Plasma total homocysteine levels in postmenopausal women with
unstable coronary artery disease
Niels Erik Nielsen
a,*, Lars Brattstro¨m
b, Bjo¨rn Hultberg
c, Finn Landgren
b,
Eva Swahn
aaDepartment of Cardiology,Heart Center,Uni6ersity Hospital,581 85 Linko¨ping, Sweden bDepartment of Medicine,County Hospital,Kalmar, Sweden
cDepartment of Clinical Chemistry,Uni6ersity Hospital,Lund, Sweden
Received 12 April 1999; received in revised form 13 September 1999; accepted 29 September 1999
Abstract
An elevated plasma total homocysteine (tHcy) level is considered a risk factor for coronary artery disease (CAD), but the relationship between plasma tHcy and well-defined CAD in women is still unclear. Plasma tHcy concentrations and the covariates serum folate, vitamin B12, and creatinine were analysed in 157 angiographically examined postmenopausal women with unstable CAD and in 101 healthy controls. At coronary angiography, 16% had normal vessels and 84% had coronary atherosclerosis. Mean plasma tHcy concentration (mmol/l, 95% confidence interval) did not differ in patients compared to controls (13.1 (12.3 – 13.8) vs. 12.5 (11.6 – 13.5)) or in patients with or without coronary atherosclerosis (13.3 (12.4 – 14.1) vs. 12.0 (10.8 – 13.2)). A trend to an increasing plasma tHcy with increasing degree of coronary atherosclerosis was attenuated after adjustment for age and the previous mentioned covariates. Odds ratio for the risk of coronary artery disease and coronary atherosclerosis in hyperhomocysteinemic patients (]90th percentile in controls) was approximately 3. However, the confidence interval included unity in half of the groups and the significance was therefore difficult to judge. Receiver operating characteristics showed age to be the only variable with a significant discriminatory ability regarding the presence of coronary atherosclerosis (area 0.77). Mild hyperhomocysteinemia seems not to be related to the risk of unstable CAD in postmenopausal women. The trend towards higher plasma tHcy with increasing degree of coronary atherosclerosis may be a marker of the disease. In future studies adjustment for age and the other three covariates should be considered. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Homocysteine; Women; Unstable coronary artery disease; Coronary angiography; Odds ratio; Covariates; ROC curve
www.elsevier.com/locate/atherosclerosis
1. Introduction
In homocysteinurias, severely increased plasma total homocysteine concentration (tHcy) causes vascular in-jury, arteriosclerosis and venous thrombosis [1]. Nu-merous studies, both retrospective and prospective, have shown that mildly elevated plasma tHcy concen-tration is associated with increased risk of myocardial infarction and coronary atherosclerosis [2 – 16]. It also seems to be a strong predictor of mortality in patients with coronary artery disease (CAD) [17,18]. The
associ-ation between modestly increased plasma tHcy concen-tration and other atherosclerotic and thromboembolic cardiovascular disease is also well documented [19 – 21]. However, there are also negative reports [22 – 26] and it is still unclear whether mild hyperhomocysteinemia is causally linked to the development of cardiovascular disease. In most of the above mentioned studies both men and women participated, but women were often in a minority. Furthermore, coronary angiography was often not performed, adding uncertainty to the diagno-sis of CAD, considering that 20 – 40% of women with chest pain of typical angina character do not have signs of coronary atherosclerosis at coronary angiography [27]. Only a few of the studies regarding the association of plasma tHcy and CAD in women involve patients, who were catheterized [7,11,14,16,17,25,26].
* Corresponding author. Tel.: +46-13-222000; fax: + 46-13-138731.
E-mail address:[email protected] (N.E. Nielsen)
Receiver operating characteristics (ROC) provide a simple, direct, yet comprehensive representation of the clinical or diagnostic accuracy of a test, i.e. its funda-mental ability to discriminate between two alternative states of health or conditions. The shape and position of the plot is a quantitative graphic picture of accuracy and the area under the curve quantitates accuracy and provides a measure of the discriminatory performance useful for the comparison of the accuracies among two or more tests [28]. ROC have been used, for instance, when analyzing lipids [29 – 33] and in tests for periph-eral artery disease [34], but to our knowledge none of the previous studies on homocysteine have used ROC for the analysis of the results.
We measured concentrations of plasma tHcy and its important determinants, serum folate, serum vitamin B12 and serum creatinine in postmenopausal women with unstable CAD and in age-matched female con-trols. All the patients underwent coronary angiography. Mean plasma concentrations were compared, the asso-ciation to unstable CAD of plasma tHcy was evaluated in a logistic regression model and the discriminatory ability of the different variables regarding the presence of coronary atherosclerosis was tested using ROC. The primary objective of the study was to see whether patients with or without angiographic coronary atherosclerosis differ with regard to plasma tHcy con-centration. A secondary objective of the study was the confirmation of previous studies, i.e. that plasma tHcy concentration is higher in patients with CAD than in control subjects.
