Effect of enhanced ultraviolet-B radiation on pollen
germination and tube growth of 19 taxa in vitro
Huyuan Feng
a, Lizhe An
a,b, Lingling Tan
a, Zongdong Hou
a,
Xunling Wang
a,c,*
aSchool of Life Science,State Key Laboratory of Arid Agrioecology,Lanzhou Uni6ersity,Lanzhou,730000,PR China bState Key Laboratory of Frozen Soil Engineering,Geocryology Lanzhou Institute of Glaciology and Geocryology,CAS.Lanzhou,
730000,P.R.China
cDepartment of Biology,Northwest Uni6ersity,Xi’an,710069,P.R.China
Received 17 February 1999; received in revised form 1 September 1999; accepted 3 September 1999
Abstract
In order to determine the response of pollen to UV-B irradiation and cumulative effects of UV-B exposure time on pollen germination and tube growth, 19 taxa of higher plants were investigated in vitro concerning the exposure of pollen grains to two levels of enhanced ultraviolet-B (UV-BBE) (280 – 320 nm, 350 and 500 mW/m2 biologically effective UV-B radiation) simulating 8 and 21% stratospheric ozone depletion in Lanzhou, China (36.04°N, 1550 m) and to no UV-B (control group). Compared with the control, enhanced UV-B radiation significantly inhibited pollen germination and tube growth in most species. Higher UV-B flux rate caused a greater inhibitory effect than lower UV-B radiation level. Several taxa exhibited insensitivity of pollen germination and tube growth to UV-B and were even stimulated by UV-B. Reduction in pollen germination rates and tube growth increased with longer exposure time and this indicated a cumulative effect of UV-B radiation. It is concluded that changes in pollen susceptibility to UV-B would lead to severe ecological consequences under natural conditions. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Pollen germination; Pollen tube growth; Ultraviolet-B (UV-B) radiation
www.elsevier.com/locate/envexpbot
1. Introduction
Anthropogenic emissions of chloroflorocarbons (CFCs) and nitrogen oxides result in depletion of the stratospheric ozone layer, which protects life
on earth against deleterious short-wave solar radi-ation. As a result, the amount of biologically effective UV-B irradiance (UV-BBE) reaching the earth’s surface increases (Kerr, 1993). Recent mathematical models predict a further increase in solar UV-B radiation (Madronich et al., 1995). Recently an ‘ozone hole’ has been discovered above the Qinghai-Tibet Plateau in China (Zhou et al., 1995).
* Corresponding author.
E-mail address:[email protected] (X. Wang)
Since Johnston (1971) first reported the poten-tial stratospheric ozone reduction over two decades ago, UV-B effects on higher plants have been the subject of considerable research (Cald-well et al., 1995). A wide range of biochemical, physiological, morphological, anatomical and growth responses have been extensively investi-gated, but little is known about UV-B effect on reproductive biology (Demchik and Day, 1996; Van de Staaij et al., 1997; Torabinejad et al., 1998). In some species an effect of elevated UV-B radiation on total plant seed production has been found (Van de Staaij et al., 1997). Although pol-len of open flowers appears to be well shielded from solar UV-B when still within the anther sacs (Flint and Caldwell, 1983), it may be exposed to natural UV-B radiation following dehiscence until successful germination and stigma penetration oc-cur. The exposure time of individual pollen tubes to natural UV-B flux rates depends on the time required for individual pollen tube to grow from the germinated pollen grains up to penetration of stigma. This time in vivo varies considerably among species and pollen grain types, averaging 13 – 57 min (summarized by Torabinejad et al., 1998). Moreover, pollen grain walls can transmit as much as 20% of the UV-B (Stadler and Uber, 1942), therefore, most of the studies concerning UV-B effects on reproductive characteristics of higher plants have been focused on pollen.
