Temporal and spatial activity of a promoter from a pea enzyme
inhibitor gene and its exploitation for seed quality improvement
T. Welham, C. Domoney *
John Innes Centre,Norwich Research Park,Norwich NR4 7UH,UK
Received 31 March 2000; received in revised form 28 July 2000; accepted 31 July 2000
Abstract
The promoter from one of the two seed-expressed genes encoding trypsin/chymotrypsin inhibitors (TI) has been isolated and characterised in transgenic pea lines, following its re-introduction byAgrobacterium-mediated transformation, as a TI promoter-b -glucuronidase (GUS) gene fusion. The promoter from this gene (TI1) directed expression of GUS enzyme at late stages of embryogenesis, comparable to those determined for activity of the homologous native TI genes. GUS expression was detected in roots of plants subjected to drought stress conditions, indicating that theTI1 gene, normally seed-specific in its expression, can be induced under these conditions. A second gene construct utilised theTI1 gene promoter to direct expression of an antisense
TI gene. Seed TI activities in some lines transformed with this construct were reduced significantly. A limitation of the pea transformation methodology for antisense manipulations, in particular, is the observed frequency of non-transmission of transgenes from primary transformants (up to 80%). © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Antisense gene; Drought response; Pea transformation;Pisum sati6um; Trypsin inhibitor
www.elsevier.com/locate/plantsci
1. Introduction
Legume seeds contain a range of components which are considered to be antinutritional. Among these are the enzyme inhibitor proteins that can be potent inhibitors of animal digestive enzymes. As a result, these proteins can limit the extent to which legume seeds are included in animal diets. However, several plant enzyme inhibitors have been linked to protective functions in plants, par-ticularly as protective agents against insect pests [1 – 3] and bacterial pathogens [4] and many hibitors are wound-inducible [5]. Protease in-hibitors may also be involved in protecting seed proteins, either by preventing premature hydroly-sis of storage proteins or by acting as enzyme-sta-bilising agents [6]. Some classes of legume protease
inhibitors are rich in sulphur-containing amino acids and therefore are of nutritionally desirable composition.
Many legume seed products are processed by heating and/or pelleting in order to denature and inactivate inhibitors, processes that are not only expensive but can be damaging to other nutrients. Genetic manipulation offers the potential to sup-press inhibitor gene activity through the introduc-tion and expression of an appropriate antisense gene. However, suppression of inhibitor synthesis in seeds would need to be achieved ideally without interfering with the expression of the same or related genes in other parts of the plant as a protective response. It is important, therefore, to establish the roles played by individual inhibitor genes in order to determine the scope for interfer-ing with the expression of some without beinterfer-ing detriment to the plant.
In pea seeds, the major enzyme inhibitor proteins are capable of inhibiting the digestive enzymes, trypsin and chymotrypsin, and belong to
The TI1 gene sequence is accession number AJ276900 in the EMBL database.
* Corresponding author. Tel.: +44-1603-450000; fax: + 44-1603-450045.
E-mail address:[email protected] (C. Domoney).
the Bowman – Birk class of inhibitors, based on sequence homology [7,8]. Two closely linked genes encode the major pea seed inhibitors (TI) and map to a genetic locus on linkage group V [8,9]. A proportion of the primary products of the two genes is post-translationally processed at the C-ter-minus to produce isoforms which are more potent inhibitors of digestive enzymes [8]. Neither of the two genes is expressed in vegetative organs of plants grown under normal environmental condi-tions. However, one of the two was found to be expressed in roots of pea plants grown under drought conditions [10], suggesting a possible physiological function for some of these proteins in dehydrating tissue.
The high degree of homology between the two genes encoding the major seed TI suggests that a single antisense gene could interfere with the ex-pression of both genes. However, an antisense TI gene would need to be controlled by a promoter specifying its activity during the very late stages of embryogenesis when expression of the two ho-mologous (sense) genes is maximal. Since such a promoter had not been characterised previously, we have isolated the promoter from one of theTI genes for construction of an antisense gene. We have used the promoter from the TI gene for which the corresponding RNA had not been de-tected in any organ other than seeds. In this paper we describe the behaviour of this promoter as a fusion with a GUS reporter gene during embryo development in transgenic pea lines and in trans-genic plants subjected to drought stress. The ex-ploitation of this promoter for an antisense TI gene and its introduction into transgenic peas is also reported.
2. Materials and methods
2.1. Plant material
Seeds for transformation experiments were Pisum sati6um cultivar (cv.) Puget (wrinkled-seeded), obtained from Advanta Seeds UK, Lin-coln, and the genotype BC1/17 (round-seeded), a gift from C. Hedley, John Innes Centre. Both batches of seeds were free from fungal contami-nants when used in tissue culture experiments.
