C RC P R E S S
Boca Raton London New York Washington, D.C.
EDITED BY
Alexa Riehle and Eilon Vaadia
MOTOR CORTEX
This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use.
Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher.
All rights reserved. Authorization to photocopy items for internal or personal use, or the personal or internal use of specific clients, may be granted by CRC Press, provided that $1.50 per page photocopied is paid directly to Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923 USA. The fee code for users of the Transactional Reporting Service is ISBN 0-8493-1287-6/05/$0.00+$1.50. The fee is subject to change without notice. For organizations that have been granted a photocopy license by the CCC, a separate system of payment has been arranged.
The consent of CRC Press does not extend to copying for general distribution, for promotion, for creating new works, or for resale. Specific permission must be obtained in writing from CRC Press for such copying.
Direct all inquiries to CRC Press, 2000 N.W. Corporate Blvd., Boca Raton, Florida 33431.
Trademark Notice: Product or corporate names may be trademarks or registered trademarks, and are used only for identification and explanation, without intent to infringe.
Visit the CRC Press Web site at www.crcpress.com © 2005 by CRC Press
No claim to original U.S. Government works International Standard Book Number 0-8493-1287-6
Library of Congress Card Number 2004057046 Printed in the United States of America 1 2 3 4 5 6 7 8 9 0
Printed on acid-free paper
Library of Congress Cataloging-in-Publication Data Motor cortex in voluntary movements : a distributed system for distributed functions /
edited by Alexa Riehle and Eilon Vaadia. p. cm.
Includes bibliographical references and index. ISBN 0-8493-1287-6 (alk. paper)
1. Motor cortex. 2. Human locomotion. I. Riehle, Alexa. II. Vaadia, Eilon. III. Series.
QP383.15.M68 2005
Methods & New Frontiers
in Neuroscience
Our goal in creating the Methods & New Frontiers in Neuroscience series is to present the insights of experts on emerging experimental techniques and theoretical concepts that are or will be at the vanguard of the study of neuroscience. Books in the series cover topics ranging from methods to investigate apoptosis to modern techniques for neural ensemble recordings in behaving animals. The series also covers new and exciting multidisciplinary areas of brain research, such as compu-tational neuroscience and neuroengineering, and describes breakthroughs in classical fields such as behavioral neuroscience. We want these to be the books every neuro-scientist will use in order to graduate students and postdoctoral fellows when they are looking for guidance to start a new line of research.
Each book is edited by an expert and consists of chapters written by the leaders in a particular field. Books are richly illustrated and contain comprehensive bibli-ographies. Chapters provide substantial background material relevant to the partic-ular subject; hence, they are not only “methods” books. They contain detailed tricks of the trade and information as to where these methods can be safely applied. In addition, they include information about where to buy equipment and about Web sites that are helpful in solving both practical and theoretical problems.
We hope that as the volumes become available, the effort put in by us, by the publisher, by the book editors, and by the individual authors will contribute to the further development of brain research. The extent to which we achieve this goal will be determiend by the utility of these books.
Preface
Voluntary movement is undoubtedly the overt basis of human behavior. Without movement we cannot walk, nourish ourselves, communicate, or interact with the environment. This is one of the reasons why the motor cortex was one of the first cortical areas to be explored experimentally. Historically, the generation of motor commands was thought to proceed in a rigidly serial and hierarchical fashion. The traditional metaphor of the piano presents the premotor cortex “playing” the upper motoneuron keys of the primary motor cortex (M1), which in turn activate with strict point-to-point connectivity the lower motoneurons of the spinal cord. Years of research have taught us that we may need to reexamine almost all aspects of this model. Both the premotor and the primary motor cortex project directly to the spinal cord in highly complex overlapping patterns, contradicting the simple hierarchical view of motor control. The task of generating and controlling movements appears to be subdivided into a number of subtasks that are accomplished through parallel distributed processing in multiple motor areas. Multiple motor areas may increase the behavioral flexibility by responding in a context-related way to any constraint within the environment. Furthermore, although more and more knowledge is accu-mulating, there is still an ongoing debate about what is represented in the motor cortex: dynamic parameters (such as specific muscle activation), kinematic param-eters of the movement (for example, its direction and speed), or even more abstract parameters such as the context of the movement. Given the great scope of the subject considered here, this book focuses on some new perspectives developed from con-temporary monkey and human studies. Moreover, many topics receive very limited treatment.
reflect the indirect and spatio-temporally imprecise nature of the fMRI signal, but these studies remain informative by virtue of the fact that usually the whole brain is covered. Not only does fMRI reveal plausible brain regions for the control of localized effects, but the distribution of response foci and the correlation of effects observed at many different sites can assist in the guidance of detailed studies at the mesoscopic or microscopic spatio-temporal level. A prudently modest view might conclude that fMRI is at present primarily a tool of exploratory rather than explan-atory value.
10-, 20-, and 40-Hz motor cortical oscillations are associated with constant, sustained muscle contractions, again a static condition. Sigma band oscillations of about 14 Hz may be indicative of maintained active suppression of a motor response. The dynamic phase at the onset of an intended movement is preceded by a marked decrease in oscillatory power, but not all frequencies are suppressed. Fast gamma oscillations coincide with movement onset. Moreover, there is increasing evidence that oscilla-tory potentials of even low frequencies (4–12 Hz) may be linked to dynamic episodes of movement. Most surprisingly, the 8-Hz cortical oscillation — the neurogenic component of physiological tremor — is emerging as a major factor in shaping the pulsatile dynamic microstructure of movement, and possibly in coordinating diverse actions performed together. In Chapter 8, Riehle discusses the main aspects of preparatory processes in the motor cortex. Preparation for action is thought to be based on central processes, which are responsible for maximizing the efficiency of motor performance. A strong argument in favor of such an efficiency hypothesis of preparatory processes is the fact that providing prior information about movement parameters or removing time uncertainty about when to move significantly shortens reaction time. The types of changes in the neuronal activity of the motor cortex, and their selectivity during preparation, are portrayed and compared with other cortical areas that are involved in motor behavior. Furthermore, linking motor cortical activity directly to behavioral performance showed that the trial-by-trial correlation between single neuron firing rates and reaction time revealed strong task-related cortical dynamics. Finally, the cooperative interplay among neurons, expressed by precise synchronization of their action potentials, is illustrated and compared with changes in the firing rate of the same neurons. New concepts including the notion of coor-dinated ensemble activity and their functional implication during movement prepa-ration are discussed. In the last chapter of Section II, Chapter 9, Jeannerod poses the question of the role of the motor cortex in motor cognition. The classical view of the primary motor cortex holds that it is an area devoted to transferring motor execution messages that have been elaborated upstream in the cerebral cortex. More recently, however, experimental data have pointed to the fact that the relation of motor cortex activity to the production of movements is not as simple as was thought on the basis of early stimulation experiments. This revision of motor cortical function originated from two main lines of research, dealing first with the plasticity of the somatotopic organization of the primary motor cortex, and second with its involve-ment in cognitive functions such as motor imagery.