Pollen germination and tube elongation in most species or cultivars are inhibited by enhanced UV-B radiation in vitro. For instance, Flint and Caldwell (1984) reported partial inhibition of in vitro pollen germination in Scrophularia pere -grina, Geranium 6iscosissimum, Papa6er rhoeas, andCleome luteaunder elevated UV-B radiation. Musil (1995) reported reduced pollen germination in two out of eight species tested. Decrease of in vitro pollen tube growth under UV-B has been observed for Nicotiana tabacum and Petunia hy -brida(Feder and Shrier, 1990). For four dicotyle-donous Asteraceae and four monocotyledicotyle-donous Iridaceae tested, pollen tube growth of all di-cotyledonous species and one Iridaceae species was reduced (Musil, 1995). In a recent report, Torabinejad et al. (1998) detected differences among 34 species under two levels of UV-B
expo-sure to in vitro pollen grains, and found that the length of pollen tube of more than 50% of the taxa tested was significantly reduced and the pol-len germination of five taxa was significantly in-hibited. In comparison with other physiological and/or ecological effects of enhanced UV-B irra-diance, less than 70 species or cultivars of higher plants have been examined regarding the effect of elevated UV-B on pollen. More studies in this field are obviously necessary to gain a better understanding of the effect of increased UV-B radiation.
Previous research has often been conducted un-der low levels (or in the absence) of visible light. This prohibits the process of photorepair of the UV-B induced damage to the DNA (Pang and Hays, 1991). Therefore a radiation regime in which UV-B radiation was combined with suffi-ciently visible light was used in this experiment.
At present it is not clear whether UV-B damage accumulates over time. Zea mays(Pfahler, 1973), Brassica rapa (Demchik and Day, 1996) and Di -morphotheca sinuata(Musil, 1996) only have been studied. In the present study we subjected mature pollen from 19 plant taxa to different UV-B regimes to determine: (i) the interspecific response of pollen germination and tube elongation in vitro to enhanced UV-B radiation, and (ii) whether there was a cumulative effect of UV-B exposure duration on pollen germination and tube growth. Here we hypothesize that the cumulative effect of UV-B over time exists and that as a consequence, pollen germination and tube elongation under longer exposure to UV-B will be inhibited more seriously than under shorter exposure time.
2. Material and methods
2.1. Plant materials
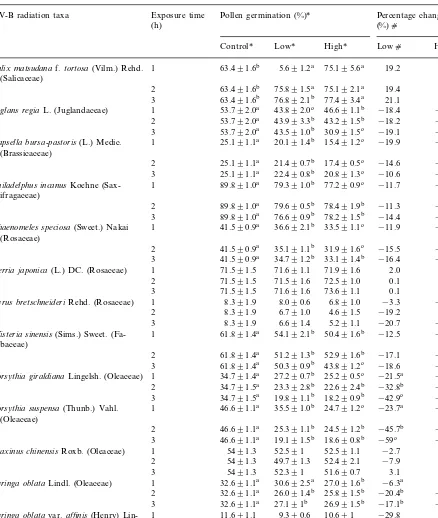
Table 1
Effect of enhanced UV-B radiation on pollen germination and percent change of 19 taxa from Lanzhou (36.04°N, 1550 m), China (means9S.D.)d
UV-B radiation taxa Exposure time Pollen germination (%)* Percentage change (%)c
(h)
Low* High* Lowc Highc
Control*
63.491.6b 5.691.2a 75.195.6a 19.2 18.3 Salix matsudanaf.tortosa(Vilm.) Rehd. 1
(Salicaceae)
75.891.5a 75.192.1a
2 63.491.6b 19.4 18.3
63.491.6b 76.892.1b 77.493.4a 21.1 22.1 3
43.892.0c 46.691.1b
Juglans regiaL. (Juglandaceae) 1 53.792.0a −18.4 −13.2a