All plants produced from transformation experi-ments were grown in John Innes No. 1 compost
with 30% extra grit. All primary transformants (T1 plants) and the majority of T2 and T3 plants were grown in a controlled environment room with 16 h light at 15°C, 8 h dark at 12°C and 70% relative humidity. A small number of T2 plants were grown in a glasshouse with light supplementation during winter.
Immature embryos were harvested from plants which were grown in the controlled environment rooms. Flowers were tagged on the day they were fully open and embryos harvested at 33, 42 and 53 days after flowering.
Plants subjected to drought stress were sown in the controlled environment room and watered daily for 26 days. After this period, some plants (control) were watered as before, whereas water was withheld from a proportion of the plants. After a further 16 days, roots, leaves and stems were harvested from control and drought-treated plants, frozen in liquid nitrogen and stored at −70°C.
2.2. Vectors and bacterial strains
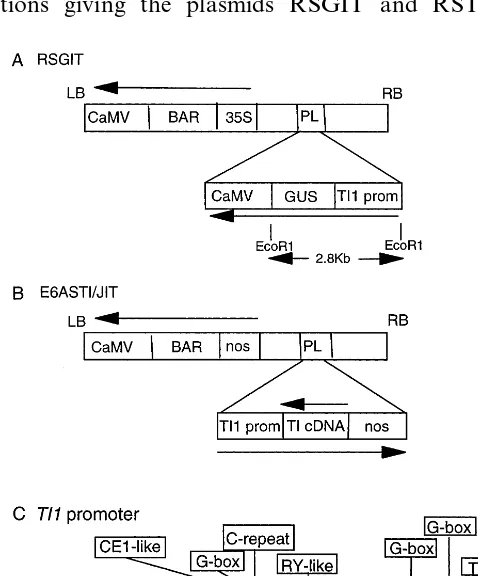
2.2.1. TI promoter — glucuronidase gene construct (RSGIT)
Several genomic clones that hybridised to the insert from a TI cDNA clone (pTI 4 – 41), corre-sponding to one of two seed-expressed TI genes [8,9], were isolated from a genomic library. The library, constructed using DNA from the pea cv. Birte [11], was a gift from C. Forster and R. Casey. Clones that hybridised strongly to the probe were analysed in detail; the transcribed re-gion of the TI gene in one clone (TI1) was identi-cal to the cDNA, pTI 12 – 36, representing the second seed-expressed TI gene [8]. Most of the region immediately upstream of the transcribed sequence was isolated from this genomic clone, following subcloning into a Bluescript vector, as a
1 kb KpnI-BglII promoter fragment. The se-quence between the BglII site in the promoter and the transcription start in the TI gene was synthe-sised as a pair of complementary oligonucleotides with the BglII site at one end and aHindIII site at the other, the latter site to facilitate ligation to marker genes.
following removal of the double 35S CaMV pro-moter from this vector with KpnI and HindIII. The resulting plasmid, pJP04, therefore, contained a TI gene promoter that included an oligonucle-otide linker to the GUS gene, but no TI gene transcribed sequence. The TI promoter-GUS gene was excised from pJP04 using XhoI and cloned into the binary vector RS66; the vector RS66 is one of a series of vectors (that includes E6 nos bar; see below) and contains a 35S CaMV pro-moter-bar gene-CaMV terminator cassette (Fig. 1A). The bar gene encodes a modifying enzyme, phosphinothricin acetyltransferase, that facilitates selection of transformed plants [13]. The TI-GUS (TIG) gene fusion was inserted in both orienta-tions giving the plasmids RSGIT and RSTIG;
only results obtained with RSGIT are reported here. Both RSTIG and RSGIT plasmids were shown to be active in a transient expression assay based on GUS enzyme detection in pea axes fol-lowing particle bombardment [14].
2.2.2. TI promoter-antisense TI construct (E6ASTI)
A near-full-length TI cDNA in pUC 19 (pTI 38-38) was isolated from a library as previously described [9]. The sequence of this cDNA is identi-cal to that of pTI 5-72 [8], except that it is two bases shorter at the 5% end. A SphI site that exists midway in the mature protein sequence was used to clone theTI1 gene promoter as aHindIII –SphI fragment such that the cDNA was in antisense orientation relative to the promoter. For this cloning, the TI1 promoter was isolated as a HindIII –BglII fragment and complementary oligonucleotides used as above to provide the re-gion between the BglII site and the transcription start in the TI1 gene; in this case, a BglII –SphI fragment was provided by the annealed oligonu-cleotides. TheTI1-antisenseTIgene was cloned as a HindIII –BamHI fragment into the binary vec-tor, E6 nos bar (one of a series described by Schneider et al.[15]); the vector employed here contained a bar gene with a CaMV terminator under the control of a nopaline synthase (nos) promoter, giving the plasmid E6ASTI. The BamHI site (at the 5%end of the cDNA) was used to clone a nos terminator as a BamHI –BglII fragment from the plasmid, pJIT 2 (a gift from P. Mullineaux), giving the plasmid E6ASTI/JIT (Fig. 1B); only results obtained with E6ASTI/JIT are reported here.