with emphasis on the neural basis of learning. In Chapter 11, Shadmehr, Donchin, Hwang, Hemminger, and Rao deal with internal models that transform the desired movement into a motor command. When one moves the hand from one point to another, the brain guides the arm by relying on neural structures that estimate the physical dynamics of the task. Internal models are learned with practice and are a fundamental part of voluntary motor control. What do internal models compute, and which neural structures perform that computation? The authors approach these questions by considering a task where the physical dynamics of reaching movements are altered by force fields that act on the hand. Many studies suggest that internal models are sensorimotor transformations that map a desired sensory state of the arm into an estimate of forces; i.e., a model of the inverse dynamics of the task. If this computation is represented as a population code via a flexible combination of basis functions, then one can infer activity fields of the bases from the patterns of gener-alization. Shadmehr and colleagues provide a mathematical technique that facilitates this inference by analyzing trial-by-trial changes in performance. Results suggest that internal models are computed with bases that are directionally tuned to limb motion in intrinsic coordinates of joints and muscles, and this tuning is modulated multiplicatively as a function of static position of the limb. That is, limb position acts as a gain field on directional tuning. Some of these properties are consistent with activity fields of neurons in the motor cortex and the cerebellum. The authors suggest that activity fields of these cells are reflected in human behavior in the way that we learn and generalize patterns of dynamics in reaching movements. In the last chapter of Section III, Chapter 12, Padoa-Schioppa, Bizzi, and Mussa-Ivaldi address the question of the cortical control of motor learning. In robotic systems, engineers coordinate the action of multiple motors by writing computer codes that specify how the motors must be activated for achieving the desired robot motion and for compensating unexpected disturbance. Humans and animals follow another path. Something akin to programming is achieved in nature by the biological mech-anisms of synaptic plasticity — that is, by the variation in efficacy of neural trans-mission brought about by past history of pre- and post-synaptic signals. However, robots and animals differ in another important way. Robots have a fixed mechanical structure and dimensions. In contrast, the mechanics of muscles, bones, and liga-ments change in time. Because of these changes, the central nervous system must continuously adapt motor commands to the mechanics of the body. Adaptation is a form of motor learning. Here, a view of motor learning is presented that starts from the analysis of the computational problems associated with the execution of the simplest gestures. The authors discuss the theoretical idea of internal models and present some evidence and theoretical considerations suggesting that internal models of limb dynamics may be obtained by the combination of simple modules or “motor primitives.” Their findings suggest that the motor cortical areas include neurons that process well-acquired movements as well as neurons that change their behavior during and after being exposed to a new task.
present and discuss the recent research in the field of brain–machine interfaces (BMI) conducted mainly on nonhuman primates. In fact, this research field has supported the contention that we are at the brink of a technological revolution, where artificial devices may be “integrated” in the multiple sensory, motor, and cognitive represen-tations that exist in the primate brain. These studies have demonstrated that animals can learn to utilize their brain activity to control the displacements of computer cursors, the movements of simple and elaborate robot arms,and, more recently, the reaching and grasping movements of a robot arm. In addition to the current research performed in rodents and primates, there are also preliminary studies using human subjects. The ultimate goal of this emerging field of BMI is to allow human subjects to interact effortlessly with a variety of actuators and sensory devices through the expression of their voluntary brain activity, either for augmenting or restoring sen-sory, motor, and cognitive function. In the last chapter, Chapter 14, Pfurtscheller, Neuper, and Birbaumer deal with BMIs, which transform signals originating from the human brain into commands that can control devices or applications. BCIs provide a new nonmuscular communication channel, which can be used to assist patients who have highly compromised motor functions, as is the case with patients suffering from neurological diseases such as amyotrophic lateral sclerosis (ALS) or brainstem stroke. The immediate goal of current research in this field is to provide these users with an opportunity to communicate with their environment. Present-day BCI systems use different electrophysiological signals such as slow cortical potentials, evoked potentials, and oscillatory activity recorded from scalp or subdural electrodes, and cortical neuronal activity recorded from implanted electrodes. Due to advances in methods of signal processing, it is possible that specific features automatically extracted from the electroencephalogram (EEG) and electrocortico-gram (ECoG) can be used to operate computer-controlled devices. The interaction between the BCI system and the user, in terms of adaptation and learning, is a challenging aspect of any BCI development and application.
It is the increased understanding of neuronal mechanisms of motor functions, as reflected in this book, that led to the success of BCI. Yet, the success in tapping and interpreting neuronal activity and interfacing it with a machine that eventually executes the subject’s intention is amazing, considering the limited understanding we have of the system as a whole.
Perhaps ironically, the proof of our understanding of motor cortical activity will stem from how effectively we, as external observers of the brain, can tap into it and make use of it.
Dedication
Editors
Alexa Riehle received a B.Sc. degree in biology (main topic: deciphering microcir-cuitries in the frog retina) from the Free University, Berlin, Germany, in 1976, and a Ph.D. degree in neurophysiology (main topic: neuronal mechanisms of temporal aspects of color vision in the honey bee) from the Biology Department of the Free University in 1980.
From 1980 to 1984, she was a postdoctoral fellow at the National Center for Scientific Research (CNRS) in Marseille, France (main topic: neuronal mechanisms of elementary motion detectors in the fly visual system). In 1984, she moved to the Cognitive Neuroscience Department at the CNRS and has been mainly interested since then in the study of cortical information processing and neural coding in cortical ensembles during movement preparation and execution in nonhuman primates.
Eilon Vaadia graduated from the Hebrew University of Jerusalem (HUJI) in 1980 and joined the Department of Physiology at Hadassah Medical School after post-doctoral studies in the Department of Biomedical Engineering at Johns Hopkins University Medical School in Baltimore, Maryland.
Contributors
Department of Brain and Cognitive Sciences
Massachusetts Institute of Technology Cambridge, Massachusetts
Niels Birbaumer
Institute of Medical Psychology and Behavioral Neurobiology University of Pittsburgh School of
Medicine
Ferdinando A. Mussa-Ivaldi
Ludwig Boltzmann Institute of Medical Informatics and Neuroinformatics
Natinoal Center for Scientific Research (INCM-CNRS) Department of Anatomy and Cell
Biology
Canadian Institutes of Health Research Group in Sensory-Motor Systems
Veterans Affairs Medical Center for the Neural Basis of Cognition
Department of Neurobiology University of Pittsburgh Pittsburgh, Pennsylvania
Ivan Toni
Table of Contents
SECTION I
Functional Neuroanatomy and
Imaging
Chapter 1 Motor Areas in the Frontal Lobe: The Anatomical Substrate for the Central Control of Movement
Richard P. Dum and Peter L. Strick
Chapter 2 Functional Magnetic Resonance Imaging of the Human Motor Cortex
Andreas Kleinschmidt and Ivan Toni
SECTION II
Neuronal Representations in the
Motor Cortex
Chapter 3 Motor Cortex Control of a Complex Peripheral Apparatus: The Neuromuscular Evolution of Individuated Finger Movements
Marc H. Schieber, Karen T. Reilly, and Catherine E. Lang
Chapter 4 Neuronal Representations of Bimanual Movements
Eilon Vaadia and Simone Cardoso de Oliveira
Chapter 5 What Is Coded in the Primary Motor Cortex?
James Ashe
Chapter 6 Conceptual Frameworks for Interpreting Motor Cortical Function: New Insights from a Planar Multiple-Joint Paradigm
Stephen H. Scott
Chapter 7 Wheels of Motion: Oscillatory Potentials in the Motor Cortex
Chapter 8 Preparation for Action: One of the Key Functions of the Motor Cortex
Alexa Riehle
Chapter 9 Is the Motor Cortex Only an Executive Area? Its Role in Motor Cognition
Marc Jeannerod
SECTION III
Motor Learning and Performance
Chapter 10 The Arbitrary Mapping of Sensory Inputs to Voluntary and Involuntary Movement: Learning-Dependent Activity in the Motor Cortex and Other Telencephalic Networks
Peter J. Brasted and Steven P. Wise
Chapter 11 Learning Dynamics of Reaching
Reza Shadmehr, Opher Donchin, Eun-Jung Hwang, Sarah E. Hemminger, and Ashwini K. Rao
Chapter 12 Cortical Control of Motor Learning
Camillo Padoa-Schioppa, Emilio Bizzi, and Ferdinando A. Mussa-Ivaldi
SECTION IV
Reconstruction of Movements Using
Brain Activity
Chapter 13 Advances in Brain–Machine Interfaces
Jose M. Carmena and Miguel A.L. Nicolelis
Chapter 14 Human Brain–Computer Interface
Section I
0-8493-1287-6/05/$0.00+$1.50 © 2005 by CRC Press LLC
1
Motor Areas in the
Frontal Lobe: The
Anatomical Substrate
for the Central Control
of Movement
Richard P. Dum and Peter L. Strick
CONTENTS
1.1 Introduction 1.2 Functional Anatomy
1.2.1 Primary Motor Cortex
1.2.1.1 Organization Based on Intracortical Stimulation 1.2.1.2 Output of Single Corticomotoneuronal Cells 1.2.1.3 Peripheral Input to M1
1.2.2 Premotor Areas
1.2.2.1 Identification by Direct Projections to M1
1.2.2.2 Somatotopic Organization Based on Connections with M1
1.2.2.3 Corticospinal Output
1.2.2.4 Somatotopic Organization Based on Corticospinal Output: Forelimb and Hindlimb Representation 1.2.2.5 Somatotopic Organization Based on Corticospinal
Output: Proximal and Distal Arm Representation 1.2.2.6 Organization Based on Intracortical Stimulation 1.2.3 Corticospinal Terminations
1.2.3.1 Primary Motor Cortex 1.2.3.2 Premotor Areas 1.3 Cortical Inputs to the Motor Areas
1.3.2.1 Interconnections among the Motor Areas 1.3.2.2 Parietal Cortex
1.3.2.3 Pre-Premotor Cortex 1.3.2.4 Prefrontal Cortex 1.3.2.5 Limbic Cortex
1.3.3 Summary of Cortical Connections 1.4 Subcortical Inputs
1.5 Summary and Conclusions Acknowledgments
References
1.1 INTRODUCTION
The objective of this chapter is to describe the major components of the structural framework employed by the cerebral cortex to generate and control skeletomotor function. We will focus on motor areas in the frontal lobe that are the source of corticospinal projections to the ventral horn of the spinal cord in primates. These cortical areas include the primary motor cortex (M1) and the six premotor areas that project directly to M1. We will begin by examining anatomical and physiological evidence that demonstrates how each of these cortical areas directly accesses spinal cord mechanisms involved in the generation and control of movement. This evidence suggests that all these cortical areas have some direct involvement in movement execution. Then we will examine how the pattern of cortical and subcortical inputs could shape the functional role of each cortical area in motor control. We will show that each of these cortical areas receives a unique pattern of cortical and subcortical input. Taken together, these results have led to an emerging view that motor commands can arise from multiple motor areas and that each of these motor areas makes a specialized contribution to the planning, execution, or control of voluntary movement. In this chapter, we will describe some of the relevant anatomical and physiological evidence that has led to this viewpoint.
