expressed as mean6S.D. (whole brain, blood, n55). Statistical differences between scrapie-infected group and control group were calculated by Student’s t-test: *, P,0.05.
by 28% (P,0.05) in the scrapie-infected group compared and brainstem (20%, P,0.05) of the infected group to the control group (Fig. 1A). There was no significant compared to controls. Interestingly, the highest iron level difference in blood levels of iron between scrapie-infected was seen in striatum. There were slight increases in iron and the control group (Fig. 1B). Analysis of dissected levels in cerebellum and hippocampus of the infected brain regions revealed that iron levels were increased in group, but the differences from controls were not signifi-cerebral cortex (24%, P,0.05), striatum (35%, P,0.01), cant (Fig. 2).
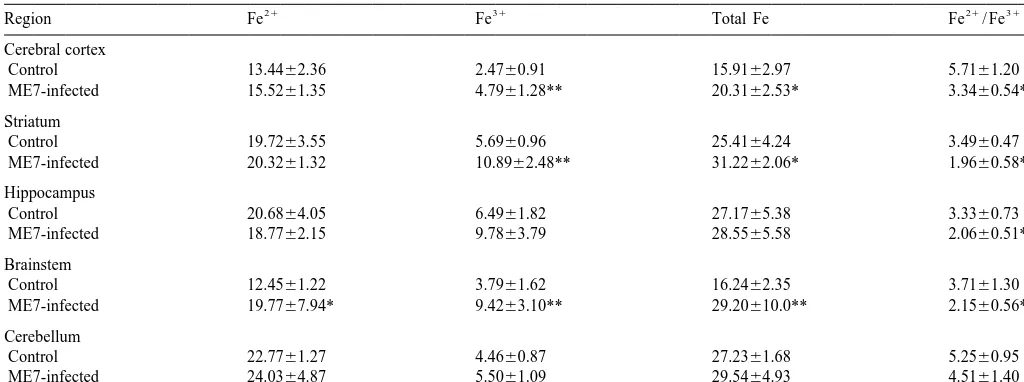
N.-H. Kim et al. / Brain Research 884 (2000) 98 –103 101 Table 1
a
Ferrous, ferric and total iron in control and ME7-infected C57BL mouse brains
21 31 21 31
Region Fe Fe Total Fe Fe / Fe
Cerebral cortex
Control 13.4462.36 2.4760.91 15.9162.97 5.7161.20
ME7-infected 15.5261.35 4.7961.28** 20.3162.53* 3.3460.54**
Striatum
Control 19.7263.55 5.6960.96 25.4164.24 3.4960.47
ME7-infected 20.3261.32 10.8962.48** 31.2262.06* 1.9660.58**
Hippocampus
Control 20.6864.05 6.4961.82 27.1765.38 3.3360.73
ME7-infected 18.7762.15 9.7863.79 28.5565.58 2.0660.51**
Brainstem
Control 12.4561.22 3.7961.62 16.2462.35 3.7161.30
ME7-infected 19.7767.94* 9.4263.10** 29.20610.0** 2.1560.56*
Cerebellum
Control 22.7761.27 4.4660.87 27.2361.68 5.2560.95
ME7-infected 24.0364.87 5.5061.09 29.5464.93 4.5161.40
a
Data, given inmg / g of fresh wet weight, are the mean6S.D. (control n57, ME7-infected n55). Statistical differences between control group and ME7-infected group were calculated by Student’s t-test; **, P,0.01, *, P,0.05.
3.2. Level of ferrous, ferric and total iron in brains 4. Discussion
21
To determine whether there was a change in the Fe / Iron is needed for all cells to survive, grow and divide
31
Fe ratio, we measured ferrous, ferric and total iron in the [12]; however, excess iron is very toxic and iron-induced brains of scrapie-infected mice compared to controls. oxidative damage can occur [10]. In this study, the level of There were no significant differences in the ferrous iron total iron and the ferric / ferrous ratio were increased in levels of any regions between infected and control groups cerebral cortex, striatum and brainstem of ME7 scrapie-except for brainstem, whereas ferric iron levels were infected mice compared to controls.
