Dioscorins, the major tuber storage proteins of yam (
Dioscorea
batatas
Decne), with dehydroascorbate reductase and
monodehydroascorbate reductase activities
Wen-Chi Hou, Hsien-Jung Chen, Yaw-Huei Lin *
Institute of Botany,Academia Sinica,Nankang,Taipei115,Taiwan,ROC
Received 26 April 1999; received in revised form 26 July 1999; accepted 26 July 1999
Abstract
Dioscorins, the major storage proteins of yam (Dioscorea batatas Decne), were purified from the tubers according to the methods of Hou et al. (W.C. Hou, J.S. Liu, H.J. Chen, T.E. Chen, C.F. Chang, Y.H. Lin, Dioscorin, the major tuber storage protein of yam (Dioscorea batatas Decne), with carbonic anhydrase and trypsin inhibitor activities, J. Agric. Food Chem. 47 (1999) 2168 – 2172.). Dioscorins reduced dehydroascorbate (DHA) in the presence of glutathione to regenerate ascorbate (AsA). However, without glutathione, dioscorins has very low DHA reductase activity. Intermolecular thiol – disulfide interchanges of dioscorins on SDS – PAGE gels were found during DHA reduction in the absence of glutathione. AsA was oxidized by AsA oxidase to generate monodehydroascorbate (MDA) free radicals. MDA was also reduced by dioscorins to AsA in the presence of NADH mimicking the MDA reductase catalyzed reaction. These data suggest that dioscorins have both DHA reductase and MDA reductase activities. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Dehydroascorbate; DHA reductase;Dioscorea batatasDecne; Dioscorin; Monodehydroascorbate; MDA reductase
www.elsevier.com/locate/plantsci
1. Introduction
Ascorbic acid (AsA) plays an important role in protecting plant cells [2 – 5] and insect cells [6] against the action of reactive oxygen species. In plants, peroxide-scavenging was accomplished through the AsA – glutathione pathway, a coupled series of redox reactions involving four enzymes: AsA-specific peroxidase (EC 1.11.1.11), monohydroascorbate (MDA) reductase (EC 1.6.5.4), de-hydroascorbate (DHA) reductase (EC 1.8.5.1), and glutathione reductase (EC 1.6.4.2) [7 – 10].
This pathway has been studied mainly in chloro-plasts, in which the possible reactive oxygen spe-cies produced by PS I during photosynthesis might cause serious damage. However, the AsA – glu-tathione pathway has also been found in cytosol [2,8,9,11 – 13], mitochondria, and peroxisomes [14]. When AsA functions as an antioxidant in cells, it is univalently oxidized to MDA free radical, and MDA reductase catalyzes the reduction of MDA back to AsA with NAD(P)H [15]. Heber et al. [16] pointed out that MDA was a sensitive endogenous index of oxidative stress in leaf tissues. Transgenic tobacco plants expressing antisense RNA of cyto-solic ascorbate peroxidase showed increased sus-ceptibility to ozone injury [17].
Patatin, the potato tuber storage protein, has been demonstrated to have lipid acyl hydrolase and acyltransferase activities that are involved in tuber tissue response to wounding [18]. The soy-bean vegetative storage proteins VSPaand VSP b Abbre6iations: APS, ammonium persulfate; AsA, ascorbate; Bis,
N,N%-methylenediacrylamide; CBB G-250, coomassie brilliant blue G-250; DHA, dehydroascorbate; mBBr, monobromobimane reagent; MDA, monodehydroascorbate; 2-ME, 2-mercaptoethanol; SDS – PAGE, sodium dodecylsulfate – polyacrylamide gel electrophoresis; TEMED,N,N,N%,N%-tetramethylenediamine; TI, trypsin inhibitor.
* Corresponding author. Tel.: +886-2-27899590 ext. 320; fax:
+886-2-27827954.