Given the breadth of the subject considered here, our review will focus on new perspectives developed from contemporary primate studies. Even with this focus, many topics will receive limited treatment. For instance, the physiological and behavioral studies that provide evidence of differential involvement of each motor area in the generation and control of movement are beyond the scope of this chapter. For further insight into the historical development of this field and a broader coverage of related issues, numerous reviews on this and related topics are available.1–11 In addition, the corticospinal system has been the subject of a recent book.12
1.2 FUNCTIONAL ANATOMY
1.2.1 PRIMARY MOTOR CORTEX
to cytoarchitectonic area 4, which is identified by the presence of giant pyramidal cells in cortical layer V.16–18 Based on these definitions, M1 is located in the anterior bank of the central sulcus and on the adjacent caudal portion of the precentral gyrus (Figure 1.1). (For more complete reviews, see References 4,5,9,12.)
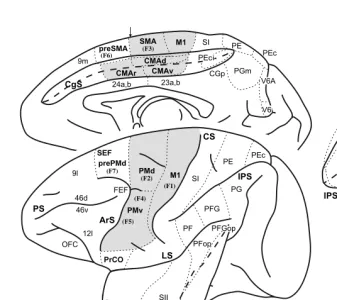
FIGURE 1.1 Identification of cortical areas in the macaque monkey. The cingulate sulcus (CgS), lateral sulcus (LS), and intraparietal sulcus (IPS) are unfolded and each fundus is indicated by a dashed line. The borders between cytoarchitectonic areas are delineated with dotted lines. M1 and the premotor areas are shaded. Abbreviations: AIP, LIP, MIP, VIP: anterior, lateral, medial, and ventral intraparietal areas; ArS: arcuate sulcus; CGp: posterior cingulate gyrus; CMAd, CMAv, CMAr: dorsal, ventral, and rostral cingulate motor areas; CS: central sulcus; F1 to F7: cytoarchitectonic areas in the frontal lobe according to Matelli et al.77,248; FEF: frontal eye fields; Ig: granular insular cortex; M1: primary motor cortex;
OFC: orbital frontal cortex; PMd: dorsal premotor area; PMv: ventral premotor area; PrCO: precentral opercular cortex; prePMd: pre-premotor area, dorsal; preSMA: presupplementary motor area; PS: principal sulcus; SEF: supplementary eye field; SI: primary somatosensory cortex; SII: secondary somatosensory cortex; SMA: supplementary motor area; PE, PEc, PEci, PF, PFG, PFop, PG, PGm, Pgop: parietal areas after Pandya and Selzer249; V6A, V6: posterior
parietal areas after Galletti et al.177; 9m, 9l, 46d, 46v, 12l: prefrontal areas after Walker181 and
1.2.1.1 Organization Based on Intracortical Stimulation
Our view of the organization of M1 as based on electrical stimulation has evolved with advances in stimulation techniques. Classically, surface stimulation suggested that M1 contained a “motor map” that was a single, contiguous representation of the body.14,15 (For reviews, see References 4 and 12.) In this map, the leg, trunk, arm, and face formed a medial to lateral procession across M1 with the distal musculature of each limb located in the central sulcus. Electrical stimulation with microelectrodes inserted into the cortex lowered the amount of current necessary to evoke movement by a factor of 100.19 Although this advance allowed a much more detailed exploration of the cortex, intracortical stimulation confirmed the overall somatotopy of leg, arm, and face representation described by surface stimulation.19–32 Thus, electrical stimulation of M1 generated a somatotopic motor map with relatively sharp boundaries between major body parts.
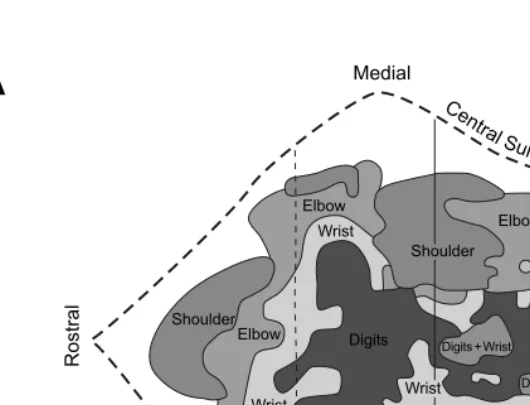
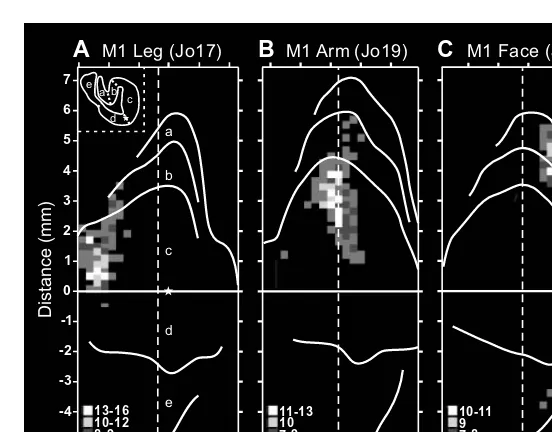
The organization of movements generated by intracortical stimulation within each major body part, however, was more complex than that produced by surface stimulation (Color Figure 1.2).* A consistent observation was that the same move-ment could be evoked at multiple, spatially separate sites.22–32 Although this obser-vation precluded an orderly somatotopy, the general features of this map were reproducible. Within the arm representation of macaque monkeys, distal limb move-ments (fingers and wrist) tended to form a central core that was surrounded by a horseshoe of proximal limb movements (elbow and shoulder) (Color Figure 1.2A).22,33 Some intermingling of distal and proximal limb movements occurred at the borders. This organizational structure has been confirmed with single-pulse, stimulus-triggered averaging (Color Figure 1.2B).34 The presence of multiple repre-sentations of an individual movement/muscle in M1 has been proposed as an arrange-ment that allows a muscle to engage in multiple synergies with other muscles acting at the same or different joints. (See Reference 35.)
Other studies utilizing intracortical stimulation20,26,28,32 reported even more com-plex patterns of muscle activation. For example, stimulation at some sites in M1 evoked reciprocal activation of wrist antagonists, whereas at other sites it caused their co-contraction.26 Some stimulus locations evoked movements of several joints at barely differing thresholds. Thus, multiple-joint movements could also be evoked by relatively localized stimulation. These more complex relationships may allow “automatic” coordination of postural stabilization of the proximal limb during object manipulation by the distal limb musculature.