The study was performed in postmenopausal women because the incidence of CAD in premenopausal women is very low and also to avoid the possible variations in menstrual cycle.
2. Subjects and methods
2.1. Patients
During a 2 year period all postmenopausal women (]12 months since last menstruation) with a history of unstable CAD entering the coronary care unit were requested to participate in the study if they fullfilled the diagnostic criteria. These were new or increasing angina during the previous 2 months or ongoing chest pain suggesting ischemia, in conjunction with transient or persistent ST-depression and/or T-wave inversion of at least 0.1 mV in at least two adjacent leads. Most of the participants were part of the multicenter Fragmin dur-ing Instability in Coronary Artery Disease (FRISC) study [35].
Exclusion criteria were left bundle branch block, left ventricular hypertrophy or pacemaker rhythm in rest-ing ECG, suspected myocarditis or endocarditis,
car-diomyopathy, hemodynamically important aortic valve disease, planned percutaneous transluminal coronary angioplasty, planned or previously performed coronary artery bypass grafting, systolic blood pressure B90 mm Hg, fever and diseases with a poor prognosis e.g. malignant disease.
Patients who were stabilised on conventional medical therapy and had no signs of severe ischemia on a predischarge exercise test were rescheduled for coronary angiograhpy within 2 months after discharge. Other-wise this was done in the acute phase.
An age-matched control group randomly selected from the population register was also examined. They had to be anamnestically and clinically healthy with normal routine laboratory tests, normal ECG and to be taking no medications. We did, however, consider it unethical to perform a coronary angiography on these healthy subjects.
2.2. Blood samples
Blood samples for the measurement of myocardial enzymes according to local routines (troponine-T, cre-atine kinase MB, crecre-atine kinase B or crecre-atine kinase) were obtained on admission and every 6th hour until the maximum level was passed with a minimum of three samples.
Blood samples for the analysis of plasma tHcy (mmol/l), serum folate (nmol/l), vitamin B12 (pmol/l) and serum creatinine (mmol/l) were obtained within 24 h after admission in the fasting state and after 15 min recumbency. For amino acid measurements venous blood was collected in evacuated tubes containing EDTA, immediately placed on ice, centrifuged within 15 min at 3000×g for 5 min at −4°C, and the supernatants were stored frozen at −70°C until analy-sis. Plasma tHcy was measured with the method of Andersson [36]. Blood samples for analysis of serum folate, serum vitamin B12, and serum creatinine were sent to the hospital biochemical laboratory for routine analysis.
2.3. Coronary angiography
2.4. Statistics
Results are given as means with 95% confidence intervals unless otherwise indicated. Normally dis-tributed data were tested using unpaired Student’s t -test. For non-parametric data we used Mann – Whitney U test. As the distribution of plasma tHcy was skewed to the right we present logarithmic transformed values as well. Multiple comparison was tested using the Bon-ferroni test. Correlation was tested by using a bivariate Spearman’s nonparametric rank correlation test. Sig-nificance of trend was evaluated using linear regression. The association of plasma tHcy and unstable CAD was evaluated in a logistic regression model by calculat-ing odds ratios and 95% confidence intervals for the whole patient group versus the control group, for pa-tients with coronary atherosclerosis versus the control group and for patients with significant coronary steno-sis versus the control group using quintiles for controls as reference. Furthermore, odds ratios were estimated with plasma tHcy as a continuous variable for all three groups. In the logistic regression model we first ad-justed for the classical risk factors: Age (continuous), current smoking (categorical), hyperlipidemia (categori-cal), hypertension (categori(categori-cal), diabetes mellitus (cate-gorical), and body-mass index (continuous). In a second model we added the serum values (continuous) of folate, vitamin B12 and creatinine.
ROC analyses and plotting were performed with ab version of the program (Rockit 0.9.1B). The ROC plot gives the full spectrum of sensitivity/specificity pairs, corresponding to all of the possible decision levels, obtainable for a test in a particular clinical application. The area under the ROC curve varies from 1.0, which corresponds to perfect discrimination (upper left cor-ner) to 0.5 where no discrimination exists. Swets [37] has suggested the following guidelines for interpreting the area: 0.5 – 0.7, rather low accuracy; 0.7 – 0.9, accu-racy useful for some purposes; and greater than 0.9, rather high accuracy. For tHcy and its covariates we calculated percentiles for all the participants in the study and for the patient group, respectively. We used the former percentiles to calculate sensitivity/specificity pairs for the patient group versus the control group regarding unstable CAD or not. Likewise, the latter percentiles were used to calculate sensitivity/specificity pairs for patients with normal coronary arteries versus patients with coronary stenosis regarding significant coronary stenosis or not. A two-sided z-test was used to compare the areas under the ROC curves and areas are given with 95% confidence intervals.