significantly increased in cerebral cortex (P,0.01), The origin of the excess iron is not known, but it could striatum (P,0.01) and brainstem (P,0.01) of the infected result from changes in the blood–brain barrier (BBB). group. Ferric iron levels in hippocampus and cerebellum Under normal conditions, iron does not readily cross the were slightly increased, but the differences were not BBB, but breakdown could lead to an increase in various significant. Except for the cerebellum, the ferrous / ferric blood-derived components including iron. Wisniewski et ratio was changed in all regions of the infected group al. [29] reported that BBB of mice is altered by scrapie
compared to controls (Table 1). agent. Recently, we found that inflammatory cytokines,
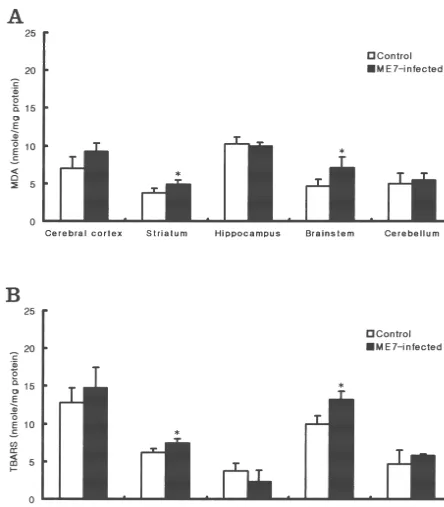
IL-1a, IL-1b, TNF-a and IL-6 were increased in 87 V scrapie-infected mice [18]. The increase in cytokines may 3.3. Analysis of lipid peroxidation be important because cytokines, e.g. IL-1, IL-6, TNF-a, induce the disruption of the BBB in vitro [28]. Castelnau et To determine if oxidative stress occurred in scrapie- al. [4] reported that BBB was damaged and that total iron infected brain, we analyzed the level of lipid peroxidation. concentration and lipid peroxidation were increased in The concentration of thiobarbituric-acid-reactive sub- GFAP-IL-6 transgenic mice (C57BL6 / SJL). These results stances (TBARS) increased in striatum and brainstem of suggest that the increase in cytokine expression induced by the infected group compared to controls. In the cerebral scrapie infection may result in breakdown of BBB. There-cortex region, TBARS was increased, but not significantly. fore, we suggest that iron accumulation may be a sec-Other regions did not show changes in TBARS levels (Fig. ondary event following BBB breakdown and cytokine 3B). Because TBA analysis can show non-specific re- induction. The excess iron could then participate in
21 sponses to many substances other than MDA, we em- oxidative stress in the following manner: free Fe would ployed HPLC analysis to measure the free MDA level be available to interact with H O and drive the Fenton2 2
more specifically. This analysis showed that the free MDA reaction, resulting in the liberation of cytotoxic hydroxyl
31 31
content in homogenate was also significantly higher in radical (OHE) and Fe . The increased amount of Fe
Fig. 3. Content of MDA (A) and TBARS (B) in control and ME7-infected brains. Data are expressed as mean6S.D. (n54). Statistical differences between control group and scrapie-infected group were calculated by Student’s t-test: *, P,0.05.
the presence of H O , resulting in further formation of2 2 nigra of PD [21]. Also, the GSH level is reduced resulting OHE. Thus, a condition for participation of iron in OHE in accumulation of hydrogen peroxide in this region [26].
21
formation and lipid peroxidation is a change in the Fe / Lastly, the level of lipid peroxidation (LP) was increased,
31 31
Fe ratio in favor of Fe [2]. and there was iron accumulation [16,29]. In these results,
N.-H. Kim et al. / Brain Research 884 (2000) 98 –103 103 [8] S.I. Choi, W.K. Ju, E.K. Choi, J. Kim, H.Z. Lea, R.I. Carp, H.M.
metabolism and / or iron redox status in favor of ferric iron
Wisniewski, Y.S. Kim, Mitochondrial dysfunction induced by
oxida-may induce dopaminergic neuronal cell death [13].
tive stress in the brains of hamsters infected with the 263 K scrapie
In our study, we found that ferric iron was increased and agent, Acta Neuropathol. 96 (1998) 279–286.
21 31
the Fe / Fe ratio was changed in cerebral cortex, [9] S.J. DeArmond, S.B. Prusiner, Etiology and pathogenesis of prion brainstem and striatum of scrapie-infected mice (Table 1). diseases, Am. J. Pathol. 146 (1995) 785–811.
[10] R.A. Floyd, J.M. Carney, Free radical damage to protein and DNA:
We also found that GSH level (data not shown) was
mechanisms involved and relevant observations on brain undergoing
decreased in these regions in scrapie-infected mice. These
oxidative stress, Ann. Neurol. 32 (1992) S22–S27.
results show that changes of iron redox states in favor of
[11] D.C. Gajdusek, Unconventional virus and the origin and
disappear-ferric iron were observed in areas where LP level was ance of kuru, Science 197 (1977) 943–960.
increased; the exception was the cerebral cortex, in which [12] B.B. Gelman, Iron in CNS disease, J. Neuropathol. Exp. Neurol. 54 ferric iron level was increased significantly, but the (1995) 477–486.
increase in LP level was not significant (Table 1, Fig. 3). [13] M. Gerlach, D. Ben-Schachar, P. Riederer, M.B.H. Youdim, Altered brain metabolism of iron as a cause of neurodegenerative diseases?,
Thus, we suggest that the change of iron ratio brought on
J. Neurochem. 63 (1994) 793–807.
by ferric iron overload and the decreased GSH level may
[14] B. Halliwell, Oxidants and the central nervous system: some
induce LP in the brainstem and striatum of scrapie-infected fundamental questions, Acta Neurol. Scand. 126 (1989) 23–33.
mice. [15] J.M. Hill, R.C. Switzer, The regional distribution and cellular
In conclusion, we suggest the following scenario involv- localization of iron in the rat brain, Neuroscience 11 (1984) 595– 603.
ing iron deposition in brains of ME7 scrapie-infected mice:
[16] P. Jenner, Oxidative stress as a cause of Parkinson’s disease, Acta
Initially, ME7 scrapie infection induces astrocytosis and
Neurol. Scand. 136 (Suppl.) (1991) 6–15.
the reactive astrocytes secrete inflammatory cytokines.