More recently, long trains (0.5 to 1.0 sec) of supra-threshold intracortical stim-ulation have been reported to evoke coordinated forelimb movements in the awake primate (Color Figure 1.2C).36 Each stimulation site produced a stereotyped posture in which the arm moved to the same final position regardless of its posture at the initiation of stimulation. In the most complex example, the monkey formed a frozen pose with the hand in a grasping position in front of the open mouth. The map of final hand location in the workspace in front of the monkey included both M1 and the premotor cortex (Color Figure 1.2C). In many respects, these results were a more
detailed equivalent of observations made initially by Ferrier37 who reported that in M1 “long-continued stimulation brings the hand to the mouth, and at the same time the angle of the mouth is retracted and elevated.” The interpretation of these complex movements is limited by the fact that intracortical stimulation primarily activates neurons trans-synaptically, and thereby enlarges its sphere of activation.38,39 (See also References 40,41.) At the extreme, long stimulus trains and high stimulus intensities open the route for interactions at multiple levels, including local, cortical, subcortical, and spinal. Thus, intracortical stimulation is unable to determine the FIGURE 1.2 (see color figure) Intracortical stimulation maps of M1 in macaque monkeys. Note that in each map, hand movements form a central core (red). (A) Summary map of the movements evoked by intracortical stimulation (2–30 µA) in an awake macaque monkey. (Adapted with permission from Reference 22.) (B) Summary map of muscle representation in M1 derived from stimulus-triggered averages of rectified EMG activity (15 µA at 15 Hz) in an awake monkey. Sites that influenced only proximal muscles are indicated by light shading, those that influenced only distal muscles by dark shading, and those sites that influenced both proximal and distal muscles by intermediate shading. Sites of significant stimulus-triggered averages of rectified EMG activity for the shorthead of biceps (BIS, blue) and extensor digitorum communis (EDC, red) are indicated with size-coded dots (3, 4, 5, 6 S.D. levels above pre-trigger level baseline activity). (Adapted with permission from Ref-erence 34.) (C) Summary of hand and arm postures produced by long train (0.5 sec), high intensity (25–150 µA) intracortical stimulation in M1, the PMd, and the PMv of an awake monkey. Arm sites evoked postures involving the arm but without changes in the configuration of the hand. Hand + arm indicates sites where stimulation evoked postures involving both the hand and arm. Hand to mouth indicates sites that evoked grasp-like movements of the hand which was brought to the mouth. Bimodal/defensive indicates sites where neurons received visual input and stimulation moved the arm into a defensive posture. See text for further explanation. (Adapted with permission from Reference 36.)
output structure of M1 unambiguously or to ascertain the functional organization of a cortical motor area.
1.2.1.2 Output of Single Corticomotoneuronal Cells
A more focused approach to examining the output structure of M1 has been to determine the axonal branching patterns of single corticospinal neurons. Both phys-iological and anatomical studies provide evidence that single corticospinal neurons may have a rather widespread influence in the spinal cord. A substantial proportion of corticospinal neurons (43%) innervates several segments of the spinal cord.42 Reconstruction of individual corticospinal axons filled with an intracellular tracer reveals terminal arbors located in as many as four separate motor nuclei.43 Thus, a single corticospinal axon can directly influence several muscles.
The size of the branching patterns of individual CM cells appears to be related to the muscles they innervate. CM cells that influence both proximal and distal muscles have wider branching patterns than those that project to either proximal or distal muscles.49 In addition, half of the CM cells that facilitate intrinsic hand muscles targeted just one of the muscles sampled.48 These observations suggest that CM cells have more restricted branching to distal muscles than they do to proximal muscles. Lemon and colleagues50–52 have emphasized, on the basis of electrophysiological data from macaque and squirrel monkeys, that direct CM projections are important for the control of grasp. Although Schieber35 has argued that restricted branching is not a requirement for producing individuated finger movements, the restricted branching of some CM cells suggests that they may be specialized to control individual finger muscles.
The limited branching patterns of some CM neurons as well as the observation that small clusters of CM neurons tend to innervate the same motoneuron pool42,46 may explain why intracortical stimulation can evoke contractions of a single muscle at threshold.19 This raises the possibility that a framework for muscle representation exists at the level of small clusters of neurons. On the other hand, the highly divergent projections of many CM neurons are consistent with some of the more complex, multiple-joint movements observed with other variations of the intracortical stimu-lation technique.26,36 Thus, adjustment of the parameters of intracortical stimulation may promote access to different structural features of the output organization of M1 as well as other portions of the motor system.
1.2.1.3 Peripheral Input to M1
Another type of map within M1 concerns the responses of its neurons to peripheral somatosensory stimulation. In both New and Old World primates, neurons in the caudal part of the forelimb representation of M1 were activated by peripheral input predominantly from cutaneous afferents.25,53–55 In contrast, neurons in the rostral part of the M1 forelimb representation were driven by peripheral afferents originating largely from muscles or joints. A similar segregation of peripheral input has been observed in the hindlimb representation of M1 in the macaque.24 Strick and Preston54 have proposed that the segregation of peripheral inputs within M1 may represent a functional specialization designed to solve tasks demanding high levels of sen-sory–motor integration. For example, the portion of the hand representation in M1 that receives largely cutaneous input may be specialized to control finger coordina-tion during object manipulacoordina-tion. Thus, the internal organizacoordina-tion of M1 is quite complicated and may include multiple, overlapping maps of sensory input and motor output.
1.2.2 PREMOTOR AREAS
designated premotor cortex turned out to be functionally heterogeneous. For example, electrical stimulation of area 6 on the medial wall revealed a complete motor map of the body in a region that has been subsequently subdivided into the supplementary motor area (SMA) and presupplementary motor area (preSMA) (Figure 1.1).15,63 (See below.) On the lateral surface, attempts to define the boundaries of the premotor cortex using electrical stimulation or cytoarchitectonic criteria failed to produce a consensus.9,61
1.2.2.1 Identification by Direct Projections to M1
A more recent approach for determining the location of premotor cortex has been based on its neuroanatomical connections. The premotor cortex in non-human pri-mates has been operationally defined as consisting of those regions in the frontal lobe that have direct projections to M1 (For review see References 9,59,60,64–66.) According to this definition, the frontal lobe contains at least six spatially separate premotor areas (Figures 1.1 and 1.3A). For example, the arm representation of M1 receives projections from two rostrally adjacent regions on the lateral surface: the ventral premotor area (PMv) and the dorsal premotor area (PMd) (Figure 1.3A). The PMv is located in the portion of area 6 that is lateral to the arcuate spur and extends rostrally into the posterior bank of the inferior limb of the arcuate sulcus. The PMd occupies the portion of area 6 that is medial to the fundus of the arcuate spur and caudal to the genu of the arcuate sulcus. Its caudal extent typically includes the cortex within the superior precentral sulcus (Figures 1.1, 1.3A, and 1.4).
Four premotor areas are located on the medial wall of the hemisphere (Figures 1.1, 1.3A, and 1.4). These premotor areas include the SMA and three motor areas located within the cingulate sulcus: the rostral, dorsal, and ventral cingulate motor areas (CMAr, CMAd, and CMAv). The SMA is confined to the portion of area 6 on the mesial surface of the superior frontal gyrus that lies between the arcuate genu rostrally and the hindlimb representation in M1 caudally. The CMAr is located within area 24c on the dorsal and ventral banks of the cingulate sulcus at levels largely anterior to the genu of the arcuate sulcus. The CMAd occupies area 6c on the dorsal bank of the cingulate sulcus at levels caudal to the genu of the arcuate sulcus. The CMAv lies on the ventral bank of the cingulate sulcus in area 23c, mostly at the same levels as the CMAd. Thus, the premotor cortex, as defined by its anatomical connections to M1, is more complicated than previously recognized (for review see References 2,3,8,15,57,62) and is composed of multiple, spatially separate premotor areas (Figures 1.1, 1.3, and 1.4).59,60,67–69 (See also References 70–76.)
