www.elsevier.com / locate / livprodsci
Review article
Nutritional influences on the hormonal control of reproduction
in female pigs
*
A. Prunier , H. Quesnel
Station de Recherches Porcines, I.N.R.A., 35590 Saint-Gilles, France
Received 8 October 1998; received in revised form 14 April 1999; accepted 30 April 1999
Abstract
Effects of nutrition on age at puberty, return to oestrus after weaning, ovulation rate and embryo survival have been observed in the pig. Nutrition may influence reproduction at the three levels of the hypothalamic–pituitary–ovarian axis via neuroendocrine pathways and / or variation in metabolic clearance of reproductive hormones. For instance, undernutrition impairs the gonadotrophin-releasing hormone (GnRH) pulse generator while refeeding restores luteinizing hormone (LH) secretion. Nutrition influences follicular growth and maturation; for example, the percentage of small (1–3 mm) healthy follicles and the ovulation rate are decreased under feed restriction. Detection of hormone receptors in tissues and both in vitro and in vivo studies suggest that insulin, cortisol, thyroid hormones and hormones from the somatotrophic axis could be mediators of the effects of nutrition on reproduction. These hormones are able to alter folliculogenesis directly at the ovarian level as either hormones controlling nutrition of the cells, growth factors (insulin and insulin-like growth factor-I) or amplifiers of the action of the gonadotrophins. Insulin and cortisol, at least, may influence gonadotrophin secretion through action at the hypothalamic–pituitary level. Decreased metabolic clearance of progesterone resulting in increased plasma concentrations of this hormone could be involved in the inhibition of gonadotrophin release and reduction of the ovulation rate occurring in feed-restricted cyclic gilts. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Reproduction; Pig; Gonadotrophin; Ovarian follicle; Metabolic status
1. Introduction voluntary feed intake of sows is generally insuffi-cient to meet their nutrient requirements (Noblet et
Peripubertal gilts are often submitted to stressful al., 1990). This risk of nutrient deficit is particularly
conditions (transportation, vaccination, mixing with acute in young sows which have lower appetite than
unfamiliar animals, etc.) which may have marked multiparous sows despite similar milk production
negative effects on their appetite. During lactation, (Dourmad, 1988). The nutrient deficit may increase
in subsequent years for sows of all parities if the current trend for improving prolificacy is maintained. Indeed, both milk production and appetite increase
*Corresponding author. Tel.: 133--9928-5056; fax: 1
33-2-with litter size but the augmentation of feed intake is
9928-5080.
E-mail address: [email protected] (A. Prunier) not sufficient to compensate for nutrient
ments (O’Grady et al., 1985). Inadequate nutritional 2. Main characteristics of the hypothalamic–
intake may influence reproductive performance of pituitary–ovarian axis
female pigs in various ways: it may delay puberty
attainment and return to oestrus after weaning, 2.1. Gonadotrophin secretion
decrease ovulation rate and either reduce (nutritional
inadequacy occurring before ovulation) or improve Synthesis and release of luteinizing hormone (LH)
(nutritional inadequacy occurring after ovulation) by the pituitary cells are tightly controlled by
embryonic survival (for reviews, see Den Hartog and gonadotrophin-releasing hormone (GnRH) of
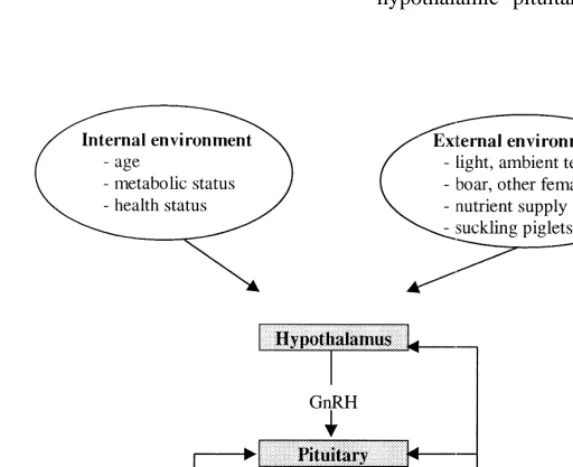
hypo-van Kempen, 1980; Aherne and Kirkwood, 1985; thalamic origin (Fig. 1). LH is secreted in a pulsatile
Kirkwood and Aherne, 1985; Dourmad et al., 1994; manner and each pulse of LH coincides with a pulse
Cosgrove and Foxcroft, 1996; Foxcroft, 1998). of GnRH (for reviews, see Kraeling and Barb, 1990;
These effects of undernutrition on reproductive Foxcroft et al., 1994). Therefore, LH secretion is
efficiency of the female pig may be related to principally under the influence of factors acting on
physiological mechanisms acting at various points the GnRH neurons. These factors include numerous
along the hypothalamic–pituitary–ovarian–uterine neuropeptides (endogenous opioids, serotonin,
cat-axis. They may be mediated by nutrients, hormones echolamines, neuropeptide Y, excitatory amino acids,
or neuropeptides primarily involved in the control of etc.) from intra- and extra-hypothalamic origin which
nutritional function. In the present review, we briefly allow the animal to integrate influences from internal
describe the main characteristics of reproductive origin (age, metabolic and health status, etc.) and the
function before focussing on the endocrine mecha- external environment (light, ambient temperature,
nisms that may explain the effects of nutrition on it. nutrient supply, social and physical environments,
Nutritional effects on embryonic survival, which etc.).
have been reviewed recently (Foxcroft, 1998), will Ovarian steroids exert feedback effects on the
not be discussed. hypothalamic–pituitary axis (Fig. 1). Progesterone
always has an inhibitory influence. Oestrogens (and forming-growth factor) or a negative (follistatin)
more particularly oestradiol-17b) which are synthet- influence. Therefore, FSH is generally high when
ized by the follicles mainly exert negative effects on ovaries are quiescent (during infancy in prepubertal
LH secretion. However, when concentrations are gilts: Camous et al., 1985) and decreases when antral
sufficiently high, oestradiol can act as a positive follicles become more numerous and differentiated
feedback signal to elicit the preovulatory LH surge. (during the waiting phase in prepubertal gilts:
Cam-This level of oestradiol is attained during the follicu- ous et al., 1985; during the mid-follicular phase in
lar phase of the oestrous cycle when follicles are cyclic gilts: Prunier et al., 1987; Flowers et al.,
developed and differentiated enough to secrete high 1991).
amounts of oestrogens (see Section 2.2.).
As the central nervous system and the gonads 2.2. Folliculogenesis
considerably influence the hypothalamic–pituitary
axis, frequency of the LH pulses varies between In prepubertal gilts, as well as in cyclic, pregnant,
physiological stages and between animals (Table 1). lactating and weaned females, groups of primordial
For instance, LH pulsatility is 2-fold higher during follicles are continuously activated and start to
the early follicular phase than during the mid-luteal develop. Most of these growing follicles undergo
phase, increasing concomitantly with the decrease in atresia and only a minority (less than 1%) will
progesterone (Flowers et al., 1991; Kemp et al., ovulate in cyclic females. An activated primordial
1998). Similarly, it jumps 2- to 3-fold shortly after follicle requires several months to reach the
ovulat-weaning (Shaw and Foxcroft, 1985) due to the ory stage (Morbeck et al., 1992). Growth until
removal of the suckling stimuli exerted by the piglets antrum formation (diameter |0.4 mm according to
(for review, see Quesnel and Prunier, 1995). Quesnel et al., 1998a) is very slow and due
principal-Follicle-stimulating hormone (FSH) is also ly to oocyte growth and to multiplication of
sur-synthetized and released by the pituitary, and is also rounding cells. Final growth from 1–4 mm to the
under the positive control of GnRH. However, ovulatory size (6–10 mm) is very rapid and requires
patterns of variation of LH and FSH are very about 4–6 days (Dailey et al., 1976; Morbeck et al.,
different in the female pig (Camous et al., 1985). In 1992). This growth is due to cell proliferation
fact, FSH secretion is primarily controlled by the followed by a rapid increase in volume of the
inhibitory influence of inhibin from follicular origin antrum. It is accompanied by differentiation of
which acts directly at the pituitary level (for review, follicular cells as evidenced by an increased
secre-see Foxcroft et al., 1994). Other ovarian peptides are tion of inhibin and oestradiol, and by appearance of
also probably involved in the regulation of FSH LH receptors on granulosa cells (for review, see
secretion, having either a positive (activin, trans- Foxcroft and Hunter, 1985).