E-mail address:[email protected] (Y.-H. Lin)
both have acid phosphatase activity [19]. Dioscorins, the yam tuber storage protein, has been demonstrated to have carbonic anhydrase and trypsin inhibitor activities [1]. Tru¨mper et al. [20] found that the first 17 N-terminal amino acid residues of DHA reductase purified from spinach chloroplasts are completely identical to the soy-bean trypsin inhibitor (TI). Both the reduced (thiol) form of soybean TI, which was produced by treating oxidized form of soybean TI with dithiothreitol, and the native (thiol) form of spinach chloroplast DHA reductase can reduce DHA to regenerate AsA in the presence of glu-tathione; meanwhile, the oxidized (disulfide) form of spinach chloroplast DHA reductase, which was produced by oxidized glutathione, exhibited TI activity. Hou and Lin [21] previously demon-strated that the root storage proteins (TIs) of sweet potatoes have both DHA reductase and MDA reductase activities. In this study, we present evidence to show that the purified storage proteins, dioscorins, of yam tubers also exhibit both DHA reductase and MDA reductase activities.
2. Materials and methods
2.1. Plant materials and dioscorin purification
Fresh tubers of yam (Dioscorea batatas Decne) were imported from Japan and purchased from a local market. After cleaning with water, the tubers were peeled and cut into strips immediately for dioscorin extraction. Extraction and purification processes were according to the methods of Hou et al. [1] and performed at 4°C.
2.2. DHA reductase acti6ity assay
The DHA reductase activity of dioscorins was assayed according to the method of Tru¨mper et al. [20] with some modifications. Ten milligrams DHA were dissolved in 5.0 ml of 100 mM phos-phate buffer with different pH values (pH 6.0, 6.5 and 7.0). The reaction was carried out at 30°C by adding 100 ml dioscorin solution (100 mg protein) to 0.9 ml DHA solution with or without 4 mM glutathione. The increase of absorbance at 265 nm was recorded for 5 min. Non-enzymatic reduction of DHA in phosphate buffer was measured in a
separate cuvette at the same time. A standard curve was plotted using 0.1 – 6 nmol AsA.
2.3. Protein and thiol-labeling stainings of dioscorins in 15% SDS–PAGE gels
Before and after reaction with DHA without glutathione added at pH 6.0, 6.5 or 7.0 for 30 min, dioscorins were examined by both protein and thiol-labeling stainings in 15% SDS – PAGE gels [22]. One hundred microliter samples were mixed with 25 ml sample buffer containing 60 mM Tris buffer (pH 6.8), 2% SDS, 25% glycerol and 0.1% bromothymol blue, with or without 2-mercap-toethanol (2-ME) in a final concentration of 14.4 mM, and heated at 100°C for 5 min for protein staining and thiol-labeling staining. Thiol-labeling staining in a SDS – PAGE gel followed the method of Kobrehel et al. [23] using monobromobimane (mBBr) as a probe.
2.4. MDA reductase acti6ity assay
The MDA reductase activity of dioscorins was assayed according to Hossain et al. [14] by follow-ing the decrease in absorbance at 340 nm due to NADH oxidation. MDA free radicals were gener-ated by AsA oxidase (EC 1.10.3.3) in the assay system [24]. The reaction mixtures contained 50 mM phosphate buffer (pH 6.0, 6.5 and 7.0, respec-tively), 0.33 mM NADH, 3 mM AsA, AsA oxi-dase (0.9 U), and 200 ml dioscorins solution (200
mg protein) in a final volume of 1 ml. Dioscorin solution was replaced with distilled water for controls.
2.5. MDA reductase acti6ity staining in 15%
SDS–PAGE gels
2.6. Chemicals
Ascorbic acid, dehydroascorbic acid, elec-trophoresis grade acrylamide and Bis, TEMED and APS were from E. Merck Inc. (Germany). Thiolyte® monobromobimane reagent was
pur-chased from Calbiochem International (San Diego, CA). Other chemicals and solvents were purchased from Sigma Chemical Company (St. Louis, MO). The low molecular weight kits for electrophoresis were obtained from Pharmacia (Uppsala, Sweden).
3. Results and discussion
We reported [21] recently that TIs, the major storage protein of sweet potato roots, display both DHA reductase and MDA reductase activities and
might be involved in response to environmental oxidative stresses in sweet potato roots. In this work we found that the storage proteins of yam tuber, dioscorins, also display both DHA reduc-tase and MDA reducreduc-tase activities.