66.) Thus, the current definition of premotor cortex includes multiple premotor areas located in the caudal half of area 6 as well as in additional regions within the cingulate sulcus that were historically considered part of the limbic cortex.9
1.2.2.2 Somatotopic Organization Based on Connections with M1
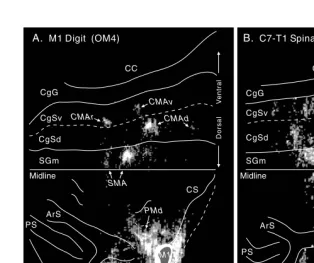
The somatotopic organization of the premotor areas has been evaluated based of their projections to the arm, leg, and face representations of M1.59,60,64,67–69,71–76,81,82 A number of general conclusions have come from these studies. Some premotor FIGURE 1.3 Identification of premotor areas in the frontal lobe. (A) Premotor areas project to M1. An unfolded map of the frontal lobe depicts the density of labeled neurons after WGA–HRP injections into the physiologically identified digit representation of M1 in the macaque monkey. (For details of the unfolding and the determination of cell density, see Dum and Strick.60) The medial wall is unfolded and reflected upward from the midline so that it
appears upside down. The lip of each sulcus (solid line) and its fundus (dashed line) are indicated. The labeled neurons in the PMv (arrow) are located in the posterior bank of the arcuate sulcus and have been projected to the surface. This projection to the surface artificially increases the displayed density. (B) Premotor areas project to the spinal cord. An unfolded map of the frontal lobe shows the density of labeled corticospinal neurons after injections of a fluorescent tracer into the C7–T1 segments of the spinal cord. Abbreviations: CC: corpus callosum; CgSd: dorsal bank of the cingulate sulcus; CgSv: ventral bank of the cingulate sulcus; SGm: medial superior frontal gyrus. (Reproduced with permission from Reference 64.)
areas lack a complete representation of the body (e.g., the PMd lacks a face area). Indeed, complete maps of the body can only be defined for the SMA, CMAv, and CMAr. On the other hand, the arm has the most widespread and robust representation within each of the premotor areas. Overall, the major representations within each premotor area originate from distinct, non-overlapping regions.
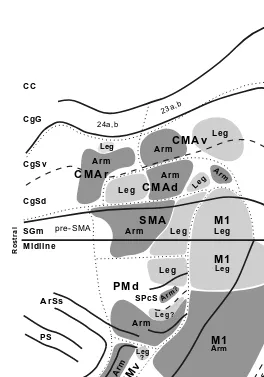
FIGURE 1.4 Somatotopy of corticospinal projections. In this map, the location of the arm representations in M1 and the premotor areas are based on the origin of neurons that project to upper and lower cervical segments. The location of the leg representations in each cortical area is based on the origin of neurons that project to lower lumbosacral segments. For conventions and abbreviations see Figures 1.1 and 1.3. ArSi: arcuate sulcus, inferior limb; ArSs: arcuate sulcus, superior limb. (Adapted with permission from Reference 84. Also adapted with permission from Reference 85.)
1.2.2.3 Corticospinal Output
Russell and DeMyer83 first demonstrated that area 6 contributes about the same number of axons to the pyramids as does area 4. However, the importance of corticospinal projections from the premotor areas has only been appreciated recently. With the advent of retrograde and anterograde neuronal tracing techniques, numerous authors were able to demonstrate that each premotor area has direct access to the spinal cord (Figures 1.3B and 1.4).59,60,84,85 (See also References 86–93.) The distri-bution of corticospinal neurons in the premotor areas that projected to cervical segments of the spinal cord corresponded remarkably well to the distribution of neurons in the premotor areas that projected directly to the arm representation in M1 (Figures 1.3A and 1.3B). These results suggest that each premotor area has the potential to influence the generation and control of movement directly at the level of the spinal cord, as well as at the level of the primary motor cortex.
Numerically, the overall contribution of the premotor areas to the corticospinal tract is equivalent to or greater than that of M1. This is most apparent for corticospinal projections to the cervical segments of the spinal cord. After tracer injections con-fined to the cervical segments (arm representation), the percentage of the total number of corticospinal neurons in the frontal lobe that originated in the premotor areas was always equal to or greater than the percentage of corticospinal neurons in M1 (premotor mean = 56%, range 50–70%, n = 6).60,84,85 For tracer injections confined to the lumbosacral segments (leg representation), the percentage of corti-cospinal neurons in the frontal lobe that originated in the premotor areas was less than the percentage of corticospinal neurons in M1 (premotor mean = 43%, range 39–46%, n = 2).85 These observations reinforce the view that the arm representation within the premotor areas is more robustly developed than is the leg representation. In other measures of the relative strength of corticospinal projections, M1 clearly dominates but the premotor areas still make significant contributions. For example, each premotor area had some localized regions in which the density of corticospinal neurons was equivalent to that found in M1. In fact, the relative density of corti-cospinal neurons in the SMA, CMAd, CMAv and PMd was similar to that found in M1.60 (See also References 84,85.) With respect to the distribution of large and small corticospinal neurons, most large corticospinal neurons (79%) were concentrated in M1.60 The remaining large corticospinal neurons were located in the PMv, PMd, SMA and CMAd.60 Large corticospinal neurons, which comprise less than 20 percent of the total,60,88,94,95 are thought to be especially important for mediating corticomo-toneuronal synapses. (See Reference 11.) Taken together, the observations on the number, density, and size of corticospinal neurons indicate that the premotor areas make a substantial contribution to the corticospinal system.
1.2.2.4 Somatotopic Organization Based on Corticospinal Output: Forelimb and Hindlimb Representation
“leg” representations of a cortical area also can be identified on the basis of the origin of their projections to the cervical or lumbosacral segments of the spinal cord, respectively. This is possible because only 0.2% of corticospinal neurons branch and innervate both the cervical and lumbosacral levels of the spinal cord.85 Corticospinal projections from all of the premotor areas displayed a high degree of topographic organization. The origin of corticospinal neurons in the premotor areas that projected to cervical or to lumbar segments of the spinal cord corresponded remarkably well to the origin of neurons in the premotor areas that projected directly to the M1 arm or to the M1 leg representations, respectively (Figures 1.3A, 1.3B, and 1.4).59,60,76,84,85 Thus, the origins of corticospinal and cortico-cortical projections to M1 are in the somatotopic register.
Five premotor areas projected to the cervical and to the lumbosacral segments of the spinal cord (Figure 1.4). In the PMd, SMA, CMAd, and CMAv, the origin of projections to cervical segments did not overlap with the origin of projections to the lumbosacral segments. In the CMAr, the arm and leg representations were not as clearly separated, whereas in the PMv, most of the corticospinal neurons projected only to the upper cervical segments.84 Thus, at least four premotor areas contained arm and leg representations that appear to be as distinct as those found in M1.
1.2.2.5 Somatotopic Organization Based on Corticospinal Output: Proximal and Distal Arm Representation
The topography of the “proximal” and “distal” arm representations has been exam-ined by injecting different fluorescent tracers into upper cervical and lower cervical segments of the spinal cord.84,85 In general, lower cervical segments are primarily involved in the control of the hand and wrist muscles, whereas upper cervical segments are largely involved in the control of the neck, elbow, and shoulder muscles. (He et al.84 have discussed the topographic organization of the spinal cord motor nuclei.) All of the premotor areas projected to upper and lower cervical segments, but only 5% of corticospinal neurons innervated both the upper and lower cervical segments.85 In each premotor area, the densest concentrations of corticospinal neu-rons that projected to upper cervical segments were separate from the densest concentrations of neurons that projected to lower cervical segments.84,85 This same pattern was also evident in M1. These results suggest that some of the premotor areas have proximal and distal representations of the arm that are as distinct as those in M1.
1.2.2.6 Organization Based on Intracortical Stimulation
The anatomical framework outlined above firmly establishes that the premotor areas are important components in the central mechanisms of skeletomotor control. Intra-cortical stimulation with microelectrodes has been used to assess the potential of each premotor area to generate movements and to construct a map of the body parts represented in each area. Significantly, intracortical stimulation has evoked move-ment in each of the premotor areas. Typically, the average threshold for evoking movement with intracortical stimulation in a premotor area is somewhat higher than that in M1, and the probability of evoking movement at any given site is lower in the premotor areas than in M1.64,76,98–101 In most respects, the body maps produced by intracortical stimulation within the premotor areas are congruent with the topo-graphic organization revealed by anatomical methods.