The plasmids containing the above constructs were transformed into Agrobacterium tumefaciens EHA 105 [16] by electroporation.
2.3. Pea transformation
Peas were transformed as described in [17]. Briefly, cotyledonary meristems of germinating seeds were inoculated by making cuts with a scalpel blade which had been dipped in a culture of A. tumefaciens carrying either RSGIT or E6ASTI plasmids. Inoculated explants (cotyledon pieces with attached axes) were cultured on selec-tion medium according to [17]. Shoots produced by explants were excised and placed directly onto
selective media containing 3.75 mg/l phos-phinothricin (PPT). Shoots surviving selection were grafted onto root stocks provided by non-transgenic seedlings.
2.4. Analysis of transgenic plants and seeds
Primary transformants (T1) were analysed by painting 2 – 4 leaflets with the herbicide, Herbiace, at 3 mg/ml [17].
2.4.1. GUS acti6ity measurements of plants transformed with RSGIT
Mature T2seeds were assayed for GUS activity. A small meal sample was taken from individual seeds by drilling a small hole in the cotyledons away from the axis. Meal was extracted with 100 mM Tris, 150 mM NaCl pH 8 (250 mg meal per ml buffer) for 1 h at 4°C with continuous stirring. The meal residue was removed by centrifugation and GUS activity in the supernatant was esti-mated using a GUS-light™ kit (Tropix). Between 1 and 5 ml of the extracts were assayed using 200 ml diluted glucuron substrate, according to the manufacturer’s instructions. Emitted light was measured as relative light units (RLU) using a luminometer (Lumat 9501). The total protein con-centration of supernatants was determined using a dye-binding kit (Bio-Rad) and bovine serum albu-min as a standard.
Immature embryos from T2 plants transformed with the RSGIT construct were harvested at three different stages of development (measured by days after flowering). Three embryos from every stage were ground using a pestle and mortar and ex-tracted with 100 mM Tris, 150 mM NaCl pH 8 (200 mg fresh weight per ml buffer). GUS activity was measured using supernatants as before.
Vegetative organs from drought-treated and control watered plants were freeze-dried and ground to a fine powder using a pestle and mortar. The samples were extracted with buffer and GUS activity measured as described for mature seeds.
2.4.2. Southern blot analysis
DNA was prepared from 1 to 2 leaves using a small scale DNA preparation method [18]. DNA was digested withEcoRI and run on 0.8% agarose gels. DNA was blotted onto nitrocellulose and hybridised [19] with a [32P]-labelledbargene probe and, where appropriate, a GUS gene probe. Bar
and GUS DNA for labelling was isolated from the plasmids pJIT 84 and pJIT 166, respectively, using appropriate restriction enzymes and labelled using an oligolabelling kit (Pharmacia). Blots were washed in 0.1× SSC, 0.1% SDS at 50°C and exposed to pre-flashed X-ray film at −70°C.
2.4.3. Northern blot analysis
Total RNA was extracted from organs of plants subjected to drought or control conditions and analysed by Northern hybridisation as previously described [20]. Blots were hybridised as outlined for Southern blots using a GUSprobe or a trypsin inhibitor cDNA probe; the latter was derived from the plasmid pTI 4 – 41 [8,9]. Blots were washed in 0.1× SSC, 0.1% SDS at 50°C and exposed to pre-flashed X-ray film at −70°C. Autoradio-graphs were scanned using a Joyce-Loebl Chromoscan.
2.4.4. Western blot analysis
Organs from drought-treated and control plants were extracted directly in sample buffer (50 mg freeze-dried and ground material per ml buffer) and boiled, or were extracted with 100 mM Tris/ 150 mM NaCl pH 8 (50 mg freeze-dried and ground material per ml buffer) and the protein concentration of supernatants determined as above; supernatants were diluted 1:1 with 2× sample buffer and boiled. Sample aliquots, based on equivalent dry weights or equivalent protein amounts, were analysed on sodium dodecyl sul-phate-polyacrylamide gels (SDS-PAGE). Condi-tions for SDS-PAGE were as described in [21], with the exception that mercaptoethanol in the sample buffer was replaced by dithiothreitol (DTT) at 17mM. Separated polypeptides were electroblotted onto nitrocellulose and blots pro-cessed [21], using anti-ß-glucuronidase-rabbit IgG (Molecular Probes) as a primary antibody for detection of GUS protein on blots.
2.4.5. Trypsin inhibitor (TI) measurements
2.5. Statistical methods
Segregation ratios were analysed using a X2 analysis.
A two-sample t-test (Minitab) was applied in the analysis of TI activity measurements and com-parison of populations of T3 seeds.