Table 1
a
Comparison of LH pulsatility between physiological stages in female pigs Number of LH pulses / 6 h
Prepubertal gilts Infancy (|1 month of age) Juvenile (|3 months of age)
0.5–1 1.5–2.5
Cyclic gilts Luteal phase Follicular phase
1–2 2.5–5
Sows Lactation 0–12 h after weaning
0.5–1.5 2–6
a
Growth of the follicles up to 2–3 mm does not effect of nutrition on the hypothalamic–pituitary axis
require gonadotrophin support (Driancourt et al., may have consequences on folliculogenesis and
¨
1995; Brussow et al., 1996) but such growth seems ovulation.
to be controlled mainly by local ovarian factors such
as growth factors. Among healthy 1- to 4-mm 3.1. Influence of nutrition on circulating levels of
follicles present at luteolysis or at weaning, 15–25 gonadotrophins
are recruited and selected to undergo preovulatory
growth and to ovulate 4–7 days later whereas the 3.1.1. Influence of feed restriction
others become atretic. In the female pig, there is no The effects of feed restriction on circulating levels
clear evidence showing that the signal for recruit- of LH and / or FSH have been evaluated in numerous
ment and selection of these follicles is an increase in experiments involving premature and cyclic gilts, as
FSH as has been proposed for numerous other well as lactating and weaned sows (Table 2). In
mammalian species. Guthrie and Bolt (1990) and these studies, control females are fed either ad
Hunter et al. (1992) have not associated any vari- libitum (Kirkwood et al., 1987; Mullan et al., 1991;
ation in gonadotrophin secretion with preovulatory Cosgrove et al., 1992; Booth et al., 1994; Zak et al.,
growth and have suggested that, in cyclic gilts, the 1997, 1998) or close to ad libitum (Flowers et al.,
decline in progesterone at luteolysis is the signal for 1989; Baidoo et al., 1992a; Prunier et al., 1993a,b;
recruitment and selection. However, ovulation can be Peltoniemi et al., 1997; Quesnel et al., 1998a,b), or
induced during the luteal phase by exogenous gona- receive 125–150% of the energy requirements for
dotrophins showing that preovulatory growth may maintenance (Armstrong and Britt, 1987; Prunier and
occur despite high levels of progesterone (Caldwell Peintre, unpublished data). In most studies, restricted
et al., 1969). An increase in LH pulsatility around females receive 10–65% of the control feed intake
luteolysis (van de Wiel et al., 1981; Flowers et al., which is below, equal or above energy requirements
1991; Kemp et al., 1998) or immediately after for maintenance (Table 2). In young females
(pre-weaning (for review, see Quesnel and Prunier, pubertal, mature and early pregnant gilts), growth is
1995), without any clear variation in FSH, has been retarded (Flowers et al., 1989; Prunier et al., 1993a;
observed in numerous studies. Moreover, experi- Peltoniemi et al., 1997) and may even be suppressed
ments with gonadotrophin deprivation have shown (Armstrong and Britt, 1987; Cosgrove et al., 1992;
that FSH is necessary to support follicular growth Booth et al., 1994, 1996) by feed restriction. During
beyond 2–3 mm and LH beyond 4 mm (Driancourt lactation, the level of feeding of restricted sows is
¨
et al., 1995; Brussow et al., 1996). Therefore, it can never sufficient to meet energy requirements for
be concluded that recruitment and selection of the maintenance plus milk production and feed intake of
preovulatory follicles can occur only when the control sows is also below requirements in most
equilibrium between stimulatory factors (at least LH studies (Table 2).
and FSH) and inhibitory factors (e.g. progesterone in Detailed analyses of the data show that LH
cyclic females or hormones induced by suckling in pulsatility is inhibited during the period of feed
lactating sows) is displaced in favour of the stimulat- restriction in a majority of experiments regardless of
ory factors. As selected follicles develop, oestradiol the physiological stage (seven out of 11 experiments,
and inhibin secretion increases, inhibiting further LH Table 2). The influence of feed intake on mean LH
and FSH release (see Section 2.1), which probably plasma concentrations is less clear and mean LH is
induces atresia of the smaller follicles of the cohort. significantly reduced during feed restriction in only a
minority of experiments (four out of 14 experiments, Table 2). Such a difference is probably due to
3. Main effects of nutrition on the methodological reasons as plasma LH between
hypothalamic–pituitary axis pulses is very low and often close to the limit of detection of assays. After cessation of feed
restric-Since the ovarian function is under the control of tion, the inhibitory effect of underfeeding on LH
Table 2
a
Influence of feed restriction on the number of LH pulses / 6 h and on mean concentrations of LH and FSH (ng / ml)
c b
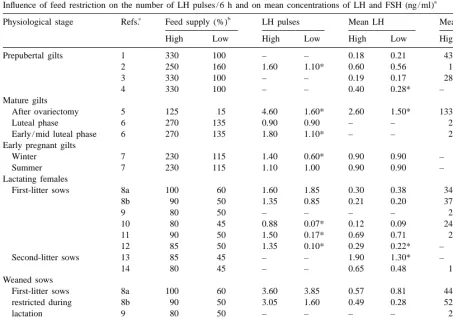
Physiological stage Refs. Feed supply (%) LH pulses Mean LH Mean FSH
High Low High Low High Low High Low
Prepubertal gilts 1 330 100 – – 0.18 0.21 43.6 44.7
2 250 160 1.60 1.10* 0.60 0.56 1.53 2.07
3 330 100 – – 0.19 0.17 28.4 30.0
4 330 100 – – 0.40 0.28* – –
Mature gilts
After ovariectomy 5 125 15 4.60 1.60* 2.60 1.50* 133 144
Luteal phase 6 270 135 0.90 0.90 – – 2.4 2.2
First-litter sows 8a 100 60 1.60 1.85 0.30 0.38 34.1 43.5*
8b 90 50 1.35 0.85 0.21 0.20 37.1 60.4*
First-litter sows 8a 100 60 3.60 3.85 0.57 0.81 44.7 46.0
restricted during 8b 90 50 3.05 1.60 0.49 0.28 52.6 59.1*
lactation 9 80 50 – – – – 2.4 2.4
11 90 50 0.83 0.50 0.86 0.87 2.57 2.77
12 85 50 3.25 1.90* 0.45 0.36* – –
Multiparous sows
restricted after weaning 15 150 50 – – 0.95 0.87 2.64 2.25
a
Note that, in weaned sows, feed restriction occurred before gonadotrophin measurement in most studies in contrast to other physiological stages.
b
Feed supply (%) is the estimated ratio between metabolic energy intake and requirements for maintenance (MAINT in prepubertal, mature and pregnant gilts, and in sows restricted after weaning) or for maintenance1milk production (MAINT1MILK in lactating sows). When this ratio was not indicated in the publication, it was necessary to calculate it. The following equations derived from Noblet et al.
0.60
(1990,1999) were used: metabolic energy intake50.953digestible energy intake; MAINT (MJ)51.03liveweight (kg) for prepubertal
0.75
and mature gilts; MAINT (MJ)50.443liveweight (kg) for pregnant gilts and weaned sows; MAINT1MILK (MJ)50.463liveweight
0.75
(kg) 128.593daily gain of the litter (kg)20.52 for lactating sows.
c
List of references: (1) Cosgrove et al., 1992; (2) Prunier et al., 1993a; (3) Booth et al., 1994; (4) Booth et al., 1996; (5) Armstrong and Britt, 1987; (6) Flowers et al., 1989; (7) Peltoniemi et al., 1997; (8) Mullan et al., 1991 (a: six piglets, b: 12 piglets); (9) Prunier et al., 1993b (10 piglets); (10) Zak et al., 1997 (six piglets); (11) Quesnel et al., 1998a (9–10 piglets); (12) Zak et al., 1998 (8–10 piglets); (13) Kirkwood et al., 1987 (11 piglets); (14) Baidoo et al., 1992a (eight piglets); (15) Prunier and Peintre, unpublished data (8–10 piglets).