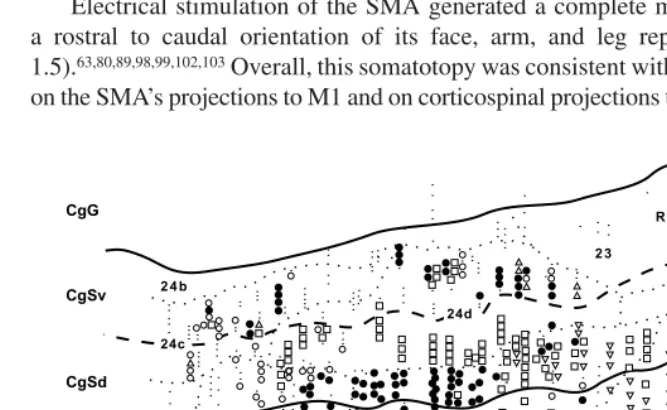
Electrical stimulation of the SMA generated a complete map of the body with a rostral to caudal orientation of its face, arm, and leg representations (Figure 1.5).63,80,89,98,99,102,103 Overall, this somatotopy was consistent with the body map based on the SMA’s projections to M1 and on corticospinal projections to different segmental
FIGURE 1.5 Intracortical stimulation map of the medial wall of the hemisphere. The medial wall is unfolded and reflected upward to display the medial wall in an “upside down” orientation. The boundaries between cytoarchitectonic areas (dotted lines), the fundus of the cingulate sulcus (dashed line), and the lips of the cingulate sulcus (solid lines) are indicated. Movements were evoked by short- or long-train intracortical stimulation in a macaque monkey. All movements were contralateral to the stimulated hemisphere. For conventions and abbre-viations see Figures 1.1 and 1.3. ArSs: rostral limit of the superior limb of the arcuate sulcus; Fgc: frontal granular cortex; Skc: somatic koniocortex. (Adapted from Reference 99.)
levels (Figures 1.4 and 1.5) (see above).67,75,76,84,85 The reported organization of body parts within the face, arm, and leg representations has been less consistent, perhaps due to the fact that complex movements involving multiple joints or noncontiguous joints were evoked at some sites in the SMA. Nevertheless, sites within the arm representation of the SMA that evoked movements of distal joints tended to be located ventral to sites where movements of proximal joints were evoked.80,89,98,99 Correspondingly, the origin of corticospinal neurons projecting to lower cervical segments tended to be located ventral to the origin of corticospinal neurons projecting to upper cervical segments.85
Intracortical stimulation reinforced the distinction between the SMA and the preSMA. Intracortical stimulation with parameters that were effective in the SMA did not evoke movement in the preSMA which lies just rostral to the SMA on the medial wall of the hemisphere (Figures 1.1 and 1.5).79,80,99,104–107 Movements of the arm and rarely the face were evoked at some sites within the preSMA when higher currents and longer pulse trains were applied.80,99,102,106 The movements were also different in character from those evoked in the SMA. Movements elicited in the preSMA were typically slow, involved multiple joints, and resembled natural postural movements. PreSMA neurons often responded to visual but not to somatosensory stimuli, whereas SMA neurons had the opposite characteristics, responding to somato-sensory but not visual stimuli.79,80 The requirement for higher currents and longer stimulus trains is consistent with the fact that the preSMA lacks direct projections to the spinal cord60,85 and to M1.59,60,74,78,79
The major features of the body maps generated with intracortical stimulation in the cingulate motor areas are in many respects consistent with anatomically defined somatotopy (compare Figures 1.3, 1.4, and 1.5). The intracortical stimulation maps, however, are more fractured, are punctuated with nonresponsive areas, and reflect a lower sampling frequency than in the anatomical experiments. Movements of the arm and leg were elicited in each cingulate motor area, but face movements were evoked only, and infrequently, in the CMAr (Figure 1.5).98,99,101–103 In addition, proximal and distal arm movements have been evoked within the arm representation of each cingulate motor area.99 The evoked movements, like those elicited in M1, were usually limited to fast, brief contractions at a single joint.
evoked in the rostral portion of the region corresponding to the CMAv (Figure 1.5)99 (see also Reference 109), but few penetrations have been made in the caudal portion of the CMAv where a leg representation was reported to be located.72,76,82,85,108 Thus, there is a reasonable correspondence between the maps generated by intracortical stimulation and those generated using anatomical methods.
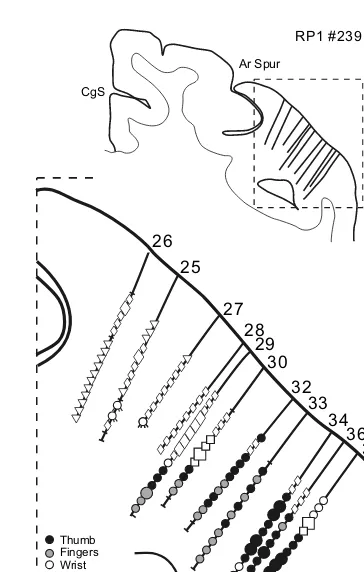
Systematic mapping of the PMd with intracortical stimulation has been limited to one study,101 although numerous studies have reported the results of partial explorations of this region.22,36,76,110 In general, leg movements were evoked in the region of the PMd that was medial to the superior precentral sulcus (dimple) and arm movements were evoked in the region that was lateral to this sulcus.101 Distal and proximal arm movements were evoked within this region.22,101,110 Within the PMd, the threshold for evoking movements is highest rostrally and decreases cau-dally.101,111,112 These results parallel the increase in the density of corticospinal neurons in the caudal portion of the PMd.60,84 Eye movements were evoked by stimulation in the prePMd which lies just rostral to the PMd.112 Thus, here again, the border between the prePMd and the PMd defined by intracortical stimulation corresponds to the border defined using connections to M1 and the spinal cord.60,64,76,84 Intracortical stimulation has defined arm and face representations within the PMv.101 Distal arm movements dominated the portion of the arm representation that was buried medially within the inferior limb of the posterior bank of the arcuate sulcus (Figure 1.6).64,100,101 This portion of the PMv projected almost exclusively to upper cervical segments of the spinal cord.60,84,113 The distal movements evoked by stimulation at this site must therefore be mediated either by propriospinal connec-tions from upper to lower cervical segments (for discussions see References 84,114–116) or through connections with the hand area of M1.117,118 Proximal arm movements tended to be evoked on the surface near the arcuate spur.100,101 The PMv face representation was located lateral to the arm representation both on the surface and within the posterior bank of the arcuate sulcus (not shown in Figure 1.6).101 Information regarding the internal organization of the face area is limited, although laryngeal muscles appear to be represented laterally along the inferior limb of the arcuate sulcus.119
1.2.3 CORTICOSPINAL TERMINATIONS
1.2.3.1 Primary Motor Cortex
The pattern of corticospinal terminations is one indicator of a cortical area’s potential influence on different spinal mechanisms. (A complete discussion of this issue is provided by Kuypers.1) Corticospinal efferents from M1 project to the intermediate zone (laminae V–VIII) of the spinal cord where interneurons that innervate moto-neurons are located as well as directly to the portions of the ventral horn where motoneurons are located (Figure 1.7).1,97,121–129 On the other hand, corticospinal projections from somatosensory and posterior parietal cortex terminate primarily in the dorsal horn.1,121,122,124–126 Evidence from physiological studies suggests that these corticospinal efferents modulate neural processing in ascending somatosensory path-ways. (For review, see References 12,130.) Thus, both anatomical and physiological FIGURE 1.6 Intracortical stimulation map of the arm representation of the PMv. A cross-section from a macaque brain (Macaca nemestrina) illustrates the location of electrode penetrations and the movements evoked at each stimulation site within the posterior bank of the inferior limb of the arcuate sulcus. Thresholds for evoking movement are indicated by symbol size (large symbols = #15 µA, small symbols = 16–50 µA). (Reproduced with permission from Reference 64.)
30 32
33 34
35 36
2 mm 29
Thumb Fingers Wrist Elbow, Shoulder Orofacial Upper Trunk Eye Blink No Response
28
RP1 #239
CgS
Ar Spur
26
evidence suggest that the pattern of corticospinal terminations reflects the differential involvement of these cortical areas in motor output or in somatosensory processing. The extent of M1 terminations within the motor nuclei of the spinal cord changes during development129,131,132 and varies between different species.1,133 This variation appears to correlate with an animal’s manual dexterity.127,132–136 For example, cebus monkeys, which grasp small objects and manipulate tools with a modified “precision grip,”137–139 have abundant direct projections from M1 to spinal motor nuclei.127 In contrast, squirrel monkeys, which pick up small items with all fingers grasping in concert,139,140 have sparse monosynaptic corticospinal terminations that are located remotely on motoneuron dendrites.50,127 Thus, the extent of monosynaptic projections from M1 to spinal motoneurons appears to be part of the neural substrate necessary to make highly skilled and relatively independent movements of the fingers.50,127,129 (For review, see References 1,12.)