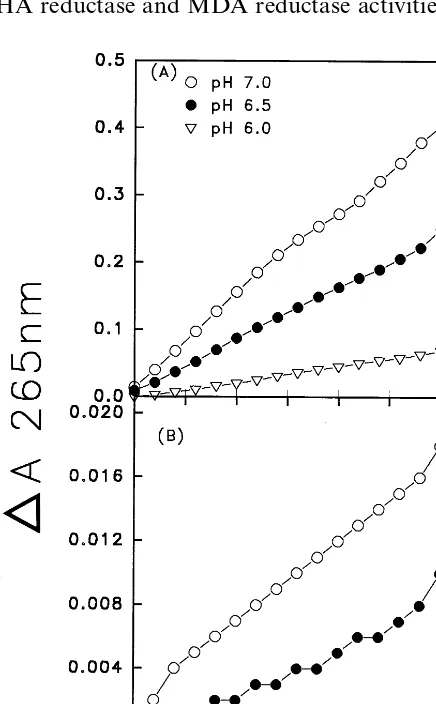
From our previous report [1], the dioscorins were purified from yam tubers by ammonium sulfate fractionation (45 – 75% saturations), DE-52 ion exchange chromatography and Sephadex G-75 gel filtration. Two protein bands (28 kD and 82 kD) were found in non-reducing SDS – PAGE gels, and only one band (32 kD) was found in reducing ones. The first 21 amino acids in N-terminal re-gion of 28 kD dioscorin showed high sequence homology to the deduced sequence of dioscorin cDNA from another yam species (Dioscorea cayenensisL.) [27,28] except at positions 1 and 11. These purified dioscorins from yam tubers were used to examine DHA reductase activity. Fig. 1 shows AsA regeneration (DA 265nm) from DHA at different pH values (pH 6.0, 6.5 and 7.0) with (A) or without (B) glutathione. Fig. 1A shows that dioscorins exhibited DHA reductase activity and could reduce DHA back to AsA. The specific activities of DHA reductase for dioscorins in the presence of glutathione were 1.08, 4.60 and 7.65 nmol AsA produced/min/mg protein at pH 6.0, 6.5 and 7.0, respectively. However, in the absence of glutathione, very low DHA reductase activities of dioscorins were found (Fig. 1B): 0.023 and 0.041 nmol AsA produced/min per mg protein at pH 6.5 and 7.0, respectively. DHA reductase of dioscorins in the absence of glutathione represent only one 200th of those in the presence of glu-tathione (Fig. 1A). It was reported that thiore-doxin m and thioredoxin f from spinach chloroplast and thioredoxin from Escherichia coli
exhibit very low DHA reductase activities without glutathione [20].
Fig. 2 shows protein and thiol-labeling stainings of dioscorins in SDS – PAGE gels before (lanes 1 and 5) and after reacting with DHA for 30 min in phosphate buffer at pH 6.0 (lanes 2 and 6), pH 6.5 (lanes 3 and 7) and pH 7.0 (lanes 4 and 8) without glutathione. Protein and thiol-labeling are shown in Figs. 2A and 2B, respectively. For electrophore-sis, both samples of lanes 1 – 4 in sample buffer without 2-ME and samples of lanes 5 – 8 in sample buffer with 2-ME were heated at 100°C for 5 min. There were higher amounts of dioscorins with Mr
36 and 82 kD and lesser amounts of dioscorins
Fig. 2. Protein (A) and thiol-labeling (B) stainings of dioscorins in 15% SDS – PAGE gels. Before (lanes 1 and 5, in distilled water) and after reacting with dehydroascorbate for 30 min in phosphate buffer at pH 6.0 (lanes 2 and 6), pH 6.5 (lanes 3 and 7) and pH 7.0 (lanes 4 and 8) in the absence of glutathione. Samples in lanes 1 – 4 were incubated in sample buffer without 2-ME; while in lanes 5 – 8, samples were incubated in sample buffer with 2-ME in a final concentration of 14.4 mM. ‘M’ represents the molecular weight marker and 10mg protein was loaded in each well.