*
P,0.05 between high and low groups, within hormonal criteria.
weaned sows (Quesnel et al., 1998a) and in prepub- antisera used in FSH radioimmunoassays differs
ertal gilts (Booth et al., 1996). from one antiserum to the other. However, within a
Mean concentrations of plasma FSH differ greatly given experiment, a single antiserum is used, thus
between experiments ranging from less than 2 ng / ml allowing the comparison between treatment groups.
to more than 50 ng / ml (Table 2). Numerous iso- Mean concentrations of plasma FSH are not clearly
forms of FSH are present in the blood and it is likely modified by feed restriction: no effect or a significant
increase in plasma FSH in restricted sows as shown by Mullan et al. (1991) may reflect a lower secretion of inhibin due to the inhibition of folliculogenesis.
3.1.2. Respective influence of protein and energy The influence of protein restriction on LH secre-tion in females receiving high amounts of energy and, reciprocally, the influence of energy deficit in females receiving adequate amounts of proteins have been investigated in few studies. Some researchers tried to analyse the influence of lysine which is the principal limiting amino acid in the pig (Tokach et al., 1992a; Jones and Stahly, 1995). However, they increased the intake of protein and of numerous other amino acids together with that of lysine. Results
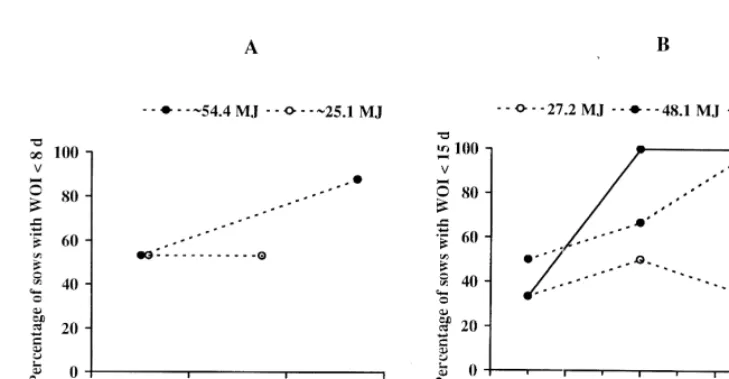
Fig. 2. Predicted influence of daily lysine and energy intakes
obtained in primiparous sows which were protein (27.2, 48.1 and 69.0 MJ of metabolic energy / day) on LH
restricted during lactation suggest that LH secretion secretion in lactating sows (adapted from Tokach et al., 1992a).
is inhibited during lactation and that this inhibition is Increasing amounts of lysine are confounded with increasing
intakes of proteins.
still present after weaning (Table 3). Similarly, pulsatility and mean plasma concentrations of LH
can be reduced by energy restriction alone in lactat- production), the LH response to the lysine / protein
ing sows (Koketsu et al., 1996). intake is suppressed in lactating sows.
King and Williams (1984a) as well as Tokach et
al. (1992a) investigated the influence of lysine / pro- 3.2. Influence of nutrition on the pituitary stores of
tein intake under various levels of energy intake in gonadotrophins
lactating primiparous sows. They showed that the
effects of lysine / protein and energy intake are Feed restriction induces an increase in the pituitary
interdependent: LH secretion (Fig. 2) and the per- content of LH in cyclic gilts (Cooper et al., 1973). In
centage of sows with a short weaning-to-oestrus prepubertal intact gilts, as well as in ovariectomized
interval (Fig. 3) increase with the lysine / protein mature gilts, LH and FSH release after GnRH
intake only when energy is sufficient, and vice versa. administration is higher in animals having the lower
From these data, it can be calculated that, below 50% feed intake (Armstrong and Britt, 1987; Cosgrove et
of the total energy requirements (maintenance1milk al., 1992; Prunier et al., 1993a). These observations,
Table 3
Influence of protein / lysine restriction during lactation on LH secretion of primiparous sows observed during lactation or after weaning
Reference Experimental group
Control Protein restricted Number of pulses / 6 h
Lactation King and Martin, 1989 (7–8 piglets) 0.68 0.43
Jones and Stahly, 1995 1.22 0.48*
Post-weaning King and Martin, 1989 (7–8 piglets) 1.62 1.65
Mean plasma LH (ng / ml)
Lactation King and Martin, 1989 (7–8 piglets) 0.54 0.38*
Jones and Stahly, 1995 0.24 0.17*
Post-weaning King and Martin, 1989 (7–8 piglets) 0.94 0.74*
*
Fig. 3. Influence of daily energy (25.1–69.0 MJ of metabolic energy) and lysine / protein intakes during lactation on the percentage of sows with a normal weaning-to-oestrus interval (WOI). (A) Adapted from King and Williams (1984a); (B) adapted from Tokach et al. (1992a).
coupled with those concerning LH pulsatility, sug- any difference between experimental groups. In
gest that feed restriction inhibits LH release more addition, Barb et al. (1991) observed that the LH
than LH synthesis. This might result in an increase in response to GnRH was lower in prepubertal gilts
the pituitary stores of gonadotrophins in under- receiving an intravenous bolus of glucose than in
nourished females. Therefore, inhibition of the those injected with saline. Therefore, the effect of
GnRH pulse generator system could be one of the glucose on LH secretion is not clear. Moreover, it is
mechanisms explaining the effects of nutrition on confounded with that of insulin and other hormones
reproduction in the female pig. involved in the control of glucose homeostasis
(growth hormone and cortisol for example) since
3.3. Nutritional mediators manipulation of plasma glucose has immediate
con-sequences on release of these latter hormones.
Numerous hormones and metabolites are likely to Circulating levels of insulin can also be
manipu-mediate the effects of nutrition on the hypothalamic– lated by controlling the feed intake of carbohydrates,
pituitary axis. It can also be hypothesized that by inducing diabetes mellitus (e.g.
streptozocin-in-nutritional-associated variations in hepatic portal duced diabetes) or by the administration of insulin.
blood flow have consequences on the metabolic All of these possibilities have been used in the pig to
clearance of sex steroids and hence on the negative explore the effects of insulin on reproduction.
Feed-feedback exerted by the ovaries on the hypo- ing a starch-rich diet to multiparous sows stimulates
thalamic–pituitary axis. LH pulsatility at day 7 of lactation but not at days 14
or 21 and increases the preovulatory surge of LH
3.3.1. Glucose and insulin occurring after weaning (Kemp et al., 1995). In
In order to determine the influence of glucose on normal prepubertal or cyclic gilts, administration of
LH secretion, various researchers have compared insulin, using either the intravenous or the
subcuta-plasma LH in animals infused with glucose or saline. neous routes, provokes hypoglycemia whereas it has
Results from these studies are conflicting and dif- variable effects on LH release: it may stimulate or
ficult to interpret. Booth (1990) observed a positive have no effect (Cox et al., 1987; Matamoros et al.,
effect of glucose on LH pulsatility in feed-restricted 1991). In diabetic mature gilts which were intact or
gilts; this was in contrast to Tokach et al. (1992b) ovariectomized, LH secretion remains relatively high
(Cox et al., 1994). Therefore, lack of insulin does contribution of high levels of FFA to the effects of
not seem to alter LH secretion during a relatively undernutrition on the hypothalamic–pituitary axis of
long interval of time (4–6 days). Such an hypothesis the female pig.
is in agreement with the results of Rojkittikhun et al.