1.2.3.2 Premotor Areas
The relationship between the terminations of corticospinal efferents and the motor, sensory, and interneuronal systems of the spinal cord has been studied only for premotor areas on the medial wall of the hemisphere.97,128,141,142 In general, the pattern of corticospinal terminations from the SMA was quite similar to that of M1 (Figure 1.7).97,128 The densest terminations of efferents from the SMA and M1 were located FIGURE 1.7 Corticospinal terminations in C7 of a macaque monkey. Digital photomicro-graphs of spinal cord sections viewed under dark-field illumination with polarized light. The gray matter and spinal laminae are outlined. (A)SMA efferents terminate densely in inter-mediate zone of the gray matter of the cervical spinal cord. Arrow points to terminations in the dorsolateral part of lamina IX that contains motoneurons. (B) M1 efferents terminate in the same regions as do SMA efferents. Compared to SMA terminations, M1 terminations are denser and more extensive in lamina IX, and extend further into the base of the dorsal horn. (Adapted with permission from Reference 9.)
A
A
SMA to C7B
B
M1 to C7in the intermediate zone (laminae V–VIII) of the cervical spinal cord. Terminations here were concentrated at three locations: (1) the dorsolateral portion of laminae V–VII; (2) the dorsomedial portion of lamina VI at the base of the dorsal columns; and (3) the ventromedial portion of lamina VII and adjacent lamina VIII. The cingulate motor areas (CMAr, CMAd, CMAv) also terminated most densely within the intermediate zone.128,142 However, the density of their terminations was noticeably lower than those from the SMA. In addition, terminations from the CMAr and CMAd were concentrated in the dorsolateral portions of the intermediate zone whereas CMAv terminations were most dense in the dorsomedial portions.128,142 This differ-ential pattern of terminations suggests that the CMAr, CMAd, and CMAv innervate specific sets of spinal interneurons and thereby influence different spinal mechanisms for controlling forelimb movements.
All of the medial wall premotor areas, like M1, had terminations that overlapped motor nuclei in the ventral horn of the cervical segments (Figure 1.7).97,128,141,142 Although the terminations from the premotor areas were less dense over lamina IX than were those from M1, all of these studies had a consistent result: the terminations of premotor areas that do overlap lamina IX were concentrated over the motor nuclei innervating muscles of the fingers and wrist. Furthermore, the presence of mono-synaptic projections onto motoneurons innervating muscles of the distal forelimb has been confirmed electrophysiologically for the SMA.97 This result implies that the presence of anterogradely labeled terminations over spinal motoneurons is an indication of direct corticomotoneuronal connections. Thus, not only the SMA but also the CMAd, CMAv, and CMAr appear to project directly to motoneurons con-trolling the distal forelimb. In summary, these results suggest that the premotor areas have the anatomical substrate required to influence the generation and control of limb movement, particularly of the hand. This influence is mediated by pathways that are parallel to and independent of those originating in M1.
1.3 CORTICAL INPUTS TO THE MOTOR AREAS
The recognition that the premotor areas as well as M1 project to the spinal cord suggests that motor commands may arise from multiple cortical areas. We have proposed that each cortical area in the frontal lobe that projects to the spinal cord could operate as a separate efferent system for the control of specific aspects of motor behavior.59 Obviously, identification of the cortical and subcortical inputs to these motor areas could provide some insight into their functional contributions to motor control.
repre-sentation actually injected. Third, the boundaries and identification of the cortical areas projecting to the motor areas are still evolving. For instance, area PE in the parietal lobe projects to several premotor areas, but these projections tend to originate from separate portions of this parietal area.81,146,148,149 These results suggest that PE may not be a single homogeneous area. Thus, precise localization and identification of the cortical areas injected with tracers and the cortical areas containing labeled neurons are essential for valid comparisons between different experiments.
Another aspect of comparing the inputs to each motor area is judging the relative importance of various inputs. A small cortical region may receive input from 40 to 70 cytoarchitectonically recognized cortical areas in the ipsilateral hemisphere alone.82,144,150,151 However, quantitative analysis of all of the inputs to a single cortical site has rarely been attempted.
To minimize the problems of cortical identification and strength of input, we focused our analysis on studies that examined the inputs to the arm representation of motor areas in macaque monkeys. We then transformed the results of these studies onto a standarized map of the frontal and parietal lobes (Figure 1.1). Next, we pooled the results from recent publications and assigned a “strength” to specific connections based on the relative number of labeled neurons and the consistency with which a projection was observed in all studies (Tables 1.1 and 1.2). Even with these con-straints, we found considerable variation in the results among studies. Consequently, our synthesis of these results reflects our consensus derived from multiple studies and may not always fit with the data reported in an individual study.
1.3.1 PRIMARY MOTOR CORTEX
1.3.1.1 Frontal Cortex
Cortical input to M1 is entirely confined to cortical regions in the frontal and parietal lobes that, like M1, are the origin of projections to the spinal cord (Figure 1.8, Table 1.1; see Figure 1.1 for area identification). These corticospinal tract (CST) projecting areas include all the premotor areas in the frontal lobe (defined above) and portions of the superior parietal lobe (SPL). M1 has no substantial connections with the prefrontal, pre-premotor or limbic cortex.
1.3.1.2 Parietal Cortex
M1.71,73,154–156 SII has heavy projections to M1 in most studies,68,71,73,154,155 but these projections were less substantial when injections were confined to the surface of the precentral gyrus.59,76
As noted earlier, neurons in M1 exhibit short latency responses to activation of cutaneous and proprioceptive receptors. However, the route by which this soma-tosensory input reaches M1 remains controversial. Lesion of the dorsal columns, a major ascending pathway to the parietal cortex, extinguishes the responsiveness of
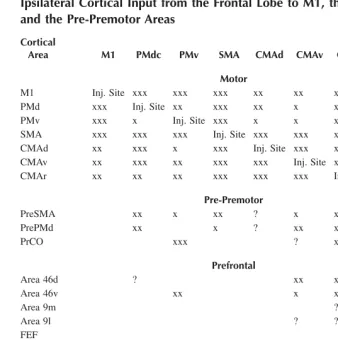
TABLE 1.1
Ipsilateral Cortical Input from the Frontal Lobe to M1, the Premotor Areas and the Pre-Premotor Areas
Cortical
Area M1 PMdc PMv SMA CMAd CMAv CMAr Pre-PMd
Pre-SMA
Motor
M1 Inj. Site xxx xxx xxx xx xx x
PMd xxx Inj. Site xx xxx xx x x xxx xx
PMv xxx x Inj. Site xxx x x xx x xx
SMA xxx xxx xxx Inj. Site xxx xxx xx x x
CMAd xx xxx x xxx Inj. Site xxx xxx
CMAv xx xxx xx xxx xxx Inj. Site xxx ?
CMAr xx xx xx xxx xxx xxx Inj. Site xxx xxx
Pre-Premotor
PreSMA xx x xx ? x xx xxx Inj. Site
PrePMd xx x ? xx xx Inj. Site xxx
PrCO xxx ? xx ?
Prefrontal
Area 46d ? xx x xxx x
Area 46v xx x x xx
Area 9m ? xx xxx
Area 9l ? ? x
FEF x
SEF x
Limbic
Area 24a,b ? ? ? x x xx xx ? xxx
Area 23a,b ? x x ?
25, 29, 30 ?
Orbital Frontal Cortex
TF, 28, 35 ? x
Prostriata ?
M1 neurons to peripheral input.157 Removal of the primary and secondary somato-sensory areas, as well as removal of the cerebellum, does not.5,158 Some authors have proposed that M1 receives input directly from a thalamic region innervated by a portion of the dorsal column pathway (for references and discussion, see Reference 5), but firm anatomical evidence for such a neural circuit remains elusive.159 Although the major route by which short latency somatosensory information reaches M1 has
TABLE 1.2
Ipsilateral Cortical Input from the Parietal Lobe to M1, the Premotor Areas and the Pre-Premotor Areas
Cortical
Area M1 PMdc PMv SMA CMAd CMAv CMAr Pre-PMd Pre-SMA
Superior Parietal Lobule
Area 3a xx x xx x ?