43.993.3b 43.291.5b −18.2
53.792.0a −19.6b
2
53.792.0a
3 43.591.0b 30.991.5c −19.1 −42.6c
25.191.1a 20.191.4b
Capsella bursa-pastoris(L.) Medic. 1 15.491.2c −19.9 −38.6
(Brassicaceae)
21.490.7b 17.490.5c
2 25.191.1a −14.6 −30.6
22.490.8b 20.891.3c −10.6 −17.1
3 25.191.1a
89.891.0a 79.391.0b 77.290.9c
1 −11.7
Philadelphus incanusKoehne (Sax- −14.1
ifragaceae)
Chaenomeles speciosa(Sweet.) Nakai 41.590.9a 36.692.1b 33.591.1c −11.9 −19.3 (Rosaceae)
1 71.691.1 71.991.6 2.0 0.6
Kerria japonica(L.) DC. (Rosaceae)
71.591.6 72.591.0 0.1
71.591.5 4.2
2
71.691.6 73.691.1 0.1 3.0
3 71.591.5
8.090.6 6.891.0 −3.3
8.391.9 −18.3
Pyrus bretschneideriRehd. (Rosaceae) 1
8.391.9
2 6.791.0 4.691.5 −19.2 44.5
6.691.4 5.291.1 −20.7 −37.0
3 8.391.9
61.891.4a 54.192.1b 50.491.6b
1 −12.5
Wisteria sinensis(Sims.) Sweet. (Fa- −18.6
baceae)
Forsythia giraldianaLingelsh. (Oleaceae) 1
34.791.5a
2 23.392.8b 22.692.4b −32.8b −44.4b 19.891.1b 18.290.9b −42.9c −49.1b
3 34.791.5a
46.691.1a 35.591.0b 24.791.2c
1 −23.7a
Forsythia suspensa(Thunb.) Vahl. −46.3a
(Oleaceae)
46.691.1a 25.391.1b 24.591.2b −45.7b −47.5a 2
3 46.691.1a 19.191.5b 18.690.8b −59c −60.0b 52.591 52.591.1 −2.7
5491.3 −2.8
1 Fraxinus chinensisRoxb. (Oleaceae)
5491.3
2 49.791.3 52.492.1 −7.9 −2.6
52.391 51.690.7 3.1 −4.3
3 5491.3
30.692.5a 27.091.6b −6.3a
32.691.1a −9.1
1 Syringa oblataLindl. (Oleaceae)
32.691.1a
Syringa oblatavar.affinis(Henry) Lin- −9.1
gelsh. (Oleaceae)
11.691.1 8.991.4 10.190.6 −23.5 −13.1 2
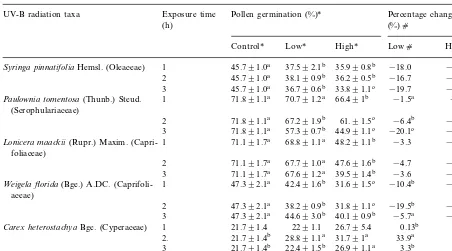
Table 1 (Continued)
Exposure time
UV-B radiation taxa Pollen germination (%)* Percentage change (%)c
(h)
Control* Low* High* Lowc Highc
45.791.0a 37.592.1b
Syringa pinnatifoliaHemsl. (Oleaceae) 1 35.990.8b −18.0 −21.4a 45.791.0a 38.190.9b 36.290.5b
2 −16.7 −20.7a
45.791.0a 36.790.6b 33.891.1c
3 −19.7 −26.2b
Paulownia tomentosa(Thunb.) Steud. 1 71.891.1a 70.791.2a 66.491b −1.5a −7.5a (Scrophulariaceae)
71.891.1a 67.291.9b 61.91.5c
2 −6.4b −15.0b
71.891.1a 57.390.7b 44.991.1c −20.1c
3 −37.5c
1 71.191.7a 68.891.1a 48.291.1b
Lonicera maackii(Rupr.) Maxim. (Capri- −3.3 −32.2a
foliaceae)
2 71.191.7a 67.791.0a 47.691.6b −4.7 −33.8a 71.191.7a 67.691.2a 39.591.4b −3.6 −44.4b 3
1 47.392.1a 42.491.6b
Weigela florida(Bge.) A.DC. (Caprifoli- 31.691.5c −10.4b −33.2b aceae)
47.392.1a 38.290.9b 31.891.1c
2 −19.5b −32.8b
47.392.1a 44.693.0b 40.190.9b −5.7a
3 −15.2a
21.791.4 2291.1 26.795.4
1 0.13b
Carex heterostachyaBge. (Cyperaceae) 23.3
2. 21.791.4b 28.891.1a 31.791a 33.9a 46.5 21.791.4b 22.491.5b 26.991.1a
3 3.3b 24.2
2
F.cirrhosaD. Don (Liliaceae) 16.791.8a 10.591.1b 7.391b −37.1 −56.3
dSignificantly difference atPB0.05 with LSD test. Values with different letter in the same row (the row with * symbol) within a species show the dose effect and in the same column (the column withcsymbol) show the accumulative effects atPB0.05 levels
F. cirrhosa. Plants chosen in this study flowered between the middle of April and the end of May. At that time ambient UV-B approximates 70 – 85% of that occurring with a clear sky at the summer solstice in Lanzhou (Jiang et al., 1998; personal communication from G.L. Ji).