with Mr 28 kD found after DHA reduction to
AsA in the absence of glutathione at pH 6.0 – 7.0 (lanes 2 – 4) than those in the control lane (lane 1). Similar results for the same protein patterns of 32 kD dioscorins (lanes 5 – 8) were observed after treating with 2-ME and heating at 100°C for 5 min, however, the 28 and 82 kD dioscorins disap-peared in lanes 5 – 8 (Fig. 2A). TIs from sweet potatoes were also reported to have intermolecular disulfide linkage changes during DHA reduction in the absence of glutathione [20]. The data imply that intermolecular thiol – disulfide interchanges of dioscorins occurred during DHA reduction. Simi-lar results have been reported for the storage proteins, sporamin, of sweet potato [29]. Two protein bands (22 and 31 kD) were detected with-out dithiothreitol treatment while only one protein band with the intermediate molecular mass (25 kD) was found in the presence of dithiothreitol in SDS – PAGE gels. Fig. 2B shows the results of thiol-labeling staining of dioscorins. From the comparisons of Fig. 2B with Fig. 2A, we may conclude that free thiol groups are detectable in all dioscorin molecules. Conlan et al. [28] reported that dioscorins had two major groups differing in the presence or absence of intra-chain disulfide bonds. Harvey and Boulter [30] reported that the intra- and intermolecular disulfide bridges were formed in dioscorins from yams at different pH values, ionic strength and protein concentrations. The 82 kD dioscorin reported in this work may come from the interactions between dioscorin molecules which disappeared under reducing con-ditions (Fig. 2A, lane 5).
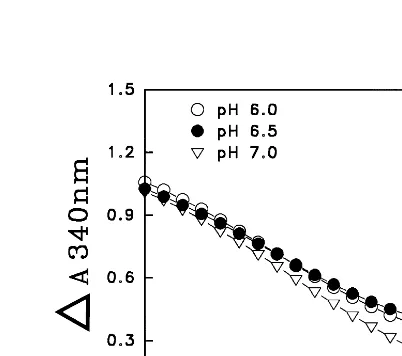
MDA was reduced to AsA in coupling with NADH oxidation (DA340nm) at pH 6.0, 6.5 and 7.0 when dioscorins purified from yam tubers were
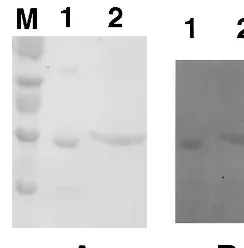
used as MDA reductase. The yam dioscorins ex-hibited MDA reductase activity at pH 6.0, 6.5 and 7.0 (Fig. 3), with higher activity at pH 7.0 than pH 6.0 or 6.5 in our assay system. From diaphorase activity staining (Fig. 4B), the major MDA reduc-tase activity was from dioscorin with Mr 28 kD
(lane 1 of both Fig. 4A, protein staining, and Fig. 4B; without 2-ME treatment) and with Mr 32 kD
(lane 2 of both Fig. 4A, protein staining, and Fig. 4B; with 2-ME treatment). MDA reductase and DHA reductase were shown to contain free thiol groups in their catalytic sites [9,11,35]. Dioscorin of 28 and 32 kD, respectively, showed diaphorase activity (lanes 1 and 2 of both Fig. 4A, protein staining, and Fig. 4B; without and with 2-ME treatment, respectively) corresponding to those molecular species with free thiol groups (lanes 1 – 4 and lanes 5 – 8, respectively, of Fig. 2B).
Fig. 4. Protein (A) and diaphorase activity (B) stainings in 15% SDS – PAGE gels for detection of monodehydroascor-bate reductase activity of dioscorins purified from yam tubers. Samples in lane 1 were incubated in sample buffer without 2-ME; while in lane 2, samples were incubated in sample buffer with 2-ME in a final concentration of 14.4 mM. ‘M’ represents the molecular weight marker and 10mg protein was loaded in each well.
Acknowledgements
The authors want to thank the financial support (NSC88-2311-B001-043) from the National Sci-ence Council, Republic of China (ROC).