3. Results
3.1. Isolation of promoter
The TI1 gene, corresponding to one of two genes encoding the major pea seed inhibitors, was isolated as a genomic clone containing approxi-mately 1 kb of promoter sequence. This promoter contained a number of elements that may influ-ence its activity (Fig. 1C). Relative to the tran-scription start, a putative TATA box (−40), a RY-like element (CCTGCATG: −467 to −473), together with a number of G-box elements, were evident. Three G-box elements, TAC ACGT ATG, TAC ACGT GTA and ATC ACGT GAC were at positions −135 to −144, −226 to −235 and −571 to −580, respectively. In the case of the most distal element, an overlapping ‘C-repeat’-like element was observed (T GACCG A: −568 to −577), together with an upstream CE1-like element (TAC CACC AC: −586 to −594). The significance of many G-box elements is unclear but their context and interaction with other elements can determine a range of promoter responses [23 – 25]. The TI1 gene promoter was used in the con-struction of transgenes designed to express either antisense TI mRNA at the appropriate stage of seed development or the marker enzyme, ß-glu-curonidase, for the assessment of the behaviour of the promoter.
3.2. Reco6ery of transformed plants
The transformation efficiency of grain legumes is consistently low; for pea, in particular, percent-age success rates of 1 – 3% have been cited, based on recoveries of primary transformed plants [15,17,26]. Two genotypes have been employed for the work described in this paper — BC1/17 and cv. Puget. Transformation efficiencies, based on recovery of individual explants producing resistant shoots, were calculated to be 0.13 and 0.8%,
re-spectively, for BC1/17 and cv. Puget. The latter value is within error of the values reported for cv. Puget [17]. Transformation efficiencies were much lower, however, when based on recovery of indi-vidual explants for which T2 transformed plants were obtained. Transmission of transgenes was observed for only 40% of explants. Multiple shoots were often recovered from individual ex-plants; since these can result from independent transformation events, all shoots were grafted (T1 plants; lines) and grown for seed production. Fur-ther details of the lines generated with the two constructs are given below.
3.2.1. Transgenic lines generated using RSGIT In all, 19 T1plants derived from explants of cv. Puget, both bar and GUS genes were detected by Southern analysis (data not shown). GUS activity could not be detected in any T2seeds tested from eight lines (up to 55 seeds per line), whereas all seeds tested from four lines (up to 15 seeds per line) were positive for GUS activity. Seeds from a further seven lines showed segregation of GUS activity (between 8 and 15 seeds tested) with ratios ranging from 1:1 to 9:1 and probabilities in the range 0.05 – 0.9 for a single locus (3:1) ratio. Where all seeds tested positive for GUS activity, a mini-mum of two loci containing transgenes could be inferred.
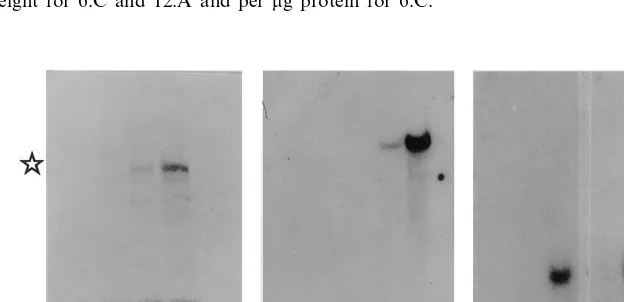
All the GUS positive seeds produced plants which were resistant to the herbicide and both bar and GUS genes were detected on Southern blot analyses. All the GUS negative seeds produced plants sensitive to the herbicide and neither bar nor GUS genes could be detected. Where there was no evidence for GUS activity in any T2 seeds from any one line, or for either bar or GUS transgenes in the T2 plants, non-transmission of the transgenes through meiosis could be assumed; this was the case for 42% of the T1 plants. Lines 6:C and 12:A, showing segregation of transgenes among T2 seeds and plants at a frequency of 9:4 (Fig. 2) and 9:1, respectively, were used for further analyses (P=0.6 and 0.3, respectively, for a 3:1 ratio).
3.2.2. Transgenic lines generated using E6ASTI/JIT
Fig. 2. Southern blot analysis of EcoRI-digested genomic DNA from thirteen (tracks 1 – 13) T2 plants from the
trans-genic line 6:C, hybridised with aGUS gene probe. A 2.8 kb fragment, predicted from Fig. 1A, was evident in plants that were resistant to the herbicide, giving a segregation ratio of 9:4 for both the transgenes. The sizes of DNA markers are indicated (kb).
ratios, respectively. Lines 1:B and 1:C were used for further analyses.
3.3. Analysis of lines transformed with RSG1T
3.3.1. GUS acti6ity in de6eloping embryos
Embryos were harvested at 33, 42 and 53 days after flowering (DAF) from line 6:C:4, which was identified as a T2 plant homozygous for the GUS transgene, following analysis of mature seed (see below). GUS activity was detected at low levels in embryos at 33 DAF but increased dramatically between 42 and 53 DAF, both on a per unit fresh weight and a per unit protein basis (Fig. 3). These data show that GUS expression in embryos, under the control of theTI1 promoter, follows that of TI activity, previously shown to increase dramatically during the late stages of embryogenesis in geno-types grown under the same conditions [10,22].