2.2. UV-B radiation treatments and growth chamber conditions
Enhanced UV-B radiation was provided by filtered Qin brand (Baoji Lamp Factory, China) 30-W fluorescence sun lamps in a controlled envi-ronment chamber (Yue et al., 1998). The lamps were suspended above and perpendicular to the Petri dishes and filtered with either 0.13-mm thick cellulose diacetate (transmission down to 290 nm) for UV-B irradiance or 0.13-mm polyester plastic films (absorbs all radiation below 320 nm) as a control. Cellulose diacetate filters were presolar-ized. The desired irradiation was obtained by changing the distance between the lamps and the
Petri plates. The spectral irradiance from the lamps was determined with an Optronics Model 742 (Optronics Laboratories, Orlando, FL, USA) spectroradiometer and weighted with the general-ized plant action spectrum (Caldwell, 1971) nor-malized to 300 nm to obtain the biologically effective UV-B radiation (UV-BBE). The two lev-els of UV-B irradiation were 350 and 500 mW/m2,
which simulated 8 and 20% stratospheric ozone depletion, respectively, with a clear sky at the summer solstice (Lanzhou), using the model of Green et al. (1980), and no UV-B radiation was regarded as the control. In addition to UV-B radiation, visible radiation (photosynthetically ac-tive radiation, PAR 400 – 700 nm) (220 mmol/m2
per s) was also supplied. The air temperature and relative humidity in the growth chamber were maintained at 2392°C and 7595%, respectively.
2.3. Pollen culture
to dehisce. Most species used in this survey typi-cally release pollen in the morning or midday hours. The pollen grains were brushed with a clear camel’s hair brush and cultured at the den-sity of 50 grains mm2 on a solid medium
according to Brewbaker and Kwack (1963) in 9-cm diameter Petri plates, containing 15% su-crose, 1.5% bacto-agar, 0.01% H3BO3, 0.03%
Ca(NO3)2.4H2O, 0.02% MgSO4.7H2O, 0.01%
KNO3 and 0.01% KH2PO4. The seeded plates
were exposed to two levels of UV-B radiation. After 1, 2 or 3 h of exposure, the plates were incubated under white light for 7, 6 or 5 h, respectively. Control group plates were cultured for 8 h only under visible light. Growth of pollen was stopped by adding some drops of killing and preserving solution comprising water, glycerine, formaldehyde and glycerol acetic acid (72:20:5:3, v:v) to each plate. Plates were stored at 4°C until pollen germination and tube growth could be scored and measured. Three to five plates were used for each treatment per experiment, and each experiment was repeated two or three times. At least 1000 pollen grains per treatment were scored for germination and 50 were measured under a light microscope for tube length when their elon-gation was greater than pollen diameter.
2.4. Statistical analysis
Data were statistically analyzed using a one-way analysis of variance (ANOVA) to test the significance of treatment and duration exposure effect of UV-B except for F. cirrhosa because of insufficient samples. Data of germination was transformed using the arcsin transformation be-fore the analysis.
3. Results
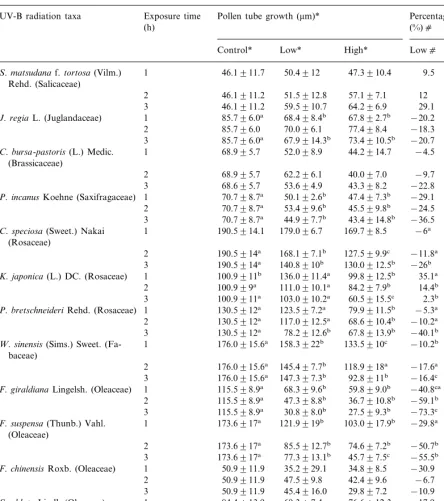
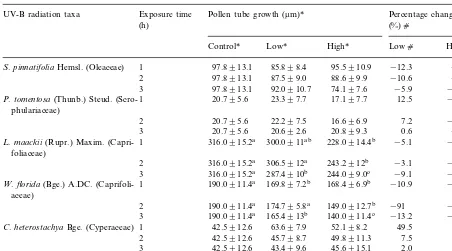
The effects of UV-B irradiation on pollen ger-mination and tube growth in vitro are shown in Tables 1 and 2.