(1993a) who showed that LH pulse frequency and 3.3.3. Adrenal hormones
mean plasma concentration are not altered in lactat- Plasma concentrations of corticosteroids may be
ing primiparous sows which were fasted for 24 h, increased under feed restriction in the pig (Rafai and
despite very low concentrations of insulin (2.5 vs. 29 Fodor, 1980; Baidoo et al., 1992a; Prunier et al.,
mIU / ml in fed sows) and glucose (2.6 vs. 6.4mmol / 1993a). These hormones have inconsistent effects on
ml in fed sows). However, the lack of insulin may be basal LH secretion in the pig: they may have positive
detrimental for LH release if it is prolonged: LH (Pearce et al., 1988), negative (Fonda et al., 1984;
pituitary stores, as well as the in vitro response of Estienne et al., 1991) or no effects (Fonda et al.,
pituitary cells to GnRH stimulation, are sharply 1983, 1984) in prepubertal and / or ovariectomized
decreased in diabetic females where insulin therapy mature gilts. However, it seems relatively clear that
has been withdrawn for 7 days (Angell et al., 1996). corticosteroids are able to decrease the LH response
Moreover, it has been demonstrated that diabetic to exogenous GnRH (prepubertal gilts: Pearce et al.,
ovariectomized gilts are not able to respond to the 1988) and to block the preovulatory surge of LH and
positive feedback of oestradiol when insulin therapy subsequent ovulation (cyclic gilts: Liptrap, 1973;
has been withdrawn for 7 days (Angell et al., 1996). Barb et al., 1982). Therefore, activation of the
Therefore, it seems that insulin has positive effects adrenal axis during acute feed restriction may be
on LH secretion but it is unlikely that variations implicated in disturbance of ovarian activity of the
associated with nutrient absorption after each meal or female pig.
with differences in level of feeding have marked
effects on LH secretion in the female pig unless 3.3.4. Other mediators
these variations are extreme. Similarly, it does not The role of neuropeptides in mediating the effects
seem that variations in glycemia, within a physiolog- of nutrition on LH secretion in the pig has received
ical range, play a role in the effects of nutrition on little attention until now. Endogenous opioids could
reproduction. be involved since it has been demonstrated that they
inhibit LH pulsatility (for review, see Kraeling and
3.3.2. Free fatty acids Barb, 1990). The only data concerning the influence
Plasma concentrations of free fatty acids (FFA) of endogenous opioids in underfed gilts have been
are elevated in fasting and in feed-restricted animals. published by Armstrong and Britt (1987) using a
Their influence on the hypothalamic–pituitary axis severe level of feed restriction, sufficient to induce
has rarely been investigated in the female pig. In cessation of oestrous cyclicity. These researchers
vitro studies have shown that oleic and linoleic acids compared LH release in control and underfed
increase basal LH release by pituitary cells whereas ovariectomized gilts before and after treatment with
they suppress the LH response to GnRH (Barb et al., naloxone, an opioid antagonist. This drug failed to
1995). These results contrast with those obtained in restore LH mean concentrations and pulsatility in
vivo: a single intravenous infusion of a high dose of feed-restricted females. Therefore, arguments to
sup-FFA has a positive influence on the LH response to port the hypothesis that endogenous opioids are
GnRH and hourly infusion of the same dose of FFA implicated in the nutritional-induced inhibition of LH
induces an augmentation of the amplitude of LH pulsatility are still lacking in the pig.
pulses without any effect on their frequency in The involvement of leptin in mediating the effects
prepubertal gilts (Barb et al., 1991); the major effect of nutrition on LH secretion is under evaluation in
of undernutrition on LH release is an inhibition of numerous species. Leptin is secreted by the adipose
the occurrence of pulses (see Section 3.2). Therefore, tissue (pigs: Ramsay et al., 1998), inhibits appetite
expenditure (mice: Pelleymonter et al., 1995). Plas-ma levels of leptin seem to be lower in feed-re-stricted than in control gilts (Prunier and Quesnel, unpublished data). Therefore, leptin may be involved in the effects of nutrition on reproduction, at least as a regulator of the nutritional status. Direct effects of leptin at the hypothalamic–pituitary level could also play a role since RNA messengers coding for the long-form leptin receptor have been detected in hypothalamic and anterior pituitary tissues of ewes and since this expression in the ventromedial and arcuate nuclei of the hypothalamus is higher in feed-restricted than in well-fed ewes (Dyer et al., 1997). Moreover, leptin exhibits a high potency to
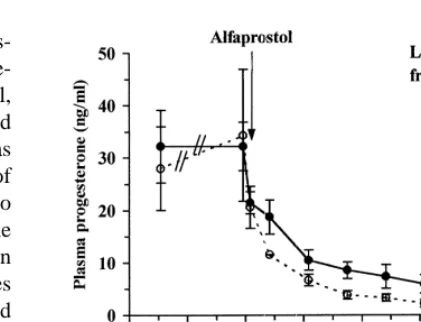
Fig. 4. Influence of daily feed intake (high vs. low: 300 vs. 80%
increase FSH and LH release from male rat
of the energy requirements for maintenance) from luteolysis
hemipituitaries incubated in vitro, and to stimulate (induced by a prostaglandin analogue, alfaprostol) on plasma
GnRH secretion from medio-basal hypothalamus concentrations of progesterone in gilts (adapted from Prunier et
explants (Yu et al., 1997). Finally, leptin is able to al., 1999).
stimulate LH secretion in vivo in mice and female rats (subcutaneous injections: Ahima et al., 1996;
intracerebroventricular injections: Yu et al., 1997). metabolic clearance of oestradiol-17b may be
de-creased by feed restriction and influence LH
secre-3.3.5. Metabolic clearance of steroids tion. However, growth and steroid secretion of the
It has been demonstrated that hepatic portal blood oestrogenic follicles are under the control of
gona-flow and metabolic clearance rate of progesterone are dotrophins (see Section 2.2) whose release is
in-decreased in ovariectomized gilts submitted to feed hibited during feed restriction. Therefore, peripheral
restriction (Prime and Symonds, 1993). Moreover, concentrations of oestradiol may not be increased
lower concentrations of progesterone have been and could even be decreased during feed restriction.
observed in well-fed than in feed-restricted gilts at Such a hypothesis is supported by the observation of
the beginning of gestation (Dyck et al., 1980; Dyck lower concentrations of oestradiol-17b in
restricted-and Kennedy, 1995; Jindal et al., 1996). Therefore, fed than in full-fed gilts at 23 and 53 h after
we have investigated in gilts whether plasma con- induction of luteolysis by alfaprostol (Prunier et al.,
centrations of progesterone around luteolysis could 1999). Similarly, concentrations of oestradiol-17b
be modified by the level of feeding (Prunier et al., were low in follicular fluid of oestrogen-active
1999). Regression of the corpora lutea was induced follicles (Spicer et al., 1991) and in plasma (Rhodes
at the end of the luteal phase by an injection of a et al., 1996) of restricted-fed compared with well-fed
prostaglandin-F2a analogue (alfaprostol). From the heifers.
time of the alfaprostol injection, gilts received either 80 or 300% of the maintenance energy requirements
for 3 days. Progesterone was significantly higher in 4. Main effects of nutrition on the ovaries
feed-restricted than in full-fed gilts from 5 to 53 h
after the induction of luteolysis (Fig. 4). Since Nutrition may influence ovarian function through
progesterone is known to inhibit LH pulsatility, gonadotrophin-mediated effects as described
previ-maintaining relatively high levels of this steroid in ously but also through direct effects of nutrients
underfed gilts around luteolysis may be detrimental and / or of metabolic hormones (insulin, hormones
for the recruitment and selection of preovulatory from the somatotrophic axis, cortisol, thyroid
4.1. Influence of nutrition on folliculogenesis 4.1.2. Late steps of folliculogenesis
Cosgrove et al. (1992) have tried to evaluate the
4.1.1. Early steps of folliculogenesis influence of feed restriction on large (2–6 mm) but
To our knowledge, there are only two reports not preovulatory follicles. They have shown that
relating the effects of nutrition to the development of diameter, antral volume and oestradiol synthesis of
small follicles (0.4–3 mm in diameter), one in the largest follicles were reduced in feed-restricted
prepubertal gilts (Dufour et al., 1985) and the other prepubertal gilts. These effects occurred despite
in reproductive sows (Quesnel et al., 1998a). In both similar plasma concentrations of LH and FSH,
studies, it was observed that feed restriction alters suggesting that nutrition influences folliculogenesis,
the repartitioning of healthy follicles between size at least in part, through gonadotrophin-independent
classes. For instance, it has been shown that the mechanisms.
proportion of 0.4–1.0 mm healthy follicles to the Numerous researchers have determined the
in-total number of antral follicles is increased whereas fluence of feed restriction on ovulation rate. Data
the proportion of 1.0–2.9 mm healthy follicles is obtained in cyclic gilts show that starting feed
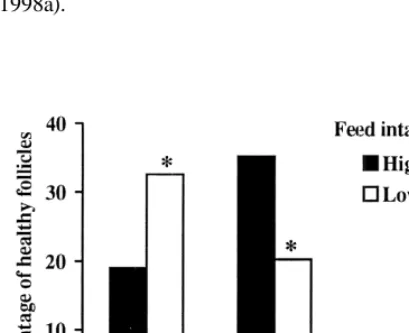
decreased in feed-restricted lactating sows (Fig. 5). restriction during the luteal phase induces a reduction
When oestrus occurs 4–7 days after weaning, pre- in the number of corpora lutea at the subsequent
ovulatory follicles are probably recruited immedi- ovulation (Table 4). In this situation, feed restriction
ately after weaning in the 1.0–2.9 mm size class. occurs at least during recruitment and selection of
Therefore, it can be hypothesized that the relatively the preovulatory follicles. In reproductive sows, most
low number of follicles from this size observed in researchers have determined the effects of feed level
feed-restricted lactating sows may result in a delayed during lactation on the ovulation rate after weaning.