3.3.2. Response of the TI1 promoter to drought stress
Populations of T3seeds from individual T2seeds from lines 6:C and 12:A were assayed for GUS activity to determine which populations were derived from T2 seeds that were homozygous for the presence or absence of the transgene or were hemizygous. Measurements were made on 30 seeds from each population. Plants from populations that were homozygous for the presence or absence of the transgene were subjected to either drought or control growth conditions. GUS activity mea-surements were made on extracts from roots and leaves harvested after the 42-day-treatment. Very low background GUS activity (B1 RLU/mg protein ×10−3) was recorded for non-transgenic plants. A low level of GUS activity was detected in roots and leaves from control transgenic plants (Fig. 4). The activity in roots from drought-treated plants was greatly elevated compared with control levels on a unit dry weight basis and on a unit protein basis (Fig. 4, line 6:C). In analyses of line 12:A, an even greater fold increase in GUS activity was observed for drought-treated roots (Fig. 4). Western analysis (Fig. 5a – d) showed a large in-crease in the amount of GUS protein (, Fig. 5c and d) in the drought-treated roots compared with those of the control plants, in agreement with activity measurements [a non-specific low molecu-lar weight protein that reacted with the antibody appeared to be constitutively present in all the
Fig. 3. GUS activity measured as relative light units (RLU; mean of 3 determinations) per mg fresh weight ( ) and per
mg protein () in developing T3embryos from the transgenic
line 6:C (T2plant 6:C:4 of Fig. 2). Embryos were harvested at
three developmental stages.
extracts when roots of plants analysed in Fig. 5a – d were extracted directly in sample buffer and in all the analyses of plants derived from line 12:A (not shown)]. Northern analysis of RNA from these plants showed increased levels of GUS mRNA in roots of drought-treated plants (Fig. 5e – h), estimated to be 10-fold by scanning autora-diographs. Control non-transgenic plants showed no hybridisation to the GUS probe (Fig. 5e and f). TI mRNA was detected in the roots of the two sets (transgenic and non-transgenic) of drought-treated plants (Fig. 5j and l) but not in either set of well-watered plants (Fig. 5i and k).
3.4. Analysis of seeds from lines transformed with E6ASTI/JIT
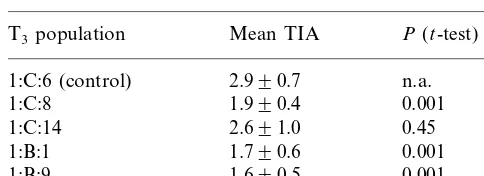
TI activity (TIA) measurements were made on T3 seeds originating from five T2 plants; seeds from the T2 plant 1:C:6, which segregated for absence of thebargene, were included as controls. Analyses of TIA measurements on these seeds showed there was a significant difference at the 0.1% level in three of the populations when com-pared with the non-transgenic control population (Table 1) with reductions in TIA of up to 45%. There was no significant difference between the
Fig. 4. GUS activity measured as relative light units (RLU; mean of 4 determinations) in leaves and roots of T3plants, derived
from the two transgenic lines 6:C (T2plant 6:C:4 of Fig. 2) and 12:A, subjected to control or drought conditions. Activities are
presented per mg dry weight for 6:C and 12:A and permg protein for 6:C.
Fig. 5. Western blot analysis (a – d) of protein (20mg) from roots of non-transgenic (a and b) and transgenic (c and d) T3plants,
derived from T2plants 6:C:1 and 6:C:4, respectively (Fig. 2), subjected to control (a and c) or drought (b and d) conditions. The
blot was developed using a commercial antibody to GUS [Mrapproximately 68 000 in c and d ()] which reacts non-specifically
Table 1
Trypsin inhibitor activity (TIA) in transgenic seeds from plants transformed with an antisense TIgenea
T3population Mean TIA P (t-test)
aMean TIA for populations of seeds from four transgenic
T2plants in comparison to seeds from a control T2plant that
segregated for absence of the transgene. The T3 seeds
analysed from the four transgenic T2 plants all gave rise to
transgenic plants. Mean TIA was based on three determina-tions for n individual seeds from the T3 populations;n=15
for C andn=10 for B populations.
second (TI1) gene were not isolated in these exper-iments [10]. The behaviour of the TI1 gene pro-moter was examined, therefore, as a marker gene construct, in transgenic peas under normal and drought stress conditions.