Enhanced UV-B radiation significantly reduced (PB0.05) pollen germination of many taxa (Table 1). The species K. japonica, P. bretschneideri and F. chinensis, S. oblata var.
affinis were insensitive to elevated UV-B (P\ 0.05). Only in K. japonicaan increase in germina-tion percentages under elevated UV-B was found. S. matsudana f. tortuosa and C. heterostachya showed only increased germination rates under the highest UV-B dose. Germination percent change (%) against the control was negative among 16 out of 19 taxa. The highest inhibitory effect on germination was found in F. giraldiana and F.suspensa. Only S.matsudana f. tortosa, C. heterostachya and K. japonica showed a positive percent change (%) against the control (Table 1). Similarly, pollen tube length of many species exhibited considerable sensitivity to UV-B (Table 2). The adverse effect of UV-B irradiance on tube growth was obvious. The most sensitive species were F. giraldiana and F. suspensa where pollen tube elongation decreased by 76.2 and 73.7%, respectively, with exposure of 3 h under high UV-B radiation level (Table 2). A total of seven species had no statistically significant reduction of pollen tube growth; they were S. matsudana f. tortuosa, C. heterostachya, C. bursa-pastoris, F. chinensis,S.pinnatifolia,S.oblatavar.affinis, and P. tomentosa. Pollen tube growth of K. japonica was stimulated significantly under the lower UV-B flux rate (PB0.05) but significantly inhibited un-der the higher UV-B radiation level (PB0.05) (Table 2).
Table 2
Effect of enhanced UV-B radiation on pollen tube growth and percent change of 19 taxa from Lanzhou (36.04°N, 1550 m), China (means9S.D.)d
Exposure time Pollen tube growth (mm)* Percentage change UV-B radiation taxa
1 46.1911.7 47.3910.4 9.5 2.6
S.matsudanaf.tortosa(Vilm.) Rehd. (Salicaceae)
12
2 46.1911.2 51.5912.8 57.197.1 22.0
29.1 39.3
J.regiaL. (Juglandaceae)
70.096.1 77.498.4 −18.3 −9.7
C.bursa-pastoris(L.) Medic. 1 68.995.7 (Brassicaceae)
62.296.1 40.097.0 −9.7 −42.0
2 68.995.7
−22.8 −37.1 43.398.2
3 68.695.7 53.694.9
47.497.3b
1 70.798.7a 50.192.6b −29.1 −32.9
P.incanusKoehne (Saxifragaceae)
53.499.6b 45.599.8b −24.5 −35.6
C.speciosa(Sweet.) Nakai 1 190.5914.1 (Rosaceae)
K.japonica(L.) DC. (Rosaceae)
84.297.9b P.bretschneideriRehd. (Rosaceae) 1 130.5912a
−10.2a −47.4 W.sinensis(Sims.) Sweet. (Fa- 1 176.0915.6a 158.3922b
baceae)
−17.6a −32.6a 145.497.7b 118.9918a
176.0915.6a 2
3 176.0915.6a 147.397.3b 92.8911b −16.4c −47.4b 59.899.0b
1 115.598.9a 68.399.6b −40.8ca −48.2a
F.giraldianaLingelsh. (Oleaceae)
−59.1b −68.2b
F.suspensa(Thunb.) Vahl. (Oleaceae)
−50.7b −57.0b 85.5912.7b 74.697.2b
173.6917a 2
−55.5b −73.7c 3 173.6917a 77.3913.1b 45.797.5c
−30.9 −31.6 34.898.5
1 50.9911.9
F.chinensisRoxb. (Oleaceae) 35.2929.1
42.499.6
2 50.9911.9 47.599.8 −6.7 −16.9
45.4916.0 29.897.2 −10.9 −41.5
3 50.9911.9
69.397.4 76.6912.3 −17.9 −9.2 S.oblataLindl. (Oleaceae) 1 84.4913.9
−9.0 −2.1 82.6911.7
76.898.2 84.4913.9
2
3 84.4913.9 70.6911.5 88.299.3 −16.4 4.5
56.697.3b −28.8 −26.3 54.796.3b
76.898.6a 1
S.oblatavar.affinis(Henry) Lin-gelsh. (Oleaceae)
2 76.898.6 67.499.3 54.6913.0 −12.2 −28.9 41.7910.0b 55911.8b −45.7 −28.4 76.898.6a
Table 2 (Continued)
UV-B radiation taxa Exposure time Pollen tube growth (mm)* Percentage change
(h) (%)c
Control* Low* High* Lowc Highc
S.pinnatifoliaHemsl. (Oleaceae) 1 97.8913.1 85.898.4 95.5910.9 −12.3 −2.4 97.8913.1 87.599.0 88.699.9
2 −10.6 −9.4
97.8913.1 92.0910.7 74.197.6 −5.9
3 −24.2
1 20.795.6 23.397.7
P.tomentosa(Thunb.) Steud. (Scro- 17.197.7 12.5 −17.8
phulariaceae)
20.795.6 22.297.5 16.696.9
2 7.2 −20.1
20.795.6 20.692.6 20.899.3 0.6
3 −3.1
1 316.0915.2a 300.0911ab 228.0914.4b
L.maackii(Rupr.) Maxim. (Capri- −5.1 −27.8
foliaceae)