Statistical significance was set at PB0.05. All statis-tical analyses were performed by a computer using the SPSS system (Statistical Package for the Social Sciences INC., Chicago, USA, 1990).
The study was approved by the local scientific ethics committee. All procedures followed the Helsinki Declaration.
3. Results
3.1. Characteristics of study population
One hundred and fifty seven patients and 101 healthy controls were included in the study. The results at coronary angiography and baseline characteristics are given in Table 1. Women with angina-like chest pain and normal coronary vessels were significantly younger than the rest of the study population. Serum myocar-dial enzyme level above reference value was found in 43%, and more frequently in patients with significant stenosis than in patients with normal vessels (50 vs. 24%, PB0.05). No patient developed heart failure. At the time of blood sampling 38% of the patients were taking betablockers, 23% calcium antagonists, 22% long-acting nitroglycerin, 20% aspirin, 17% diuretics, 6% ACE-inhibitors, and 5% digoxin. Furthermore, 37% received low-molecular-weight heparin and 5% intra-venous nitroglycerin. Sixteen per cent in the patient group and 14% in the control group were taking hor-mone replacement therapy.
All controls performed an exercise test, which was performed as previously described [38]. Seventeen of the controls had ST-depression ]0.1 mV during exercise. Apart from a lower mean body-mass-index (23.1 (21.7 – 24.5) vs. 25.1 (24.2 – 26.0), PB0.05), these women did not differ from the rest of the control group.
3.2. Plasma tHcy and co6ariates in patients and control subjects
covariates and age, there was still no difference in plasma tHcy concentration between patients and con-trol subjects. A comparison of the logarithmic trans-formed values did not show any significant differences. There was no difference in mean plasma tHcy in control subjects with or without
ST-depres-sion at exercise test (11.9 (10.5 – 13.3) vs. 12.3 (11.2 – 13.5))
Apart from age, mean plasma tHcy concentration showed no correlation to the variables in Table 1, or to raised coronary enzyme level on admission, or to current medication.
Table 1
Baseline characteristics in 157 women with unstable CAD and in 101 healthy control persons (mean (95% CI) unless otherwise indicated)a
Coronary stenosis
Normal vessels Coronary stenosis
All patients Coronary athero
Controls
]50% (n=109) (E)
B50% (n=23) (D) (n=132) (F) (n=25) (C)
(n=101) (A) (n=157) (B)
67.1 (66.0;68.2) Age (years) 66.0 (64.6;67.4) 66.1 (65.0;67.1) 60.5 67.0 (64.0;69.9) 67.2 (66.0;68.3)
(57.7;63.3)**
21
7* 19 12 22 20
Smokers (%)
26.8 (26.1;27.6) 24.7 (24.0;25.4)* 26.6 (26.0;27.3)
Body-mass-index 25.6 (24.3;26.8) 26.0 (24.7;27.3) 27.0 (26.2;27.9) (kg/m2)
0.87 (0.85;0.89) 0.86 (0.82;0.89) 0.87 (0.84;0.89)
0.82 (0.79;0.85) Waist-hip-ratio 0.81 (0.80;0.82)* 0.86 (0.84;0.87)
20 11
7 9 9
Oophorectomy 10
(%)
Diabets - 12 0*** 9 15 14
mellitus (%)
4 2
Previous heart - 3 0 13****
failure (%)
37 39
30 32
36
-Hypertension (%)
-Hyperlipidemia 6 0 9 6 7
(%)
7 7
6 0
PAD/CVI (%) - 4
20
Previous MI - 17 4*** 9 22
(%)
aNormal vessels=Angina with normal coronary vessels. Coronary athero=Patients with non-significant and significant coronary stenosis
pooled together. Hyperlipidemia=pharmacologically treated hyperlipidemia. PAD/CVI=peripheral arterial disease and/or cerebro-vascular incidence. MI=myocardial infarction.PB0.05:
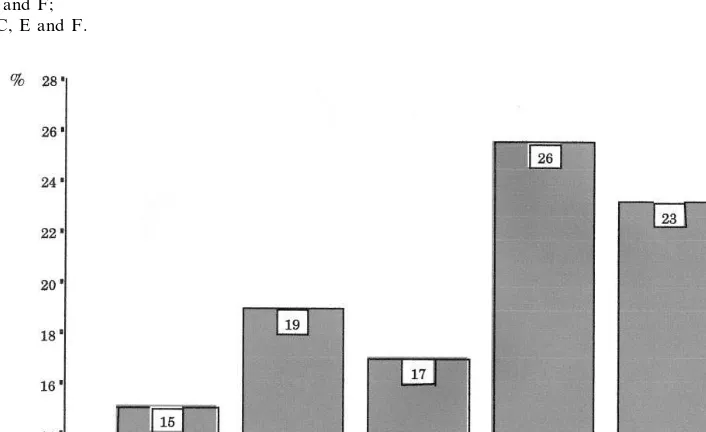
*=A versus B, E and F; **=C versus A, D, E and F; ***=C versus E and F; ****=D versus C, E and F.