References
[1] W.C. Hou, J.S. Liu, H.J. Chen, T.E. Chen, C.F. Chang, Y.H. Lin, Dioscorin, the major tuber storage protein of yam (Dioscorea batatasDecne), with carbonic anhydrase and trypsin inhibitor activities, J. Agric. Food Chem. 47 (1999) 2168 – 2172.
[2] D.A. Dalton, S.A. Russell, F.J. Hanus, G.A. Pascoe, H.J. Evans, Enzymatic reactions of ascorbate and glu-tathione that prevent peroxide damage in soybean root nodules, Proc. Natl. Acad. Sci. USA 83 (1986) 3811 – 3815.
[3] K. Kobayashi, S. Tagawa, S. Sano, K. Asada, A direct demonstration of the catalytic action of monodehy-droascorbate reductase by pulse radiolysis, J. Biol. Chem. 270 (1995) 27551 – 27554.
[4] S. Mazhoudi, A. Chaoui, M.H. Ghorbal, E.E. Ferjani, Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum, Mill.), Plant Sci. 127 (1997) 129 – 137.
[5] C.H. Foyer, H. Lopez-Delgado, J.F. Dat, I.M. Scott, Hydrogen peroxide- and glutathione-associated mecha-nisms of acclimatory stress tolerance and signalling, Physiol. Plant. 100 (1997) 241 – 254.
[6] C.B. Summers, G.W. Felton, Antioxidant role of dehy-droascorbic acid reductase in insects, Biochem. Biophys. Acta 1156 (1993) 235 – 238.
[7] C. Miyake, K. Asada, Thylakoid-bound ascorbate per-oxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radi-cals in thylakoids, Plant Cell Physiol. 33 (1992) 541 – 553. [8] K. Asada, Ascorbate peroxidase — a hydrogen peroxide scavenging enzyme in chloroplasts, Physiol. Plant. 85 (1992) 235 – 241.
[9] D.A. Dalton, L.M. Baird, L. Langeberg, C.Y. Taugher, W.R. Anyan, C.P. Vance, G. Sarath, Subcellular loca-tion of oxygen defense enzymes in soybean (Glycine max
[L.] Merr.) root nodules, Plant Physiol. 102 (1993) 481 – 489.
[10] S. De Leonardis, G. De Lorenzo, G. Borraccino, S. Dipierro, A specific ascorbate free radical reductase isozyme participates in the regeneration of ascorbate for scavenging toxic oxygen species in potato tuber mito-chondria, Plant Physiol. 109 (1995) 847 – 851.
[11] N. Yamuchi, K. Yamawaki, Y. Ueda, K. Chachin, Sub-cellular location of redox enzymes involving ascorbic acid in cucumber fruit, J. Jpn. Soc. Hort. Sci. 53 (1984) 347 – 353.
[12] G. Borraccino, S. Dipierro, O. Arrigoni, Purification and properties of ascorbate free-radical reductase from potato tubers, Planta 167 (1986) 521 – 526.
[13] M.R. Elia, G. Borraccino, S. Dipierro, Soluble ascorbate peroxidase from potato tubers, Plant Sci. 85 (1992) 17 – 21.
[14] A. Jimenez, J.A. Hernandez, L.A. del Rio, F. Sevilla, Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves, Plant Physiol. 114 (1997) 275 – 284.
[15] M.A. Hossain, Y. Nakano, K. Asada, Monodehy-droascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide, Plant Cell Physiol. 25 (1984) 385 – 395.
[16] U. Heber, C. Miyake, J. Mano, C. Ohno, K. Asada, Monodehydroascorbate radical detected by electron paramagnetic resonance spectrometry is a sensitive probe of oxidative stress in intact leaves, Plant Cell Physiol. 37 (1996) 1066 – 1072.
[17] B.L. Orvar, B.E. Ellis, Transgenic tobacco plants ex-pressing antisense RNA for cytosolic ascorbate peroxi-dase show increased susceptibility to ozone injury, Plant J. 11 (1997) 1297 – 1305.