2 316.0915.2a 306.5912a 243.2912b −3.1 −23.1 316.0915.2a 287.4910b 244.099.0c −9.1 −22.8 3
1 190.0911.4a 169.897.2b
W.florida(Bge.) A.DC. (Caprifoli- 168.496.9b −10.9 −11.6a
aceae)
190.0911.4a 174.795.8a 149.0912.7b
2 −91 −21.7b
190.0911.4a 165.4913b 140.0911.4c −13.2
3 −26.2c
42.5912.6 63.697.9 52.198.2
1 49.5
C.heterostachyaBge. (Cyperaceae) 22.7
42.5912.6
2 45.798.7 49.8911.3 7.5 17.1
42.5912.6 43.499.6 45.6915.1
3 2.0 7.3
F.cirrhosaD. Don (Liliaceae) 2 134.0911.4a 103.0910.3b 107.0919.2b −23.1 −20.1
dSignificantly difference atPB0.05 with LSD test. Values with different letters in the same row (the row with * symbol) within a species show the dose effect and in the same column (the column withcsymbol) show the accumulative effects atPB0.05 levels.
4. Discussion
Effects on pollen germination and tube growth in response to UV-B radiation found in this study have been reported by other authors and seemed to be species specific (Flint and Caldwell, 1984; Feder and Shrier, 1990; Musil and Wand, 1993; Musil, 1995; Demchik and Day, 1996; Torabine-jad et al., 1998).
Potential key targets for UV-B damage are membranes, DNA and other macromolecules (Rozema et al., 1997). These molecules absorb the short-wave solar UV-B radiation. UV-B irradi-ance can damage pollen DNA (Jackson and Linskens, 1979), injure membranes (Predieri et al., 1995) and lead to a differential transmission of alleles at various loci (Pfahler et al., 1981). Chro-mosome aberration and micronucleus effect can be induced as well (Kirby-Smith and Craig, 1957; Wang and Wang, 1999). All these and other un-known factors may contribute to the deleterious effect of UV-B on pollen.
The considerable interspecific difference of pol-len to UV-B may be due to different adaptive or defensive mechanisms to UV-B radiation, such as the capacity of repair system, the efficiency of free radicals scavenging and the content of UV-ab-sorbing compounds in sporoderm. DNA photore-pair has been proven to be effective in other plant tissues under certain visible light radiation (\5% of midday sunlight in midsummer) (Takeuchi et al., 1996). Therefore, concomitant visible light flux radiation (220mmol/m2 per s, PAR, 400 – 700
nm) used in this study should be sufficient to allow these photorepair systems to operate effectively.
60% at the lower exposures were significantly reduced while at the higher exposures, little or no effect was observed. If a nucleus controlled pollen germination and tube elongation, the activity or structure of the nucleus is altered by the lower exposures, but at the higher dose no further changes occur (Pfahler, 1973). The results suggest that simulated 8% ozone depletion at the summer solstice in Lanzhou (China) could cause the inhi-bition of pollen germination and tube growth of most taxa tested.
Longer exposure of pollen to UV-B exhibited a greater inhibitory effect on pollen germination and tube growth in more than 50% of the species investigated in this experiment. This suggests a cumulative effect of UV-B damage on pollen grains. Musil (1996) observed the accumulative effect of elevated UV-B radiation on pollen tube elongation in multiple generations of D. sinuata. Recently, Musil et al. (1999) found that in a fifth generation that was not exposed to UV-B treat-ment, the effects on reproductive performance and growth and allocation of biomass ofD.sinu -atapersisted after four generations of UV-B radi-ation. Pfahler (1973) also reported that pollen growth rate inZ.mays decreased with increasing exposure time; a similar observation was made for B. rapa (Demchik and Day, 1996). This phe-nomenon could amplify the effects of UV-B irra-diance as observed over several growing seasons in long-lived woody plants (Sullivan and Tera-mura, 1992).