Table 2
Plasma tHcy, serum folate, serum vitamin B12 and serum creatinine in patients and healthy control subjects (mean (95% CI))a
Controls All patients Normal vessels Coronary stenosis Coronary stenosis Coronary athero (n=157) (B) (n=25) (C)
(n=101) (A) B50% (n=23) (D) ]50% (n=109) (E) (n=132) (F)
12.5 (11.6;13.5) 13.1 (12.3;13.8) 12.0 (10.8;13.2) 12.7 (11.5;14.0)
Homocysteine 13.4 (12.4;14.4) 13.3 (12.4;14.1)
(mmol/l)
12.8 (12.0;13.6) 12.8 (12.1;13.5) 12.3 (10.6;14.0)
Homocysteine 12.8 (11.1;14.5) 13.0 (12.2;13.8) 13.0 (12.3;13.7)
adj.
2.48 (2.42;2.54) 2.53 (2.48;2.57) 2.46 (2.36;2.55)
Log-transformed 2.52 (2.42;2.62) 2.54 (2.49;2.60) 2.54 (2.49;2.59)
tHcy
2.50 (2.45;2.55) 2.51 (2.47;2.55) 2.49 (2.38;2.59)
Log-transformed 2.55 (2.44;2.65) 2.53 (2.48;2.58) 2.53 (2.49;2.58)
tHcy adj.
Folate (nmol/l) 17.7 (16.0;19.5) 16.2 (14.7;17.7) 15.4 (11.1;19.6) 18.7 (13.8;23.6) 15.9 (14.2;17.6 16.4 (14.8;18.0) 313 (287;340) 297 (270;323) 318 (239;397) 284 (229;340)
Vitamin B12 295 (262;327) 293 (264;321)
(pmol/l)
79 (77;81)* 83 (80;85) 84 (79;89)
Creatinine 80 (77;87) 83 (80;86) 82 (79;85)
(mmol/l)
aNormal vessels=angina with normal coronary vessels. Coronary athero=patients with non-significant and significant coronary stenosis
pooled together. Adj.=values adjusted for the four main covariates (serum folate, serum vitamin B12, serum creatinine and age). Log-tranformed tHcy=logarithmic transformed values for plasma tHcy. There were no significant differences between the different patient groups regarding plasma tHcy.PB0.05:
*=A versus E.
Table 3
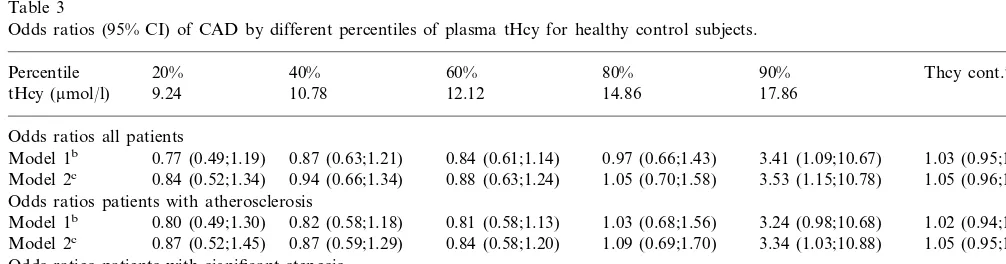
Odds ratios (95% CI) of CAD by different percentiles of plasma tHcy for healthy control subjects.
40% 60% 80% 90% Thcy cont.a
Percentile 20%
10.78 12.12 14.86
9.24 17.86
tHcy (mmol/l)
Odds ratios all patients
0.87 (0.63;1.21) 0.84 (0.61;1.14) 0.97 (0.66;1.43)
0.77 (0.49;1.19) 3.41 (1.09;10.67)
Model 1b 1.03 (0.95;1.11)
0.94 (0.66;1.34) 0.88 (0.63;1.24) 1.05 (0.70;1.58)
Model 2c 0.84 (0.52;1.34) 3.53 (1.15;10.78) 1.05 (0.96;1.15)
Odds ratios patients with atherosclerosis
0.82 (0.58;1.18) 0.81 (0.58;1.13)
Model 1b 0.80 (0.49;1.30) 1.03 (0.68;1.56) 3.24 (0.98;10.68) 1.02 (0.94;1.11)
0.87 (0.52;1.45)
Model 2c 0.87 (0.59;1.29) 0.84 (0.58;1.20) 1.09 (0.69;1.70) 3.34 (1.03;10.88) 1.05 (0.95;1.15)
Odds ratios patients with significant stenosis
0.77 (0.53;1.14) 0.74 (0.51;1.07) 1.00 (0.64;1.55) 2.83 (0.87;9.22)
Model 1b 0.85 (0.51;1.41) 1.01 (0.93;1.10)
0.86 (0.57;1.30) 0.80 (0.55;1.17) 1.11 (0.69;1.78) 2.80 (0.90;8.76) 1.04 (0.94;1.15) 0.99 (0.57;1.71)
Model 2c
atHcy cont.=plasma tHcy as a continuous variable.
bModel 1=adjusted for age, current smoking, hyperlipidemia, hypertension, diabetes mellitus and body-mass index. cModel 2=model 1+adjustment for serum values of folate, vitamin B12 and creatinine.