Area 3b x
Area 1 xx ? x ?
Area 2 xx ? xx x x
5 (PE) xx xx xxx x xx
PEip xxx xxx xx xx xx xx ?
MIP x xxx ? ? xxx ? x
PEc xxx ? x ? xx
PEci xx xx x x x xx
V6A xxx
PGm x ? ? xxx
CGp x ? ? xxx
Inferior Parietal Lobule
AIP x xxx
VIP x ? ?
LIP ? ?
7b (PF) ? ? xxx x ?
PFG ? ? ? xxx x ? x
7a (PG) ? ? x xx x
Lateral Sulcus
PFop x ? xx xxx xx
PGop ? x
SII xx xxx xx x xxx
Ig xx xx x xxx x
Idg x xx
RI ?
Temporal
STS ? x
not been determined, the interconnections between SI and M1 may be necessary for an animal to learn a new motor skill under the guidance of somatosensory cues.5,160
1.3.2 PREMOTOR AREAS
1.3.2.1 Interconnections among the Motor Areas
connections with every other premotor area as well as with M1. Second, the inter-connections among the cingulate motor areas (CMAd, CMAv, CMAr) on the medial wall are dense and equal. Third, the PMd and the PMv on the lateral surface have strong, reciprocal connections with M1 and the SMA. On the other hand, the con-nections between the PMd and PMv are more limited. The PMv is connected to the caudal portion of the PMd,58,59,68,72,81 but only sparsely to its rostral portions.58,161,162 The restricted connections between the PMd and the PMv may reflect differences in the body representation in each area. The PMd was reported to project mainly to the shoulder representation of M1, whereas the PMv projected largely to the digit representation in M1.73 Fourth, the projections from the lateral motor areas (PMd, PMv, M1) to the cingulate motor areas, particularly the CMAv and the CMAr, tend to be relatively weak.82,163,164 These patterns of connectivity suggest that the PMd and PMv are fundamentally distinct from each other and from the motor areas on the medial wall. The SMA with its broad, balanced connectivity is ideally situated to coordinate and integrate information flowing among the motor areas in the frontal lobe.
1.3.2.2 Parietal Cortex
Parietal lobe input to the premotor areas appears to follow a general trend. Most of the parietal lobe input to the premotor areas originates from posterior portions of the parietal lobe including the SPL (area 5, as described by Brodmann16), the inferior parietal lobule (area 7, as described by Brodmann16) and the secondary somatosen-sory cortex (SII) (Figure 1.8, Table 1.2). These areas are thought to be concerned with the highest levels of somatosensory processing and to participate in multimodal sensory integration, spatial attention, or visuomotor control.165 Every premotor area in the frontal lobe is richly interconnected with parts of at least one of these posterior parietal areas. On the other hand, only the SMA receives dense input from any of the subdivisions of the primary somatosensory cortex. This input to the SMA orig-inates in area 2, which is thought to be at an intermediate stage of somatosensory processing (Figure 1.8, Table 1.2).59,68,70,72,78,81,146,148,155,161–163
Portions of the superior parietal lobule project to all the premotor areas except for the CMAr. On the postcentral gyrus, the lateral portion of area PE targets the PMv, whereas its more medial portions project heavily to the SMA and CMAv. In the most caudal portion of the postcentral gyrus, area PEc supplies dense input to the PMd. Laterally within the intraparietal sulcus, area PEip has the most widespread projections. Area PEip targets five premotor areas, including the PMd, PMv, SMA, CMAd, and CMAv (Figure 1.8, Table 1.2). Despite this apparently broad divergence, the origin of projections from PEip to the PMv and PMd as well as M1 tend to arise from separate location.59,147,149,162 Medially in the intraparietal sulcus, area MIP projects densely to the PMd and the CMAv. In the caudal portion of the cingulate sulcus, area PEci provides strong input to the SMA and the PMd.59,78,146,148,149,166 Thus, although the premotor areas are broadly targeted by SPL projections, each premotor area can be distinguished by its unique mixture of inputs from the SPL (Figure 1.8, Table 1.2).
Subdivisions of area 7 are characterized by more complex forms of somatosensory processing and the presence of multimodal information that integrates visual and somatosensory inputs.167–169 Area PFop is the only IPL subdivision with strong links to more than one premotor area. It has heavy projections to the CMAd, CMAv, and CMAr. AIP in the anterior portion of the ventral bank of the intraparietal sulcus and area PF on the adjacent IPL provide dense input to the PMv.59,68,81,149,161,162 Area PFG, located just caudal to area PF, projects to the CMAv.82,163,170 Within the lateral sulcus, SII projects densely to the PMv, CMAd, and CMAr, as well as M1.58,59,70,149,162,163,171–174 Taken together, these observations indicate that each premotor area receives a unique pattern of input from the various subdivisions of the parietal lobe and provides another basis for differentiating the individual premotor areas.
The outputs from the medial posterior parietal cortex to the frontal motor areas arise from regions that are one to two synapses removed from the primary visual cortex. These regions include the PEc on the most caudal portion of the superior parietal gyrus, area V6A buried on the anterior bank of the parietal occipital sulcus, area PGm on the medial wall just rostral to the parietal occipital sulcus and the posterior cingulate gyrus (CGp) (Figure 1.1). The projections from these regions are restricted to the prePMd and PMd. The differential distribution of the projections from this posterior parietal region to regions of the PMd and prePMd suggests that these frontal lobe areas may require further parcellation. This is likely to be especially evident as more physiological studies are designed to explore the visual–spatial capabilities of these regions.146,148,175
In general, the density of projections from these “visual areas” in posterior parietal cortex increases as one proceeds from the caudal border of the PMd to the prePMd.146–149 Some have argued that these projections provide a neural substrate for the visual guidance of reaching movements to objects in extrapersonal space.146–149 It is unclear, however, whether the visual information provided by these regions is sufficient for the accurate localization of targets. For instance, area V6A has the most direct visual input to the motor areas in the frontal lobe. V6A neurons have large receptive fields located in the periphery of the visual field. Such fields seem better suited to alert the motor system and shift attention to a particular quadrant of space176–178 than to drive the visuomotor transformation required to reach out and grasp an object.
1.3.2.3 Pre-Premotor Cortex
preSMA, and PrCO are one step removed from M1 and have connections with at least one premotor area (Figure 1.8, Table 1.1). For example, all of these frontal lobe areas are interconnected with the CMAr.58,78,82,107,144,164,180 Each area is also connected with the adjacent premotor area — PrCO with the PMv, prePMd with the PMd, and preSMA with the SMA.78,107,148,161,162,180 However, the significance of the preSMA–SMA connection and the prePMd–PMd connection is unclear. Only immediately adjacent regions in these cortical areas appear to be interconnected and at times these interconnections do not appear especially dense. The organization of these connections clearly requires further investigation.
1.3.2.4 Prefrontal Cortex
Only three premotor areas — the PMv, the CMAr, and the CMAv — receive substantial input from the dorsolateral prefrontal cortex (Walker’s area 46181). The vast majority of these projections originate in the dorsal (area 46d) and ventral (area 46v) banks of the principal sulcus (Figures 1.1 and 1.8; Table 1.1).58,59,74,81,82,143,162 The PMv is the target of dense prefrontal projections that originate from area 46v in a topographic manner.59,74,81,162,182,183 The CMAv and the CMAr are the targets of projections from both the dorsal and ventral banks of principal sulcus (areas 46d and 46v). Additional projections to the CMAr arise from the medial and lateral portions of area 9.82,166 These observations indicate that specific portions of the prefrontal cortex selectively target just three of the seven motor areas in the frontal lobe. These projections of area 46 to the premotor areas link the prefrontal cortex to cortical regions with direct access to the primary motor cortex and spinal mech-anisms of motor control. Connections of the prefrontal cortex with the motor system appear to be tightly focused and designed to provide specialized information about particular aspects of cognitive and executive functions.
1.3.2.5 Limbic Cortex