Among six species of Oleaceae, two taxa of Forsythia were the most susceptible to UV-B ex-posure whileC.speciosaseemed to be more sensi-tive than other Rosaceae species (Tables 1 and 2). Torabinejad et al. (1998) found a positive correla-tion between sensitivity of pollen elongacorrela-tion to UV-B radiation and time of the year when pollen grains were collected. Genetically based adapta-tion to enhanced UV-B radiaadapta-tion is known from plant populations under a naturally high exposure to UV-B, i.e. high elevation above sea level (Lar-son et al., 1990).
Pollen germination and tube growth in several species was stimulated by enhanced UV-B, how-ever the mechanism remains unclear. Increased pollen tube growth ofIxia6iridiflora(Musil, 1995)
and Pinus syl6estris (Zelles et al., 1977) were reported under elevated UV-B radiation. In some species UV-B stimulates biomass production and other growth parameters (Musil and Wand, 1993).
The effects of increased UV-B irradiance on pollen might have severe ecological consequences. Reduction of germination and inhibition of pollen elongation caused by UV-B stress might decrease the effectiveness of pollination and fertilization, and consequently change the quantity and quality of seed (Demchik and Day, 1996; Van de Staaij et al., 1997). In the long run, the response difference of taxa to UV-B and the accumulative effects elaborated in this study may lead to a change in the competitive balance and species composition in natural communities (Caldwell et al., 1995; Musil et al., 1999).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 39670132, 39970126), State Key Laboratory of Frozen Soil Engineering, Lanzhou Institute of Glaciology and Geocryology, CAS (9808), Key project-B, CAS (KZ952-S1-216) and Fukang desert ecosystem ob-servation and experiment station of Xinjiang, CAS. We also thank Dr Professor W.H.O. Ernst (Editor-in Chief for EEB) and two anonymous referees who provided valuable comments and suggestions on the manuscript.
References
Brewbaker, J.L., Kwack, B.H., 1963. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 50, 859 – 865.
Caldwell, M.M., 1971. Solar UV-B irradiation and the growth and development of higher plant. In: Giese, A.C. (Ed.), Photophysiology, vol. 6. Academic Press, New York, pp. 131 – 137.
Caldwell, M.M., Teramura, A.H., Tevini, M., Bornman, J.F., Bjo¨rn, L., Kulandaivelu, G., 1995. Effects of increased solar ultraviolet radiation on terrestrial plants. Ambio 24, 166 – 173.
Feder, W.A., Shrier, R., 1990. Combination of UV-B and ozone reduces pollen growth more than either stress alone. Environ. Exp. Bot. 30, 451 – 454.
Flint, S.D., Caldwell, M.M., 1983. Influence of floral optical properties on the ultraviolet radiation environment of pol-len. Am. J. Bot. 70, 1416 – 1419.
Flint, S.D., Caldwell, M.M., 1984. Partial inhibition of in vitro pollen germination by simulated solar ultraviolet-B radia-tion. Ecology 65, 792 – 795.
Green, A.E.S., Cross, K.R., Smith, L.S., 1980. Improved analytic characterization of ultraviolet skylight. Pho-tochem. Photobiol. 31, 59 – 65.
Jackson, J.F., Linskens, H.F., 1979. Pollen DNA repair after treatment with the mutagens 4-nitroquinoline-1-oxide, ul-traviolet and near-ulul-traviolet irradiation, and boron depen-dence of repair. Mol. Gen. Genet. 176, 11 – 16.
Jiang, H., Ji, G.L., Shi, S.B., Beng, G.Y., Han, F., 1998. The characteristics of ultraviolet radiation variation over the northern Tibetan plateau. Acta Energ. Sol. Sin. 19 (1), 7 – 12.
Johnston, J., 1971. Reduction of stratospheric ozone by nitro-gen oxide catalysts from supersonic transport exhaust. Science 173, 517 – 522.
Kerr, R.A., 1993. The ozone hole reaches a new low. Science 262, 501.
Kirby-Smith, J.S., Craig, D.L., 1957. The induction of chro-mosome aberrations inTradescantia by ultraviolet radia-tion. Genetics 42, 176 – 187.
Larson, R.A., Garrison, W.J., Carlson, R.W., 1990. Differen-tial responses of alpine and non-alpine Aquilegia species to increased ultraviolet-B radiation. Plant Cell Environ. 13, 983 – 987.