3.3. Plasma tHcy, coronary atherosclerosis and risk of CAD
Mean plasma tHcy concentration tended to increase with an increasing degree of coronary atherosclerosis, i.e. the number of vessels involved. The increase was, however, not significant when analyzing for significance of trend (P=0.17), and the trend further weakened after adjustments for age and values of folate, vitamin B12 and creatinine in serum.
Results from our logistic regression model, as de-scribed under statistics, are presented in Table 3. We saw a tendency to significance only for the highest (\90%) percentile. Otherwise odds ratios were just around unity for all percentiles, with confidence inter-vals including the null value and no obvious graded effect.
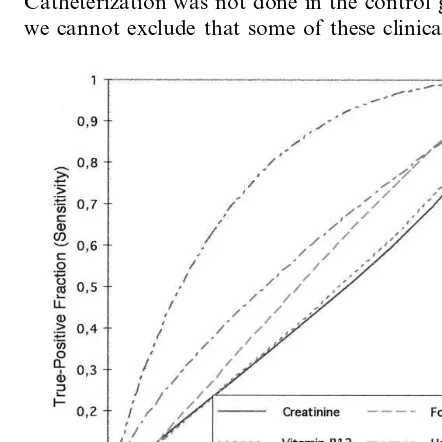
3.4. ROC analyses
Table 4
The areas under the ROC curves (95% C.I.) for diagnosing unstable CAD and significant stenosis, respectively
Patients with significant stenosis Pversus agea
All patients with unstable CAD
0.58 (0.46;0.69)
Homocysteine 0.56 (0.49;0.63) B0.05
Folate 0.43 (0.36;0.50) 0.53 (0.40;0.65) B0.03
0.44 (0.37;0.51)
Vitamin B12 0.46 (0.34;0.58) B0.01
0.45 (0.33;0.56)
0.57 (0.50;0.64) B0.01
Creatinine
Age 0.50 (0.43;0.58)b 0.77 (0.64;0.87)
aStatistics: only for patients with significant stenosis.
bExpected ROC area as patients and controls were age-matched.
4. Discussion
The results of the present study of plasma tHcy in female patients with signs and symptoms of unstable CAD and female age-matched healthy control subjects, do not support the hypothesis that mild hyperhomocys-teinemia is a risk factor for CAD in postmenopausal women. There was no difference in plasma tHcy con-centration between patients and controls, and no sig-nificant difference between patients with or without coronary atherosclerosis. In the highest percentile for plasma tHcy concentration (]90th percentile in con-trols) we saw a tendency to increased risk for unstable CAD and coronary atherosclerosis. ROC analyses showed that none of the evaluated variables were useful for discriminating women with unstable CAD from the control group. Age was by far the best variable regard-ing the ability to discriminate patients with normal coronary vessels from patients with significant coronary stenosis, although it did not have a particularly high discriminatory power.
A question to ask is whether blood sampling during the acute phase of unstable CAD has influenced the present results. Two studies have found lower plasma tHcy concentration in the acute phase of myocardial infarction than at follow-up [39,40]. We do not have follow-up analyses. However, plasma tHcy concentra-tions in those 43% of our patients who showed an increase in their myocardial enzyme level, suggesting possible myocardial damage, were not lower than in those with normal enzyme levels. Therefore, we find it reasonable to assume that the acute phase of unstable CAD has not influenced the present results.
As expected, serum folate and serum vitamin B12 were negatively correlated and age and serum creatinine positively correlated, to plasma tHcy concentration. These four variables are well-known determinants of plasma tHcy [1,41]. A tendency to increasing plasma tHcy concentration with increasing degree of coronary atherosclerosis was attenuated after adjustment for these correlates.
More than 70% of plasma homocysteine is probably metabolized by the kidney [42]. Patients with atherosclerosis are likely to have a higher serum
crea-tinine because of concomitant nephrosclerosis. This, and the well-known age-related decline in renal func-tion, makes it important to adjust the risk-estimate associated with tHcy for the levels of serum creatinine, although this measure is a poor indicator of early renal decline. We also adjusted for serum levels of folate and vitamin B12, which some may not find relevant, argu-ing that the hypothesis of homocysteine involves low values of these two vitamins. However, in more studies there are no differences between patients and controls regarding one or both of these vitamins in spite of differences in plasma tHcy [5,10,14,16,23,40]. Unfortu-nately, in most of the below mentioned studies there is scarce information about the covariates, or the authors have not adjusted for these in spite of differences between the groups studied.