return to oestrus and / or in a lower ovulation rate Therefore, they have imposed the nutritional deficit
after weaning. In fact, the resumption of follicular before recruitment of the preovulatory follicles. In
development 48 h after weaning is more variable in this situation, feed restriction has no clear effect on
feed-restricted than in control sows (Quesnel et al., the ovulation rate whereas it delays the occurrence of
1998a). oestrus in most studies (Table 4). Similarly, King
and Williams (1984b) have shown a prolonged weaning-to-oestrus interval but similar ovulation rates in primiparous sows submitted to protein restriction during lactation. Few studies have evalu-ated the effects of feed intake around ovulation or
between weaning and oestrus (5during recruitment
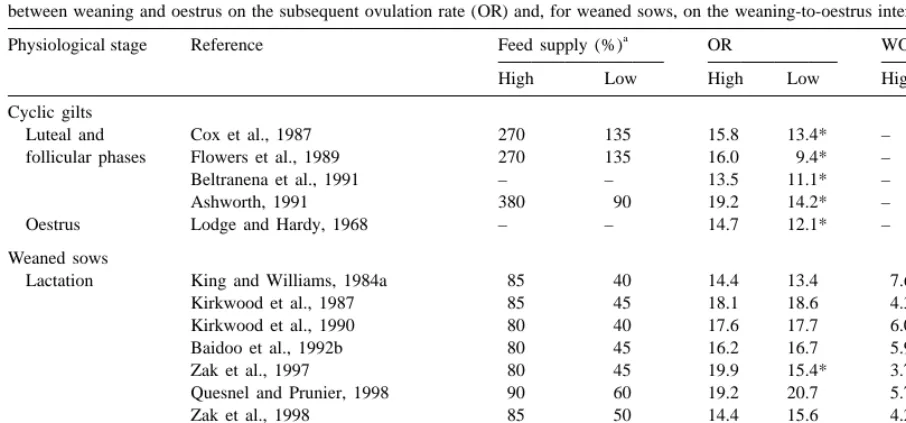
and selection). Their results do not show any clear effect of the level of feed intake on the ovulation rate (Table 4).
4.2. Nutritional mediators
4.2.1. Insulin
Insulin is a good candidate for mediating the effects of nutrition on the ovaries. There is evidence from in vitro studies that insulin stimulates uptake and utilization of nutrients and regulates growth and proliferation of pig granulosa cells (for review, see
Fig. 5. Average proportion of healthy antral follicles per size class
Booth, 1990). It also acts on differentiation, and
to the total number of antral follicles in lactating sows submitted
potentiates FSH-stimulated induction of LH
recep-to high or low levels of feeding (adapted from Quesnel et al.,
tors as well as steroid production by pig granulosa
Table 4
Influence of feed restriction during at least one part of the luteal phase and during the follicular phase or during oestrus or during lactation or between weaning and oestrus on the subsequent ovulation rate (OR) and, for weaned sows, on the weaning-to-oestrus interval (WOI, days)
a
Physiological stage Reference Feed supply (%) OR WOI
High Low High Low High Low
Cyclic gilts
Luteal and Cox et al., 1987 270 135 15.8 13.4* – –
follicular phases Flowers et al., 1989 270 135 16.0 9.4* – –
Beltranena et al., 1991 – – 13.5 11.1* – –
Ashworth, 1991 380 90 19.2 14.2* – –
Oestrus Lodge and Hardy, 1968 – – 14.7 12.1* – –
Weaned sows
Lactation King and Williams, 1984a 85 40 14.4 13.4 7.6 19.9
Kirkwood et al., 1987 85 45 18.1 18.6 4.3 5.8*
Kirkwood et al., 1990 80 40 17.6 17.7 6.0 8.9*
Baidoo et al., 1992b 80 45 16.2 16.7 5.9 7.5
Zak et al., 1997 80 45 19.9 15.4* 3.7 5.6
Quesnel and Prunier, 1998 90 60 19.2 20.7 5.7 5.9
Zak et al., 1998 85 50 14.4 15.6 4.2 6.3*
After weaning Den Hartog and van der Steen, 1981 – – 15.2 14.8 9.1 8.2
King and Williams, 1984a 285 115 14.6 13.2* 13.4 14.1
Baidoo et al., 1992b 245 155 16.6 16.2 6.0 5.9
a
Feed supply (%) is the estimated ratio between metabolic energy intake and requirements for maintenance in prepubertal, mature and pregnant gilts, and weaned sows or for maintenance1milk production in lactating sows. When this ratio was not indicated in the publication, it was calculated in the same way as for Table 2.
*
P,0.05 between control and feed-restricted groups, within criteria.
cells (May and Schomberg, 1981). Some results 4.2.2. Hormones from the somatotrophic axis
from in vivo studies also suggest that insulin stimu- In the pig, as in other species, growth hormone
lates folliculogenesis directly at the ovarian level: for (GH) secretion by the pituitary as well as insulin-like
example, Cox et al. (1987) have shown that insulin growth factor-I (IGF-I) secretion by the liver are
treatment starting in the early follicular phase in- under metabolic influences (for reviews, see Breier
creases the ovulation rate without a clear effect on and Gluckman, 1991; Thissen et al., 1994).
Under-plasma LH. However, insulin treatment may have no nutrition leads to a rise in circulating GH, to a
influence (insulin treatment in lactating sows: Ques- decrease in liver response to GH (decreased GH
nel and Prunier, 1998; insulin treatment during the binding or a post-receptor defect), to a decrease in
follicular phase of cyclic gilts: Quesnel, Jan and circulating IGF-I and to changes in binding proteins
Prunier, unpublished data) or have a negative in- (BPs) for the IGFs. In numerous studies designed to
fluence (insulin treatment between weaning and determine the influence of feed level on ovarian
oestrus: Rojkittikuhn et al., 1993b) on ovulation rate. activity in the female pig, low concentrations of
Therefore, we can conclude that insulin has a IGF-I in plasma and / or in follicular fluid have been
positive influence on nutrition, growth and develop- associated with reduced ovulation rate or impaired
ment of follicular cells, but direct evidence is still folliculogenesis (Cosgrove et al., 1992; Charlton et
missing to demonstrate that a decrease in insulin al., 1993; Booth et al., 1994; Quesnel et al.,
secretion due to feed restriction is responsible, even 1998a,b). In vitro studies have shown that IGF-I
in part, for lower ovulation rate or delayed ovulation and / or GH stimulate mitogenesis and amplify the
on steroidogenesis by granulosa cells (for review, see 4.2.4. Metabolic clearance of steroids
Booth, 1990). In vivo manipulation of GH levels has Higher concentrations of progesterone in
feed-led to contradictory results. For example, treatment restricted females (see Section 3.3) may influence
of cyclic gilts with exogenous GH may induce folliculogenesis directly at the ovarian level since
anoestrus or increase the ovulation rate (Kirkwood et progesterone inhibits preovulatory growth of the
al., 1988, 1989). GH implants have positive effects follicles (see Section 2.2). Indeed, we have recently
on the number of medium-sized follicles (4–6.9 mm) observed that plasma concentration of oestradiol
and on the concentration of IGF-I in plasma and after induced luteolysis was decreased in
feed-re-follicular fluids of prepubertal gilts (Echternkamp et stricted gilts with high concentrations of
progester-al., 1994). Over expression of GH in transgenic one suggesting that preovulatory growth of the
females has a detrimental influence on the number of follicles was impaired (Prunier et al., 1999).