Madronich, S., Mckenzie, R.L., Caldwell, M.M., Bjorn, L.O., 1995. Changes in ultraviolet radiation reaching the earth’s surface. Ambio 24, 143 – 152.
Musil, C.F., 1995. Differential effects of elevated ultraviolet-B radiation on the photochemical and reproductive perfor-mance of dicotyledonous and monocotyledonous arid-envi-ronment ephemerals. Plant Cell Environ. 18, 844 – 854. Musil, C.F., 1996. Accumulated effect of elevated ultraviolet-B
radiation over multiple generation of the arid-environment annualDimorphotheca sinuataDC. (Asteraceae). Plant Cell Environ. 19, 1017 – 1027.
Musil, C.F., Wand, S.J.E., 1993. Responses of sclerophyllous Ericaceae to enhanced levels of ultraviolet-B radiation. Environ. Exp. Bot. 33, 233 – 242.
Musil, C.F., Midgley, G.F., Wand, S.J.E., 1999. Carry-over of enhanced ultraviolet-B exposure effects to successive gener-ations of a desert annual: interaction with atmospheric CO2and nutrient supply. Global Change Biol. 5, 311 – 329.
Pang, Q., Hays, J.B., 1991. UV-B inducible and temperature-sensitive photoreactivation of cyclobutane pyrimidine dimers inArabidopsis thaliana. Plant Physiol. 95, 536 – 543. Pfahler, P.L., 1973. In vitro germination and pollen tube growth of maize (Zea maysL.) pollen grains. VII. Effects of ultraviolet radiation. Radiat. Bot. 13, 13 – 18.
Pfahler, P.L., Linskens, H.F., Mulachy, D.L., 1981. Effect of pollen ultraviolet radiation on the abortion frequency and segregation patters at various endosperm mutant loci in maize (Zea maysL.). Environ. Exp. Bot. 21, 5 – 13. Predieri, S., Norman, H.A., Krizek, D.T., Pillai, P., Mirecki,
R.M., Zimmerman, R.H., 1995. Influence of UV-B radia-tion on membrane lipid composiradia-tion and ethylene of evolu-tion in ‘Doyenne d’Hiver’ pear shoots grown in vitro under different photosynthetic photo fluxes. Environ. Exp. Bot. 35, 152 – 260.
Rozema, J., van de Staaij, J.W.M., Bjo¨rn, L.O., Caldwell, M.M., 1997. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol. Evol. 12, 22 – 28. Stadler, L.J., Uber, F.M., 1942. Genetic effects of ultraviolet
radiation in maize. IV. Comparison of monochromatic radiation. Genetics 27, 84 – 118.
Sullivan, J.H., Teramura, A.H., 1992. The effects of ultravio-let-B radiation on loblolly pine. 2. Growth of field-grown seedlings. Trends Ecol. Evol. 6, 115 – 120.
Takeuchi, Y., Munakami, M., Nakajima, N., Kondo, N., Nikailo, O., 1996. Induction and repair of damage to DNA in cucumber cotyledons irradiated with UV-B. Plant Cell Environ. 37, 181 – 187.
Torabinejad, J., Caldwell, M.M., Flint, S.D., Durham, S., 1998. Susceptibility of pollen to UV-B radiation: an assay of 34 taxa. Am. J. Bot. 85, 360 – 369.
Van de Staaij, J.W.M., Bolink, E., Rozema, J., Ernst, W.H.O., 1997. The impact of elevated UV-B (280 – 320 nm) radia-tion levels on the reproducradia-tion biology of a highland and a lowland population of Silene 6ulgaris. Plant Ecol. 128,
172 – 179.
Wang, S.Y., Wang, X.L., 1999. The Tradescantia-micronu-cleus test on the genotoxicity of UV-B radiation. Mut. Res. 426, 151 – 153.
Yue, M., Li, Y., Wang, X.L., 1998. Effects of enhanced ultraviolet-B radiation on plant nutrients and decomposi-tion of spring wheat under field condidecomposi-tions. Environ. Exp. Bot. 40, 187 – 196.
Zelles, L., Seibold, H.W., Ernst, D.E.W., 1977. Localization of the site of action of tube growth stimulated by micro-UV-irradiation of pine pollen. Radiat. Environ. Biophys. 14, 61 – 82.
Zhou, X.J., Luo, C., Li, W.L., Shi, J.E., 1995. Change of Chinese regional ozone column and its low value center in Qinghai-Tibet Plateau. Chin. Bull. Sci. 40, 1396 – 1398.