[18] D.L. Andrews, B. Beames, M.D. Summers, W.D. Park, Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a bac-ulovirus vector, Biochem. J. 252 (1988) 199 – 206. [19] D.B. Dewald, H.S. Mason, J.E. Mullet, The soybean
vegetative storage proteins VSP a and VSP b are acid phosphatases active on polyphosphates, J. Biol. Chem. 267 (1992) 15958 – 15964.
[20] S. Tru¨mper, H. Follmann, I. Ha¨berlein, A novel dehy-droascorbate reductase from spinach chloroplasts ho-mologous to plant trypsin inhibitor, FEBS Lett. 352 (1994) 159 – 162.
[21] W.C. Hou, Y.H. Lin, Dehydroascorbate reductase and monodehydroascorbate reductase activities of trypsin in-hibitors, the major sweet potato (Ipomoea batatas [L.] Lam) root storage protein, Plant Sci. 128 (1997) 151 – 158.
[22] K. Weber, M. Osborn, The reliability of molecular weight determination by dodecyl sulphate – polyacry-lamide gel electrophoresis, J. Biol. Chem. 244 (1969) 4406 – 4412.
[23] K. Kobrehel, J.H. Wong, A. Balogh, F. Kiss, B.C. Yee, B.B. Buchanan, Specific reduction of wheat storage proteins by thioredoxinh, Plant Physiol. 99 (1992) 919 – 924.
[24] I. Yamazaki, L.H. Pette, Mechanism of free radical formation and disappearance during the ascorbic acid oxidase and peroxidase reactions, Biochem. Biophys. Acta 50 (1961) 62 – 69.
[25] J.C. Kaplan, E. Beutler, Electrophoresis of red cell NADH- and NADPH-diaphorases in normal subjects and patients with congenital methemoglobinemia, Biochem. Biophys. Res. Commun. 29 (1967) 605 – 610. [26] W.C. Hou, Y.H. Lin, Activity staining on
polyacry-lamide gels of trypsin inhibitors from leaves of sweet potato (Ipomoea batatas [L.] Lam) varieties, Elec-trophoresis 19 (1998) 212 – 214.
[27] R.S. Conlan, L.A. Griffiths, J.A. Napier, P.R. Shewry, S. Mantel, C. Ainsworth, Isolation and characterization of cDNA clones representing the genes encoding the major tuber storage protein (dioscorin) of yam (Dioscorea cayenensisLam.), Plant Mol. Biol. 28 (1995) 369 – 380. [28] R.S. Conlan, L.A. Griffiths, M. Turner, R. Fido, A.
Tatham, C. Ainsworth, P.R. Shewry, Characterisation of the yam tuber storage protein dioscorin, J. Plant Physiol. 153 (1998) 25 – 31.
[29] M. Maeshima, T. Sasaki, T. Asahi, Characterization of major proteins in sweet potato tuberous roots, Phyto-chemistry 24 (1985) 1899 – 1902.
[30] P.J. Harvey, D. Boulter, Isolation and characterization of the storage protein of yam tuber (Dioscorea rotun
-data), Phytochemistry 22 (1983) 1687 – 1693.
[31] A.V. Zavialov, M. Gaestel, T. Korpela, V.P. Zav’yalov, Thiol/disulfide exchange between small heat shock protein 25 and glutathione, Biochem. Biophys. Acta 1388 (1998) 123 – 132.
[32] W.W. Wells, D.P. Wu, Y. Yang, P.A. Rocque, Mam-malian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase ac-tivity, J. Biol. Chem. 265 (1990) 15361 – 15364.
[33] C.T. Lin, M.T. Lin, Y.T. Chen, J.F. Shaw, Subunit interaction enhances enzyme activity and stability of sweet potato cytosolic Cu/Zn – superoxide dismutase purified by a His-tagged recombinant protein method, Plant Mol. Biol. 28 (1995) 303 – 311.
[34] H.M. Hassan, Determination of microbial damage caused by oxygen free radicals, and the protective role of superoxide dismutase, Methods Enzymol. 105 (1984) 405 – 412.
[35] G. Borraccino, S. Dipierro, O. Arrigoni, Interaction of ascorbate free radical reductase with sulphhydryl reagents, Phytochemistry 28 (1989) 715 – 717.