oestrogenic follicles (.5 mm) and on their ability to
synthetize oestradiol before puberty (Guthrie et al.,
1993). Therefore, hormones from the somatotrophic 5. Conclusion
axis may be favourable to folliculogenesis in a
relatively narrow range of concentrations and be Undernutrition inhibits the GnRH pulse generator
inhibitory when their secretion is too high. In system which results in lower stimulation of
fol-conclusion, hormones from the somatotrophic axis liculogenesis by LH. The nutritional mediators at the
are good candidates for mediating the effects of origin of this inhibition are still not identified. Most
nutrition on the ovarian activity in female pigs but experiments have focussed on the role of insulin.
direct evidence is still missing. Their results show that insulin has a positive
in-fluence on LH release in pigs but do not allow to
4.2.3. Other mediators conclude that a decrease in insulin secretion explains
The effects of glucocorticoids and thyroid hor- the lower LH pulsatility in feed-restricted females.
mones on maintenance, differentiation and steroido- Besides these gonadotrophin-mediated effects of
genesis of granulosa cells have been evaluated in nutrition on folliculogenesis, much data support the
vitro (for review, see Booth, 1990). Results from hypothesis that nutritional mediators act directly at
these studies suggest that thyroid hormones amplify the ovarian level. Ovaries are relatively small organs
whereas cortisol antagonizes the action of FSH. whose contribution to the overall metabolic demand
To our knowledge, data concerning the effects of is low. However, they represent a very active tissue
leptin on folliculogenesis directly at the ovarian level with permanent cell re-organization implying a high
are lacking in the female pig. Results from other rate of cell proliferation, growth and differentiation.
species suggesting a role for this hormone are not Therefore, hormones which control nutrient uptake
clear. On the one hand, Spicer and Francisco (1997, and utilization (insulin, GH, cortisol, thyroid
hor-1998) showed in vitro that leptin can attenuate mones, etc.), as well as cell mitogenesis and growth
insulin-induced steroidogenesis of granulosa and (insulin, IGF-I, etc.), are likely to influence ovarian
thecal cells from bovine ovaries. Similarly, Zachow activity. Experimental data support this hypothesis.
and Magoffin (1997) observed that leptin impairs the However, it is still not clear whether the ovarian
synergistic action of IGF-I on FSH-stimulated oes- supply in these hormones can become insufficient or
tradiol production by rat ovarian granulosa cells. In excessive to support normal folliculogenesis in
feed-addition, leptin receptor mRNA has been identified restricted females. Nutrition may influence this
sup-in human ovary tissue (Cioffi et al., 1996). However, ply by controlling the rate of secretion of these
on the other hand, this mRNA codes for the short hormones and their metabolic clearance rate as
form of the leptin receptor and doubts exist con- shown by experimental data. This supply could also
cerning the efficiency of this truncated form to be influenced by nutrition through mechanisms
transduce the hormonal signal (Houseknecht et al., which have received little attention until now. For
Beltranena, E., Foxcroft, G.R., Aherne, F.X., Kirkwood, R.N.,
flow towards the ovaries and / or affect the rate of
1991. Endocrinology of nutritional flushing in gilts. Can. J.
secretion of binding proteins to hormones (e.g.
Anim. Sci. 71, 1063–1071.
cortisol binding globulin for cortisol and progester- Booth, P.J., 1990. Metabolic influences on
hypothalamic-pituitary-one, IGFBPs for IGFs, GHBP for GH, etc.) and ovarian function in the pig. J. Reprod. Fertil. Suppl. 40,
hence the availability of these hormones at the 89–100.
ovarian level. In addition, nutrition may influence the Booth, P.J., Craignon, J., Foxcroft, G.R., 1994. Nutritional
ma-nipulation of growth and metabolic and reproductive status in
sensitivity of the ovaries to metabolic hormones
prepubertal gilts. J. Anim. Sci. 72, 2415–2424.
through altering receptor numbers. These effects
Booth, P.J., Cosgrove, J.R., Foxcroft, G.R., 1996. Endocrine and
should be taken into account in future experiments
metabolic responses to realimentation in feed-restricted
prepub-designed to understand how nutrition influences ertal gilts: associations among gonadotropins, metabolic
hor-reproductive efficiency in swine. mones, glucose, and uteroovarian development. J. Anim. Sci.
74, 840–848.
Breier, B.H., Gluckman, P.D., 1991. The regulation of postnatal growth: nutritional influences on endocrine pathways and References
function of the somatotrophic axis. Livest. Prod. Sci. 27, 77–94.
Aherne, F.X., Kirkwood, R.N., 1985. Nutrition and sow
prolifica-¨
Brussow, K.P., Torner, H., Ratky, J., Schneider, F., Kanitz, W., cy. J. Reprod. Fertil. Suppl. 33, 169–183.
¨
Kochling, W., 1996. Aspects of follicular development and Ahima, R.S., Prabakaran, D., Mantzoros, C., Qu, D., Lowell, B.,
intrafollicular oocyte maturation in gilts. Reprod. Domest. Maratos-Flier, E., Flier, J.S., 1996. Role of leptin in the
Anim. 31, 555–563. neuroendocrine response to fasting. Nature 382, 250–252.
Caldwell, B.V., Moor, R.M., Wilmut, I., Polge, C., Rowson, Angell, C.A., Tubbs, R.C., Moore, A.B., Barb, C.R., Cox, N.M.,
L.E.A., 1969. The relationship between day of formation and 1996. Depressed luteinizing hormone response to estradiol in
functional lifespan of induced corpora lutea in the pig. J. vivo and gonadotropin-releasing hormone in vitro in
ex-Reprod. Fertil. 18, 107–113. perimentally diabetic swine. Domest. Anim. Endocrinol. 13,
Camous, S., Prunier, A., Pelletier, J., 1985. Plasma prolactin, LH, 453–463.
FSH and estrogen excretion patterns in gilts during sexual Armstrong, J.D., Britt, J.H., 1987. Nutritionally-induced anestrus
development. J. Anim. Sci. 60, 1308–1317. in gilts: metabolic and endocrine changes associated with
Charlton, S.T., Cameron, B.J., Glimm, D.R., Foxcroft, G.R., cessation and resumption of estrous cycles. J. Anim. Sci. 65,
Kennelly, J.J., 1993. Insulin-like growth factor 1 (IGF-1) gene 508–523.
expression in porcine ovarian tissue. Can. J. Anim. Sci. 73, Ashworth, C.J., 1991. Embryo development. Pig News Info. 12,
253–257. 551–554.
Cioffi, J.A., Shafer, A.W., Zupancic, T.J., Smith-Gbur, J., Mikhail, Baidoo, S.K., Lythgoe, E.S., Kirkwood, R.N., Aherne, F.X.,
A., Platika, D., Snodgrass, H.R., 1996. Novel B219 / OB Foxcroft, G.R., 1992a. Effect of lactation feed intake on
receptor isoforms: possible role of leptin in hematopoiesis and endocrine status and metabolite levels in sows. Can. J. Anim.
reproduction. Nature Med. 2, 585–588. Sci. 72, 799–807.
Baidoo, S.K., Aherne, F.X., Kirkwood, R.N., Foxcroft, G.R., Cooper, K.J., BrooksP, H., Cole, D.J.A., Haynes, N.B., 1973. The 1992b. Effect of feed intake during lactation on sow reproduc- effect of feed level during the oestrous cycle on ovulation, tive performance. Can. J. Anim. Sci. 72, 911–917. embryo survival and anterior LH potency in the gilt. J. Reprod. Barb, C.R., Kraeling, R.R., Rampacek, G.B., Fonda, E.S., Kiser, Fertil. 32, 71–78.
T.E., 1982. Inhibition of ovulation and LH secretion in the gilt Cosgrove, J.R., Tilton, J.E., Hunter, M.G., Foxcroft, G.R., 1992. after treatment with ACTH or hydrocortisone. J. Reprod. Fertil. Gonadotropin-independent mechanisms participate in ovarian 64, 85–92. responses to realimentation in feed-restricted prepubertal gilts. Barb, C.R., Kraeling, R.R., Barrett, J.B., Rampacek, G.B., Camp- Biol. Reprod. 47, 736–745.
bell, R.M., Mowles, T.F., 1991. Serum glucose and free fatty Cosgrove, J.R., Foxcroft, G.R., 1996. Nutrition and reproduction acids modulate growth hormone and luteinizing hormone in the pig: ovarian aetiology. Anim. Reprod. Sci. 42, 131–141. secretion in the pig. Proc. Soc. Exp. Biol. Med. 198, 636–642. Cox, N.M., Stuart, M.J., Althen, T.G., Bennett, W.A., Miller, H.W., Barb, C.R., Kraeling, R.R., Rampacek, G.B., 1995. Glucose and 1987. Enhancement of ovulation rate in gilts by increasing free fatty acid modulation of growth hormone and luteinizing dietary energy and administering insulin during follicular hormone (LH) secretion by cultured porcine pituitary cells. J. growth. J. Anim. Sci. 64, 507–516.