Analysis of GUS activity in developing embryos showed that the TI1 promoter was active at the appropriate stage of development with a pattern of expression (Fig. 3) that agreed with that previ-ously documented for pea seed-expressed TI genes [10]. Analysis of transgenic plants subjected to the same drought conditions employed in previous studies showed, however, that the TI1 promoter responded to drought conditions with concomitant increases in GUS RNA, protein and enzyme activ-ity in roots (Figs. 4 and 5). Furthermore, a low level of GUS expression was evident in roots of control (well-watered) transgenic plants, despite the apparent inactivity of the homologous native promoter in roots from these plants (Fig. 5). Thus, the TI1 promoter-transgene is behaving in a simi-lar, but not identical, way to that of native ho-mologous TI1 genes. It is possible that sequences upstream of the 1 kb promoter fragment utilised are necessary for conferring total seed specificity; analyses of other legume seed promoters have indicated that seed specificity is conferred by re-gions upstream of a minimal promoter in the approximate region −100 to −250 with en-hancers further upstream regulating quantitative control only (see for example [29]). However, data on grain legume seed-specific promoters that are derived from studies in non-legume transgenic plants may not correspond exactly with data ob-tained in a homologous background [30]. It is also possible that sequences upstream of the promoter used suppress its induction in roots under drought conditions and that the induced TI RNA (Fig. 5j and l) reflects activity of the second TI gene only. It is more probable, however, that both the genes of this class behave in a similar fashion and that the non-isolation of cDNAs homologous to the TI1 gene in previous work was a statistical anomaly of the small number of cDNAs isolated from the root library [10].
The existence of a G-box element with adjacent CE1 and ‘C-repeat’ elements within the promoter (Fig. 1C) may explain the drought responsiveness of TI1; combinations of G-box and CE1-like ele-ments are implicated in responses to drought in many Lea (‘late embryogenesis abundant’) genes population 1:C:14 and the control. These seeds
were sown and T3 plants analysed by painting leaves with herbicide. All the plants from the four transgenic populations were resistant to the herbi-cide and thus all seeds assayed contained the transgene(s).
4. Discussion
[24,31]. C-repeats have been implicated in cold responsiveness of some Lea genes [25,32] and an overlapping G-box/C-repeat may be significant in similar responses of TI genes; ‘winter’ peas gener-ally contain much higherTI contents than ‘spring’ peas [33] and this response may further extend the analogy between TI and Lea genes [6].
The data presented here suggest that seed TI amounts can be manipulated by introduction of an antisense TI gene with TIA being reduced by up to 45% (Table 1). More extreme lines may have been identified if the number of transformants giving rise to progeny seed containing the transge-ne(s) had been greater. Non-transmission of trans-genes from T1 plants has been observed by others [15,26] and is likely to result from chimeric pri-mary transformants; in some cases, a low level of transmission of transgenes has been evident through more extensive sampling of progeny, giv-ing rise to extremely non-Mendelian segregation ratios [17]. Segregation ratios may be distorted also through silencing of transgenes; bar gene si-lencing was observed by Schroeder et al. [34], using a three-gene, but not a four-gene, construct that each included the samebargene but in differ-ent positions. We have observedbargene silencing in plants derived from one explant only in the work reported here and, in every other case, Southern analysis confirmed the results of herbi-cide paint tests. Improvements to grain legume transformation systems must include, as a priority, a reduction in the percentage of chimeric primary transformed plants and an overall increase in numbers of transgenic plants recovered among T2 plants.
It is unlikely that seed TIA would have been abolished completely with the constructs reported in this work. Other related TI genes have been identified in pea [35,36] whose expression predom-inates in non-seed organs but which make minor contributions to seed mRNA; the antisense gene utilised here would not be expected to interfere with the expression of these related genes, based on sequence homology. A total abolition of seed TIA may not be desirable, based on proposed roles for these proteins in desiccating tissue sug-gested by this and previous work [6]. The latter authors demonstrated that TI proteins have en-zyme-preserving properties that could alleviate ef-fects of desiccation and so induction of an
antisense TI gene in roots under drought-stress conditions may prevent alleviation of stress responses.
The work described here contributes to our understanding of promoters from genes that are active both during late embryogenesis and under stress conditions. The lines containing marker genes could be used in further experiments to examine responses to other stress conditions, in-cluding wounding. The TI1 promoter will form part of a resource for grain legume transforma-tion, in particular, where temporal regulation of seed-expressed transgenes is required either for seed quality improvement or for expression of valuable products. The utility of the TI1 promoter may be limited by its response to drought condi-tions but it is possible that this response could be abolished by mutation of elements discussed above. This work provides the second example of the re-introduction of a homologous promoter into pea; earlier work [34] utilised a pea vicilin gene promoter to direct expression of a foreign albumin gene in pea seeds. To date, improvements in pea seed protein quality have not been reported using biotechnology, although work on lupin [37] suggests that this should be possible. Future work on down-regulation of the major seed TI could proceed via a direct approach, exploiting different antisense constructs, or via an indirect approach, where expression of transgenes whose products are rich in sulphur-containing amino acids can result in down-regulation of native proteins of similar composition [38]. Either approach will rely heavily on improvements to transformation systems for grain legumes (outlined above) or on the primary use of model legume systems, where greater num-bers of transformants may be forthcoming, per-mitting the efficacy of multiple gene constructs to be determined quickly.