Compared to other studies on this subject our study falls in the middle regarding the size of the population studied. However, it is to our knowledge the largest study of angiographically examined female patients. Catheterization was not done in the control group, and we cannot exclude that some of these clinically healthy
women had subclinical coronary atherosclerosis. Seven-teen of the control women had ST-depression ]0.1 mV without other signs or symptoms of CAD during exercise. We do not know if the ST-depression repre-sents CAD in these healthy women. However, other investigators have shown a low prevalence, 510%, of CAD in totally asymptomatic women with ST-depres-sion in this age group [43].
Previous studies including female patients, who were catheterized, have shown diverging results. Robinson found in 103 women an increasing risk of having CAD with increasing plasma tHcy irrespective of age and gender and with no threshold effect [7], and Nyga˚rd found plasma tHcy to be a strong predictor of mortal-ity in 109 women [17]. The latter study is difficult to interpret, because it includes patients with a marked difference in risk, from patients with stable angina and no previous myocardial infarction to patients with pre-vious coronary bypass-operation and a new myocardial infarction. Furthermore, and rather surprisingly, the authors did not find an effect of coronary revascularisa-tion. Three small studies including up to 27 women showed plasma tHcy to be a risk factor for coronary atherosclerosis [11,14,16], whereas two other small stud-ies, including 36 and 58 catetherized female patients, respectively, did not show homocysteine to be related to CAD [25,26].
In women not catheterized there are positive [5,8,10,12,13,15,19] as well as negative studies [22,24], regarding plasma tHcy and cardiovascular disease. More of these studies include rather young patients [5,8,10,22,24], where one might expect less atheroscle-rotic and more thrombotic disease, and the results therefore may be difficult to interpret.
As confirmed in the present study, serum folate is an important determinant of plasma tHcy concentration [44]. Recently, a common mutation in the gene of methylenetetrahydrofolate reductase was identified [45]. 10% of the caucasian population are mutant ho-mozygotes and have reduced enzyme activity leading to an average of 2.5 mmol/l or 25% higher plasma tHcy concentration than normal homozygotes [46 – 50]. Con-sequently, the mutation was strongly suspected to be a common genetic risk factor for cardiovascular disease, which rapidly led to several studies [48 – 51]. A recently published metaanalysis of 23 of these studies showed that the mutation, although it is a major cause of mild hyperhomocysteinemia, does not increase cardiovascu-lar risk [51]. Furthermore, a study on this mutation in relation to longevity showed no relation to premature death [52].
Our study does not settle the debate on whether or not mild hyperhomocysteinemia is an independent risk factor for cardiovascular disease. However, the strength of our study is our very well-defined and homogeneous patient population, with a high prevalence of CAD
verified at coronary angiography. Our control group was age-matched and was clinically healthy and with a very low probability of CAD. We therefore find it reasonable to expect that we would have detected a difference between these two groups of diseased and healthy individuals if one such had existed.
In conclusion, our study does not support the hy-pothesis that mild hyperhomocysteinemia is a risk fac-tor for unstable CAD in postmenopausal women. We suspect that the trend towards higher plasma tHcy with increasing degree of coronary atherosclerosis may be a marker, but not a cause of the disease, although our study was not designed to differentiate between this. In future studies adjustments should be considered for the four main covariates regarding plasma tHcy, namely age and serum values of folate, vitamin B12 and crea-tinine, as minor variations in these strongly influence the statistics, as shown in this study.
Acknowledgements
Participating investigators: S Ekdahl, I Nyman (Ek-sjo¨); O Svensson, JE Karlsson, K Malmberg (Jo¨nko¨p-ing); F Landgren, B Holmberg, S Ryden (Kalmar); NE Nielsen, E Swahn, KG Sa¨fstro¨m, E Karlsson, A Bjo¨rkholm, G Wiklund, J Malmstedt (Linko¨ping); P Ahlstro¨m, BO Ryden (Motala), J Fride´n (Norrko¨ping); B Hedba¨ck, J Perk (Oskarshamn); O Lind, PA, Jo-hansson (Va¨rnamo); LE Larsson, B Sinnerstad (Va¨ster-vik). The skilful assistance of research nurse Elisabeth Logander and laboratory technician Gunnel Almroth is kindly appreciated. The evaluation of the coronary angiographies by thoracic radiologists Anders Bjo¨rkholm and Gunnar Wiklund is kindly appreciated. We thank Professor John Carstensen for valuable help with statistics. We are grateful to Professor Charles E. Metz, University of Chicago, for the provision of the Rockit program. The work was supported by grants from The Swedish Heart and Lung Foundation, nurse Siv Olsson’s Research Foundation, the Albert Pa˚hlsson Foundation and The Heart Foundation, Linko¨ping University, Sweden.
References
[1] Mudd S, Levy H, Skovby F. Disorders of transulfuration. In: Scriver CRBA, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease, 7th ed. New York: McGraw-Hill, 1995:1279 – 327.