Dailey, R.A., Clark, J.R., Staigmiller, R.B., First, N.L., Chapman, hormone concentrations after adrenalectomy and / or ovariec-A.B., Casida, L.E., 1976. Growth of new follicles following tomy in the prepuberal gilt. Endocrinology 114, 268–273. electrocautery in four genetic groups of swine. J. Anim. Sci. Foxcroft, G.R., 1998. Mechanisms mediating nutritional effects on 43, 175–183. embryonic survival in pigs. J. Reprod. Fertil. Suppl. 52, 31–46. Den Hartog, L.A., van Kempen, G.J.M., 1980. Relation between Foxcroft, G.R., Hunter, M.G., 1985. Basic physiology of follicular
nutrition and fertility in pigs. Neth. J. Agric. Sci. 28, 211–227. maturation in the pig. J. Reprod. Fertil. Suppl. 33, 1–19. Den Hartog, L.A., van der Steen, H.A.M., 1981. Reproductive Foxcroft, G.R., Cosgrove, J.R., Ding, J., Hofacker, S., Wiesak, T.,
traits in primiparous sows in relation to feeding level. Neth. J. 1994. Reproductive function: current concepts. In: Progress in Agric. Sci. 29, 285–286. Pig Science, University Press, pp. 225–252.
Dourmad, J.Y., 1988. Voluntary feed intake in lactating sows: Guthrie, H.D., Bolt, D.J., 1990. Changes in plasma follicle-numerous factors of variation. INRA Prod. Anim. 1, 141–146. stimulating hormone, luteinizing hormone, estrogen and pro-Dourmad, J.Y., Etienne, M., Prunier, A., Noblet, J., 1994. The gesterone during growth of ovulatory follicles in the pig.
effect of energy and protein intake of sows on their longevity: a Domest. Anim. Endocrinol. 7, 83–91.
review. Livest. Prod. Sci. 40, 87–97. Guthrie, H.D., Pursel, V.G., Bolt, D.J., Cooper, B.S., 1993. Driancourt, M.A., Locatelli, A., Prunier, A., 1995. Effects of Expression of a bovine growth hormone transgene inhibits gonadotrophin deprivation on follicular growth in gilts. Re- pregnant mare’s serum gonadotropin-induced follicle matura-prod. Nutr. Dev. 35, 663–673. tion in prepuberal gilts. J. Anim. Sci. 71, 3409–3413. Dufour, J.J., Fahmy, M.H., Flipot, P.M., 1985. Follicular develop- Houseknecht, K.L., Baile, C.A., Matteri, R.L., Spurlock, M.E.,
ment during the prepuberal period of different morphological 1998. The biology of leptin: a review. J. Anim. Sci. 76, types of ovaries in Hampshire and Yorkshire gilts fed two 1405–1420.
planes of nutrition. J. Anim. Sci. 61, 1201–1210. Hunter, M.G., Biggs, C., Faillace, L.S., Picton, H.M., 1992. Dyck, G.W., Palmer, W.M., Simaraks, S., 1980. Progesterone and Current concepts of folliculogenesis in monoovular and
poly-luteinizing hormone concentration in serum of pregnant gilts ovular farm species. J. Reprod. Fertil. Suppl. 45, 21–38. on different levels of feed consumption. Can. J. Anim. Sci. 60, Jindal, R., Cosgrove, J.R., Aherne, F.X., Foxcroft, G.R., 1996. 877–884. Effect of nutrition on embryonal mortality in gilts: association Dyck, G.W., Kennedy, A.D., 1995. The effect of level of diet with progesterone. J. Anim. Sci. 74, 620–624.
intake after mating on the serum concentration of thyroxine, Jones, D.B., Stahly, T.S., 1995. Impact of amino acid nutrition triiodothyronine, growth hormone, insulin and glucose, and during lactation on subsequent reproductive function of sows. embryonic survival in the gilt. Can. J. Anim. Sci. 75, 315–325. Swine Res. Rep. 1, 56–59.
Dyer, C.J., Simmons, J.M., Matteri, R.L., Keisler, D.H., 1997. Kemp, B., Soede, N.M., Helmond, F.A., Bosch, M.W., 1995. Leptin receptor mRNA is expressed in ewe anterior pituitary Effects of energy source in the diet on reproductive hormones and adipose tissues and is differentially expressed in hypo- and insulin during lactation and subsequent estrus in multipar-thalamic regions of well-fed and feed-restricted ewes. Domest. ous sows. J. Anim. Sci. 73, 3022–3029.
Anim. Endocrinol. 14, 119–128. Kemp, B., Soede, N.M., Hazeleger, W., 1998. Control of ovula-Echternkamp, S.E., Spicer, L.J., Klindt, J., Vernon, R.K., Yen, J.T., tion. In: Progress in Pig Science, University Press, pp. 287–
Buonomo, F.C., 1994. Administration of porcine somatotropin 302.
by a sustained-release implant: effects on follicular growth, King, R.H., Williams, I.H., 1984a. The effect of nutrition on the concentrations of steroids and insulin-like growth factor-I, and reproductive performance of first-litter sows. 1. Feeding level insulin-like growth factor binding protein activity in follicular during lactation, and between weaning and mating. Anim. fluid of control, lean and obese gilts. J. Anim. Sci. 72, 2431– Prod. 38, 241–247.
2440. King, R.H., Williams, I.H., 1984b. The effect of nutrition on the Estienne, M.J., Barb, C.R., Kesner, J.S., 1991. Luteinizing hor- reproductive performance of first-litter sows. 2. Protein and
mone secretion in hypophysial stalk-transected gilts given energy intakes during lactation. Anim. Prod. 38, 249–256. hydrocortisone acetate, pulsatile gonadotropin-releasing hor- King, R.H., Martin, G.B., 1989. Relationship between protein mone. Domest. Anim. Endocrinol. 8, 407–414. intake during lactation. LH levels and oestrus activity in Flowers, B., Martin, M.J., Cantley, T.C., Day, B.N., 1989. first-litter sows. Anim. Reprod. Sci. 19, 283–292.
Endocrine changes associated with a dietary-induced increase Kirkwood, R.N., Aherne, F.X., 1985. Energy intake, body com-in ovulation rate (flushcom-ing) com-in gilts. J. Anim. Sci. 67, 771–778. position and reproductive performance of the gilt. J. Anim. Sci. Flowers, B., Cantley, T.C., Martin, M.J., Day, B.N., 1991. 60, 1518–1529.
Episodic secretion of gonadotrophins and ovarian steroids in Kirkwood, R.N., Baidoo, S.K., Aherne, F.X., Sather, A.P., 1987. jugular and utero-ovarian vein plasma during the follicular The influence of feeding level during lactation on the occur-phase of the oestrous cycle in gilts. J. Reprod. Fertil. 91, rence and endocrinology of the postweaning estrus in sows.
101–112. Can. J. Anim. Sci. 67, 405–415.
Fonda, E.S., Rampacek, G.B., Kraeling, R.R., Barb, C.R., 1983. Kirkwood, R.N., Thacker, P.A., Gooneratne, A.D., Guedo, B.L., Serum luteinizing hormone in prepuberal gilts after ovariec- Laarveld, B., 1988. The influence of exogenous growth hor-tomy or adrenalechor-tomy. J. Anim. Sci. 56, 1174–1179. mone on ovulation rate in gilts. Can. J. Anim. Sci. 68, 1097– Fonda, E.S., Rampacek, G.B., Kraeling, R.R., 1984. The effect of 1103.
The effect of exogenous growth hormone on the endocrine Periestrous patterns of circulating LH, FSH and oestradiol-17b
status and the occurrence of estrus in gilts. Can. J. Anim. Sci. in the gilt. Anim. Reprod. Sci. 14, 205–218.