Acknowledgements
References
[1] J.A. Charity, M.A. Anderson, D.J. Bittisnich, M. White-cross, T.J.V. Higgins, Transgenic tobacco and peas ex-pressing a proteinase inhibitor fromNicotiana alatahave increased insect resistance, Mol. Breeding 5 (1999) 357 – 365.
[2] X. Duan, X. Li, Q. Xue, M. Abo-El-Saad, D. Xu, R. Wu, Transgenic rice plants harboring an introduced potato proteinase inhibitor II gene are insect resistant, Nat. Biotech. 14 (1996) 494 – 498.
[3] J. Graham, S.C. Gordon, R.J. McNicol, The effect of the CpTi gene in strawberry against attack by vine weevil (Otiorhynchus sulcatus F. Coleoptera: Curculion-idae), Ann. Appl. Biol. 131 (1997) 133 – 139.
[4] V. Pautot, F.M. Holzer, L.L. Walling, Differential ex-pression of tomato proteinase inhibitor I and II genes during bacterial pathogen invasion and wounding, Mol. Plant Microbe Int. 4 (1991) 284 – 292.
[5] J. Villanueva, F. Canals, S. Prat, D. Ludevid, E. Querol, F.X. Avile´s, Characterization of the wound-induced metallocarboxypeptidase inhibitor from potato — cDNA sequence, induction of gene expression, subcellu-lar immunolocalization and potential roles of the C-ter-minal propeptide, FEBS Lett. 440 (1998) 175 – 182. [6] J.-M. Lam, K.-H. Pwee, W.Q. Sun, Y.-L. Chua, X.-J.
Wang, Enzyme-stabilizing activity of seed trypsin in-hibitors during desiccation, Plant Sci. 142 (1999) 209 – 218.
[7] C. Domoney, T. Welham, C. Sidebottom, Purification and characterization ofPisumseed trypsin inhibitors, J. Exp. Bot. 44 (1993) 701 – 709.
[8] C. Domoney, T. Welham, C. Sidebottom, J.L. Firmin, Multiple isoforms ofPisumtrypsin inhibitors result from modification of two primary gene products, FEBS Lett. 360 (1995) 15 – 20.
[9] C. Domoney, T. Welham, N. Ellis, R. Hellens, Inheri-tance of qualitative and quantitative trypsin inhibitor variants inPisum, Theor. Appl. Genet. 89 (1994) 387 – 391.
[10] T. Welham, M. O’Neill, S. Johnson, T.L. Wang, C. Domoney, Expression patterns of genes encoding seed trypsin inhibitors inPisum sati6um, Plant Sci. 131 (1998)
13 – 24.
[11] C. Forster, M. Knox, C. Domoney, R. Casey, 1ox1:Ps:2, aPisum sati6um seed lipoxygenase gene, Plant Physiol.
106 (1994) 1227 – 1228.
[12] F. Guerineau, A. Lucy, P. Mullineaux, Effect of two consensus sequences preceding the translation initiator codon on gene expression in plant protoplasts, Plant Mol. Biol. 18 (1992) 815 – 818.
[13] M. De Block, J. Botterman, M. Vandewiele, J. Dockx, C. Thoen, V. Gossele´, N. Rao Movva, C. Thompson, M. Van Montague, J. Leemans, Engineering herbicide resistance in plants by expression of a detoxifying en-zyme, EMBO J. 6 (1987) 2513 – 2518.
[14] C. Domoney, T. Welham, Genetic manipulation of en-zyme inhibitors in pea seeds, in: A.J.M. Jansman, G.D. Hill, J. Huisman, A.F.B. van der Poel (Eds.), Recent Advances of Research in Antinutritional Factors in
Legume Seeds and Rapeseed, Wageningen Pers, Wa-geningen, 1998, pp. 369 – 373.
[15] A. Schneider, S.A. Walker, S. Poyser, M. Sagan, T.H.N. Ellis, J.A. Downie, Genetic mapping and functional analysis of a nodulation – defective mutant (sym19) of pea (Pisum sati6um L.), Mol. Gen. Genet. 262 (1999) 1 – 11.
[16] E.E. Hood, S.B. Gelvin, L.S. Melchers, A. Hoekema, NewAgrobacteriumhelper plasmids for gene transfer to plants, Transgen. Res. 2 (1993) 208 – 218.
[17] S.J. Bean, P.S. Gooding, P.M. Mullineaux, D.R. Davies, A simple system for pea transformation, Plant Cell Rep. 16 (1997) 513 – 519.