[2] Ueland P, Refsum H, Brattstro¨m L. Plasma homocysteine and cardiovascular disease. In: Francis Jr RB, editor. Atherosclerotic Cardiovascular Disease, Hemostasis, and Endothelial Function. New York, NY: Marcel Dekker Inc, 1992:183 – 236.
[4] Arnesen E, Refsum H, Bonaa KH, Ueland PM, Forde OH, Nordrehaug JE. Serum total homocysteine and coronary heart disease. Int J Epidemiol 1995;24:704 – 9.
[5] Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. J Am Med Assoc 1997;277:1775 – 81.
[6] Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quan-titative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid in-takes. J Am Med Assoc 1995;274:1049 – 57.
[7] Robinson K, Mayer EL, Miller DP, et al. Hyperhomocysteine-mia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation 1995;92:2825 – 30.
[8] Wu LL, Wu J, Hunt SC, et al. Plasma homocyst(e)ine as a risk factor for early familial coronary artery disease. Clin Chem 1994;40:552 – 61.
[9] Mendis S, Athauda SB, Takashi K. Association between hyper-homocysteinemia and ischemic heart disease in Sri Lankans. Int J Cardiol 1997;62:221 – 5.
[10] Schwartz SM, Siscovick DS, Malinow MR, et al. Myocardial infarction in young women in relation to plasma total homocys-teine, folate, and a common variant in the methylenetetrahydro-folate reductase gene. Circulation 1997;96:412 – 7.
[11] Murphy-Chutorian D, Alderman E. The case that hyperhomo-cysteinemia is a risk factor for coronary artery disease. Am J Cardiol 1994;73:705 – 7.
[12] Aronow WS, Ahn C. Association between plasma homocysteine and coronary artery disease in older persons. Am J Cardiol 1997;80:1216 – 8.
[13] Malinow M, Sexton G, Averbuch M, Grossman M, Wilson D, Upson B. Homocyst(e)inemia in daily practice: levels in coronary artery disease. Coron Artery Dis 1990;1:215 – 20.
[14] Verhoef P, Kok FJ, Kruyssen DA, et al. Plasma total homocys-teine, B vitamins, and risk of coronary atherosclerosis. Arte-rioscler Thromb Vasc Biol 1997;17:989 – 95.
[15] Bots ML, Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular dis-ease in the elderly: the Rotterdam study. J Int Med 1997;242:339 – 47.
[16] Dalery K, Lussier-Cacan S, Selhub J, Davignon J, Latour Y, Genest J. Homocysteine and coronary artery disease in French Canadian subjects: relation with vitamins B12, B6, pyridoxal phosphate, and folate. Am J Cardiol 1995;75:1107 – 11. [17] Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M,
Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997;337:230 – 6. [18] Wald NJ, Watt HC, Law MR, Weir DG, McPartlin J, Scott JM.
Homocysteine and ischemic heart disease: results of a prospec-tive study with implications regarding prevention. Arch Intern Med 1998;158:862 – 7.
[19] Selhub J, Jacques PF, Bostom AG, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med 1995;332:286 – 91.
[20] Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet 1995;346:1395 – 8.
[21] den Heijer M, Blom HJ, Gerrits WB, et al. Is hyperhomocys-teinaemia a risk factor for recurrent venous thrombosis. Lancet 1995;345:882 – 5.
[22] Alfthan G, Pekkanen J, Jauhiainen M, et al. Relation of serum homocysteine and lipoprotein(a) concentrations to atheroscle-rotic disease in a prospective Finnish population based study. Atherosclerosis 1994;106:9 – 19.
[23] Verhoef P, Hennekens CH, Allen RH, Stabler SP, Willett WC, Stampfer MJ. Plasma total homocysteine and risk of angina
pectoris with subsequent coronary artery bypass surgery. Am J Cardiol 1997;79:799 – 801.
[24] Evans RW, Shaten BJ, Hempel JD, Cutler JA, Kuller LH. Homocyst(e)ine and risk of cardiovascular disease in the Multi-ple Risk Factor Intervention Trial. Arterioscler Thromb Vasc Biol 1997;17:1947 – 53.
[25] Donner MG, Klein GK, Mathes PB, Schwandt P, Richter WO. Plasma total homocysteine levels in patients with early-onset coronary heart disease and a low cardiovascular risk profile. Metabolism 1998;47:273 – 9.
[26] Folsom A, Nieto J, McGovern P, et al. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B Vitamins. The Atherosclerosis risk in Communities (ARIC) Study. Circula-tion 1998;98:204 – 10.
[27] CASS Study Group. National Heart, Lung and Blood Institute Coronary Artery Surgery Study (CASS), Circulation 1981;63 (Monography 79).
[28] Metz CE. Some practical issues of experimental design and data analysis in radiological ROC studies. Invest Radiol 1989;24:234 – 45.