[17] P. Jenner, Oxidative damage in neurodegenerative disease, Lancet
These cytokines lead to a breakdown of BBB, and iron in 344 (1994) 796–798.
blood enters the brain parenchyma. Also, deposited iron [18] J.I. Kim, W.K. Ju, J.H. Choi, J. Kim, E.K. Choi, R.I. Carp, H.M. may be changed to ferric iron by the Fenton reaction or Winiewski, Y.S. Kim, Expression of cytokine genes and increased nuclear factor kappa B activity in the brains of scrapie-infected
Haber Waiss reaction and this may produce oxygen
mice, Mol. Brain. Res. 73 (1999) 17–27.
radicals. Finally, lipid peroxidation occurs because of the
[19] R.H. Kimberlin, C. Walker, Characteristics of a short incubation
increased level of ferric iron and of oxygen-free radicals.
model of scrapie in the golden hamster, J. Gen. Virol. 34 (1977) 295–304.
[20] D.W. Lee, H.O. Sohn, H.B. Lim, Y.G. Lee, Y.S. Kim, R.I. Carp,
Acknowledgements H.M. Wisniewski, Alteration of free radical metabolism in the brain of mice infected with scrapie agent, Free Rad. Res. 30 (1998) 499–507.
This work was supported by a grant (No.
1999-1-20500-[21] R.J. Martilla, H. Lorentz, U.K. Rinne, Oxygen protecting enzymes
005-3) from the Basic Research Program of the Korea
in Parkinson’s disease, J. Neurol. Sci. 86 (1988) 321–331.
Science & Engineering Foundation. [22] G. Minotti, S.D. Aust, The requirement for iron (III) in the initiation
of lipid peroxidation by iron (II) and hydrogen peroxide, J. Biol. Chem. 262 (1987) 1098–1104.
[23] A. Morita, M. Kimura, Y. Itokawa, The effect of aging on the
References
mineral status of female mice, Biol. Trace Elem. Res. 42 (1994) 165–177.
[1] S.D. Aust, L.A. Morehouse, C.E. Thomas, Role of metals in oxygen
[24] H. Ohkawa, N. Ohishi, K. Yagi, Assay for lipid peroxides in animal radical reactions, J. Free Radic. Biol. Med. 1 (1985) 3–25.
tissues by thiobarbituric acid reaction, Anal. Biochem. 95 (1979) [2] D. Ben-Shachar, P. Riederer, M.B.H. Youdim, Iron–melanin
inter-351–358. action and lipid peroxidation: implications for Parkinson’s disease,
[25] C.W. Olanow, A radical hypothesis for neurodegeneration, Trends J. Neurochem. 57 (1991) 1609–1614.
Neurosci. 6 (1993) 439–444. [3] R.I. Carp, X. Ye, R.J. Kascsak, R. Rubenstein, The nature of the
[26] P. Riederer, E. Sofic, W.D. Rausch, B. Schmidt, F.P. Reynolds, K. scrapie agent, biological characteristics of scrapie in different
Jellinger, M.B.H. Youdim, Transition metal, ferritin, glutathione and scrapie strain–host combinations, Ann. NY Acad. Sci. 724 (1994)
ascorbic acid in Parkinsonian brains, J. Neurochem. 52 (1989) 221–234.
515–520. [4] P.A. Castelnau, R.S. Garrett, W. Palinski, J.L. Witztum, I.L.
Camp-[27] J. Siedel, A.W. Wahlefeld, J. Ziegenhorn, Improved, ferrozine-based bell, Abnormal iron deposition associated with lipid peroxidation in
reagent for the determination of serum iron (transferrin iron) without transgenic mice expressing interleukin-6 in the brain, J.
Neuro-deproteinzation, Clin. Chem. 30 (1984) 975. pathol. Exp. Neurol. 57 (1998) 268–282.
[28] H.E. Vires, M.C.M. Blom-Roosemalen, M. Oosten, A.G. Boer, [5] R.L. Chandler, Encephalopathy in mice produced by inoculation
T.J.C. Berkel, D.D. Breimer, J. Kuiper, The influence of cytokines with scrapie brain material, Lancet 1 (1961) 1378–1379.
on the integrity of the blood–brain barrier in vitro, J. Neuroim-[6] R.L. Chandler, Experimental scrapie in the mouse, Res. Vet. Sci. 4
munol. 64 (1996) 37–43. (1963) 276–285.