69, 931–937. Prunier, A., Martin, C., Mounier, A.M., Bonneau, M., 1993a. Kirkwood, R.N., Baidoo, S.K., Aherne, F.X., 1990. The influence Metabolic and endocrine changes associated with
undernutri-of feeding level during lactation and gestation on the endocrine tion in the peripubertal gilt. J. Anim. Sci. 71, 1887–1894. status and reproductive performance of second parity sows. Prunier, A., Dourmad, J.Y., Etienne, M., 1993b. Feeding level, Can. J. Anim. Sci. 70, 1119–1126. metabolic parameters and reproductive performance of Koketsu, Y., Dial, G.D., Pettigrew, J.E., Marsh, W.E., King, V.L., primiparous sows. Livest. Prod. Sci. 37, 185–196.
1996. Influence of imposed feed intake patterns during lacta- Prunier, A., Quesnel, H., Quiniou, N., LeDenmat, M., 1999. tion on reproductive performance and on circulating levels of Influence of dietary intake on plasma progesterone and embryo glucose, insulin, and luteinizing hormone in primiparous sows. mortality in gilts. J. Rech. Porcine Fr. 31, 17–22.
J. Anim. Sci. 74, 1036–1046. Quesnel, H., Prunier, A., 1995. Endocrine bases of lactational Kraeling, R.R., Barb, C.R., 1990. Role of prolactin in the anoestrus in the sows. Reprod. Nutr. Dev. 35, 395–414.
regulation of ovarian function in pigs. J. Reprod. Fertil. Suppl. Quesnel, H., Pasquier, A., Mounier, A.M., Prunier, A., 1998a. 40, 3–17. Influence of feed restriction during lactation on gonadotropic Liptrap, R.M., 1973. Effect of corticotrophin and corticosteroids hormones and ovarian development in primiparous sows. J.
on oestrus, ovulation and oestrogen excretion in the sow. Res. Anim. Sci. 76, 856–863.
Vet. Sci. 15, 215–219. Quesnel, H., Pasquier, A., Mounier, A.M., Louveau, I., Prunier, Lodge, G.A., Hardy, B., 1968. The influence of nutrition during A., 1998b. Influence of feed restriction in primiparous lactating oestrus on ovulation rate in the sow. J. Reprod. Fertil. 15, sows on body condition and metabolic parameters. Reprod.
329–332. Nutr. Dev. 38, 261–274.
Matamoros, I.A., Cox, N.M., Moore, A.B., 1991. Effects of Quesnel, H., Prunier, A., 1998. Effect of insulin administration exogenous insulin and body condition on metabolic hormones before weaning on reproductive performance in feed-restricted and gonadotropin-induced follicular development in prepuber- primiparous sows. Anim. Reprod. Sci. 51, 119–129. tal gilts. J. Anim. Sci. 69, 2081–2091. Rafai, P., Fodor, E., 1980. Studies on porcine adrenocorticol May, J.V., Schomberg, D.W., 1981. Granulosa cell differentiation function. II. Effects of the ambient temperature and restricted in vitro: effects of insulin on growth and functional integrity. feeding on the peripheral cortisol level. Acta Vet. Hung. 28,
Biol. Reprod. 25, 421–431. 443–454.
Morbeck, D.E., Esbenshade, K.L., Flowers, W.L., Britt, J.H., 1992. Ramsay, T.G., Yan, X., Morrison, C., 1998. The obesity gene in Kinetics of follicle growth in the prepubertal gilt. Biol. Reprod. swine: Sequence and expression of porcine leptin. J. Anim. Sci.
47, 485–491. 76, 484–490.
Mullan, B.P., Close, W.H., Foxcroft, G.R., 1991. Metabolic state Rhodes, F.M., Entwistle, K.W., Kinder, J.E., 1996. Changes in of the lactating sows influences plasma LH and FSH before and ovarian function and gonadotrophin secretion preceding the after weaning. In: Manipulating Pig Production III, p. 32. onset of nutritionally induced anestrus in Bos indicus heifers. Noblet, J., Dourmad, J.Y., Etienne, M., 1990. Energy utilization in Biol. Reprod. 55, 1437–1443.
¨
pregnant and lactating sows: modelling of energy requirements. Rojkittikuhn, T., Uvnas-Moberg, K., Einarsson, S., 1993a. Plasma J. Anim. Sci. 68, 562–572. oxytocin, prolactin, insulin and LH after 24 h of fasting and Noblet, J., Karege, C., Dubois, S., van Milgen, J., 1999. Metabolic after refeeding in lactating sows. Acta Physiol. Scand. 148,
utilization of energy and maintenance requirements in growing 413–419.
pigs: effect of sex and genotype. J. Anim. Sci. (in press). Rojkittikuhn, T., Einarsson, S., Zilinskas, H., Edqvist, L.E., ¨
O’Grady, J.F., Lynch, P.B., Kearney, P.A., 1985. Voluntary feed Uvnas-Moberg, K., Lundeheim, N., 1993b. Effects of insulin intake by lactating sows. Livest. Prod. Sci. 12, 355–365. administration at weaning on hormonal patterns and reproduc-Pearce, G.P., Paterson, A.M., Hughes, P.E., 1988. Effect of short- tive performance in primiparous sows. J. Vet. Med. Ser. A 40,
term elevations in plasma cortisol concentration on LH secre- 161–168.
tion in prepubertal gilts. J. Reprod. Fertil. 83, 413–418. Shaw, H.J., Foxcroft, G.R., 1985. Relationships between LH, FSH Pelleymonter, M.A., Cullen, M.J., Baker, M.B., Hecht, R., Win- and prolactin secretion and reproductive activity in the weaned
ters, D., Boone, T., Collins, F., 1995. Effects of the obese gene sow. J. Reprod. Fertil. 75, 17–28.
product on body weight regulation in ob / ob mice. Science 269, Spicer, L.J., Francisco, C., 1997. The adipose obese gene product, 540–543. leptin: evidence of a direct inhibitory role in ovarian function. Peltoniemi, O.A.T., Love, R.J., Evans, G., 1997. Effect of feed Endocrinology 138, 3374–3379.
restriction and season on LH and prolactin secretion, adrenal Spicer, L.J., Francisco, C., 1998. Adipose obese gene product, response, insulin and FFA in group housed pregnant gilts. leptin, inhibits bovine ovarian thecal cell steroidogenesis. Biol. Anim. Reprod. Sci. 49, 179–190. Reprod. 58, 207–212.
Prime, G.R., Symonds, H.W., 1993. Influence of the plane of Spicer, L.J., Enright, W.J., Murphy, M.G., Roche, J.F., 1991. nutrition on portal blood flow and the metabolic clearance rate Effects of dietary intake on concentrations of insulin-like of progesterone in ovariectomized gilts. J. Agric. Sci. Cam- growth factor-I in plasma and follicular fluid, and ovarian bridge 121, 389–397. function in heifers. Domest. Anim. Endocrinol. 8, 431–437.
´
Nutrition-al regulation of the insulin-like growth factors. Endocr. Rev. Role of leptin in hypothalamic-pituitary function. Proc. Natl.
15, 80–101. Acad. Sci. USA 94, 1023–1028.
Tokach, M.D., Pettigrew, J.E., Dial, G.D., Wheaton, J.E., Crooker, Zachow, R.J., Magoffin, D.A., 1997. Direct intraovarian effects of B.A., Johnston, L.J., 1992a. Characterization of luteinizing leptin: impairment of the synergistic action of insulin-like hormone secretion in the primiparous lactating sow: relation- growth factor-I on follicle-stimulating hormone-dependent ship to blood metabolites and return-to-estrus interval. J. Anim. estradiol-17bproduction by rat ovarian granulosa cells.
Endo-Sci. 70, 2195–2201. crinology 138, 847–850.
Tokach, M.D., Pettigrew, J.E., Dial, G.D., Wheaton, J.E., Crooker, Zak, L.J., Cosgrove, J.R., Aherne, F.X., Foxcroft, G.R., 1997. B.A., Koketsu, Y., 1992b. Influence of glucose infusions on Pattern of feed intake and associated metabolic and endocrine luteinizing hormone secretion in the energy-restricted primipar- changes differentially affect postweaning fertility in primipar-ous lactating sow. J. Anim. Sci. 70, 2202–2206. ous lactating sows. J. Anim. Sci. 75, 208–216.