[29] Grover SA, Palmer CS, Coupal L. Serum lipid screening to identify high-risk individuals for coronary death. The results of the Lipid Research Clinics prevalence cohort. Arch Int Med 1994;154:679 – 84.
[30] Grover SA, Coupal L, Hu XP. Identifying adults at increased risk of coronary disease. How well do the current cholesterol guidelines work. J Am Med Assoc 1995;274:801 – 6.
[31] Avins AL, Browner WS. Improving the prediction of coronary heart disease to aid in the management of high cholesterol levels: what a difference a decade makes. J Am Med Assoc 1998;279:445 – 9.
[32] Zweig MH. Apolipoproteins and lipids in coronary artery dis-ease. Analysis of diagnostic accuracy using receiver operating characteristic plots and areas. Arch Pathol Lab Med 1994;118:141 – 4.
[33] Nielsen NE, Olsson A, Swahn E. Plasma lipoprotein particle concentrations in postmenopausal women with unstable coro-nary artery disease. Analysis of diagnostic accuracy using re-ceiver operating characteristics, J. Int. Med. 1999;in press. [34] Lijmer J, Hunink M, van den Dungen J, Loonstra J, Aj S. ROC
analysis of noninvasive tests for peripheral arterial disease. Ul-trasound Med Biol 1996;22:391 – 8.
[35] Fragmin during Instability in Coronary Artery Disease (FRISC) study group. Low-molecular-weight heparin during instability in coronary artery disease, Lancet 1996;347:561 – 568.
[36] Andersson A, Isaksson A, Brattstrom L, Hultberg B. Homocys-teine and other thiols determined in plasma by HPLC and thiol-specific postcolumn derivatization. Clin Chem 1993;39:1590 – 7.
[37] Swets JA. Measuring the accuracy of diagnostic systems. Science 1988;240:1285 – 93.
[38] Karlsson J, Bjorkholm A, Nylander E, Ohlsson J, Swahn E, Wallentin L. ST-changes in ECG at rest or during exercise indicate a high risk of severe coronary lesions after an episode of unstable coronary artery disease. Int J Cardiol 1993;42:47 – 55. [39] Egerton W, Silberberg J, Crooks R, Ray C, Xie L, Dudman N.
Serial measures of plasma homocyst(e)ine after acute myocardial infarction. Am J Card 1996;77:759 – 61.
[40] Landgren F, Israelsson B, Lindgren A, Hultberg B, Andersson A, Brattstro¨m L. Plasma homocysteine in acute myocardial infarction: homocysteine-lowering effect of folic acid. J Int Med 1995;237:381 – 8.
[41] Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Ann Rev Med 1998;49:31 – 62. [42] Refsum H, Guttormsen A, Fiskerstrand T, Ueland PM.
[43] Task Force of the European Society of Cardiology. Management of stable angina pectoris. Recommendations of the Task Force of the European Society of Cardiology, Eur. Heart. J. 1997;18:394 – 413.
[44] Brattstrom L, Lindgren A, Israelsson B, Andersson A, Hultberg B. Homocysteine and cysteine: determinants of plasma levels in middle-aged and elderly subjects. J Int Med 1994;236:633 – 41. [45] Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk
factor for vascular disease: a common mutation in methylenete-trahydrofolate reductase. Nat Genet 1995;10:111 – 3 Letter. [46] Jacques PF, Bostom AG, Williams RR, et al. Relation between
folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 1996;93:7 – 9.
[47] Harmon DL, Woodside JV, Yarnell JW, et al. The common ‘thermolabile’ variant of methylene tetrahydrofolate reductase is a major determinant of mild hyperhomocysteinaemia. Q J Med 1996;89:571 – 7.
[48] Ma J, Stampfer MJ, Hennekens CH, et al. Methylenetetrahydro-folate reductase polymorphism, plasma Methylenetetrahydro-folate, homocysteine, and risk of myocardial infarction in US physicians. Circulation 1996;94:2410 – 6.
[49] Verhoef P, Kok FJ, Kluijtmans LA, et al. The 677C--\T mutation in the methylenetetrahydrofolate reductase gene: asso-ciations with plasma total homocysteine levels and risk of coro-nary atherosclerotic disease. Atherosclerosis 1997;132:105 – 13. [50] Kluijtmans LA, Kastelein JJ, Lindemans J, et al. Thermolabile
methylenetetrahydrofolate reductase in coronary artery disease. Circulation 1997;96:2573 – 7.
[51] Brattstro¨m L, Wilcken D, O8hrvik J, Brudin L. Common methylenetetrahydrofolate reductase gene mutation leads to hy-perhomocysteinemia but not to vascular disease — the result of a meta analysis. Circulation 1998;98:2520 – 6.
[52] Brattstro¨m L, Zhang Y, Hurtig M, et al. A common methylenetetrahydrofolate reductase gene mutation (C677T/ MTHFR) and longevity. Atherosclerosis 1998;141:315 – 9.