[18] T.H.N. Ellis, Approaches to the genetic mapping of pea, in: H.-F. Linskens, J.F. Jackson (Eds.), Modern Meth-ods of Plant Analysis, Vegetables and Vegetable Prod-ucts, vol. 16, Springer, Berlin, 1994, pp. 117 – 160. [19] C. Domoney, R. Casey, Measurement of gene number
for seed storage proteins inPisum, Nucleic Acids Res. 13 (1985) 687 – 699.
[20] C. Domoney, R. Casey, Changes in legumin messenger RNAs throughout seed development inPisum sati6umL,
Planta 170 (1987) 562 – 566.
[21] R. Casey, C. Domoney, N.C. Nielsen, Isolation of a cDNA clone for pea (Pisum sati6um) seed lipoxygenase,
Biochem. J. 232 (1985) 79 – 85.
[22] C. Domoney, T. Welham, Trypsin inhibitors in Pisum: variation in amount and pattern of accumulation in developing seed, Seed Sci. Res. 2 (1992) 147 – 154. [23] F. Ishige, M. Takaichi, R. Foster, N.-H. Chua, K. Oeda,
A G-box motif (GCCACGTGCC) tetramer confers high-level constitutive expression in dicot and monocot plants, Plant J. 18 (1999) 443 – 448.
[24] Q. Shen, P. Zhang, T.-H.D. Ho, Modular nature of abscisic acid (ABA) response complexes: composite pro-moter units that are necessary and sufficient for ABA induction of gene expression in barley, The Plant Cell 8 (1996) 1107 – 1119.
[25] A.C. Cuming, LEA proteins, in: P.R. Shewry, R. Casey (Eds.), Seed Proteins, Kluwer, The Netherlands, 1999, pp. 753 – 780.
[26] A.L. Jones, I.E. Johansen, S.J. Bean, I. Bach, A.J. Maule, Specificity of resistance to pea seed-borne mosaic potyvirus in transgenic peas expressing the viral replicase (NIb) gene, J. Gen. Virol. 79 (1998) 3129 – 3137. [27] M. Cannon, J. Platz, M. O’Leary, C. Sookdeo, F.
Cannon, Organ-specific modulation of gene expression in transgenic plants using antisense RNA, Plant Mol. Biol. 15 (1990) 39 – 47.
[28] X. Good, J.A. Kellogg, W. Wagoner, D. Langhoff, W. Matsumura, R.K. Bestwick, Reduced ethylene synthesis by transgenic tomatoes expressingS-adenosylmethionine hydrolase, Plant Mol. Biol. 26 (1994) 781 – 790. [29] T.C. Hall, G. Li, M.B. Chandrasekharan, Participation
of chromatin in the regulation of phaseolin gene expres-sion, J. Plant Physiol. 152 (1998) 614 – 620.
[31] Q. Shen, T.-H.D. Ho, Functional dissection of an abscisic acid (ABA)-inducible gene reveals two independent ABA-responsive complexes each containing a G-box and a novelcis-acting element, The Plant Cell 7 (1995) 295 – 307. [32] H. Knight, E.L. Veale, G.J. Warren, M.R. Knight, The
sfr6 mutation in Arabidopsis suppresses low-temperature induction of genes dependent on the CRT/DRE sequence motif, The Plant Cell 11 (1999) 875 – 886.
[33] F. Muel, B. Carrouee, F. Grosjean, Trypsin inhibitor activity of pea cultivars: new data and a proposal strategy for breeding programmes, in: Association Europe´enne de recherche sur les Prote´agineux (AEP) (Ed.), Proceedings of the Third European Conference on Grain Legumes, Valladolid, Spain, 1998, pp. 164 – 165.
[34] H. Schroeder, S. Gollasch, L. Tabe, A. Moore, D. Spencer, T. Higgins, The expression and stability of transgenes in peas (Pisum sati6um L.), in: Association
Europe´enne de recherche sur les Prote´agineux (AEP)
(Ed.), Proceedings of the Second European Conference on Grain Legumes, Copenhagen, 1995, pp. 422 – 423. [35] C. Domoney, T. Welham, Limited proteolysis of enzyme
inhibitor proteins during seed desiccation in Pisum, J. Plant Physiol. 152 (1998) 692 – 695.
[36] C. Domoney, T. Welham, P. Mozzanega, Three classes of proteinase inhibitor gene have distinct but overlapping patterns of expressions inPisum sati6umplants, in
prepa-ration.
[37] L. Molvig, L.M. Tabe, B.O. Eggum, A.E. Moore, S. Craig, D. Spencer, T.J.V. Higgins, Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius L.) expressing a sunflower seed albumin gene, Proc. Natl. Acad. Sci. USA 94 (1997) 8393 – 8398.
[38] L. Tabe, T.J.V. Higgins, Engineering plant protein com-position for improved nutrition, Trends Plant Sci. 3 (1998) 282 – 286.