doi: 10.1152/japplphysiol.01153.2011
113:775-784, 2012. First published 12 July 2012;
J Appl Physiol
I. W. Suranadi, L. Demaison, V. Chaté, S. Peltier, M. Richardson and X. Leverve
contributes to the cardioprotective effect of GIK solution
An increase in the redox state during reperfusion
You might find this additional info useful...
56 articles, 16 of which you can access for free at: This article cites
http://jap.physiology.org/content/113/5/775.full#ref-list-1
including high resolution figures, can be found at: Updated information and services
http://jap.physiology.org/content/113/5/775.full
can be found at: Journal of Applied Physiology
about Additional material and information
http://www.the-aps.org/publications/jappl
This information is current as of September 26, 2012.
http://www.the-aps.org/.
Copyright © 2012 the American Physiological Society. ESSN: 1522-1601. Visit our website at
year (twice monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991. physiology, especially those papers emphasizing adaptive and integrative mechanisms. It is published 24 times a
publishes original papers that deal with diverse area of research in applied Journal of Applied Physiology
at INSERM on September 26, 2012
http://jap.physiology.org/
An increase in the redox state during reperfusion contributes
to the cardioprotective effect of GIK solution
I. W. Suranadi,1* L. Demaison,2,3,5* V. Chaté,3,5S. Peltier,3,5 M. Richardson,4and X. Leverve,3,5†
1Udayana University Faculty of Medicine, Denpasar, Bali, Indonesia;2Institut National de la Recherche Agronomique, Unité
de Nutrition Humaine, Clermont Université, Université d’Auvergne, Clermont-Ferrand, France;3Laboratoire de
Bioénergétique Fondamentale et Appliquée, Université Joseph Fourier, Grenoble, France;4Department of Population Health
Sciences, School of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin; and5Laboratoire de
Bioénergétique Fondamentale et Appliquée, INSERM, U1055, Grenoble, France
Submitted 13 September 2011; accepted in final form 2 July 2012
Suranadi IW, Demaison L, Chaté V, Peltier S, Richardson M, Leverve X.An increase in the redox state during reperfusion contributes to the cardioprotective effect of GIK solution. J Appl Physiol 113: 775–784, 2012. First published July 12, 2012; doi:10.1152/japplphysiol.01153.2011.—This study aimed at deter-mining whether glucose-insulin-potassium (GIK) solutions modify the NADH/NAD⫹
ratio during postischemic reperfusion and whether their cardioprotective effect can be attributed to this change in part through reduction of the mitochondrial reactive oxygen species (ROS) production. The hearts of 72 rats were perfused with a buffer con-taining glucose (5.5 mM) and hexanoate (0.5 mM). They were maintained in normoxia for 30 min and then subjected to low-flow ischemia (0.5% of the preischemic coronary flow for 20 min) followed by reperfusion (45 min). From the beginning of ischemia, the perfus-ate was subjected to various changes: enrichment with GIK solution, enrichment with lactate (2 mM), enrichment with pyruvate (2 mM), enrichment with pyruvate (2 mM) plus ethanol (2 mM), or no change for the control group. Left ventricular developed pressure, heart rate, coronary flow, and oxygen consumption were monitored throughout. The lactate/pyruvate ratio of the coronary effluent, known to reflect the cytosolic NADH/NAD⫹ ratio and the fructose-6-phosphate/
dihydroxyacetone-phosphate (F6P/DHAP) ratio of the reperfused myocardium, were evaluated. Mitochondrial ROS production was also estimated. The GIK solution improved the recovery of mechan-ical function during reperfusion. This was associated with an en-hanced cytosolic NADH/NAD⫹ratio and reduced mitochondrial ROS
production. The cardioprotection was also observed when the hearts were perfused with fluids known to increase the cytosolic NADH/ NAD⫹ratio (lactate, pyruvate plus ethanol) compared with the other
fluids (control and pyruvate groups). The hearts with a high mechan-ical recovery also displayed a low F6P/DHAP ratio, suggesting that an accelerated glycolysis rate may be responsible for increased cytosolic NADH production. In conclusion, the cardioprotection induced by GIK solutions could occur through an increase in the cytosolic NADH/NAD⫹ ratio, leading to a decrease in mitochondrial ROS
production.
cytosolic NADH/NAD⫹ ratio; ischemia-reperfusion; isolated rat
heart; glucose/insulin/potassium; mechanical dysfunction
GLUCOSE-INSULIN-POTASSIUM(GIK) solutions have been shown to
protect the heart in several pathological situations such as acute myocardial infarction, cardioplegia, cardiac surgery, and septic
shock (26, 37). In acute myocardial infarction, they are effi-cient when administrated at the beginning of reperfusion (15, 17, 29, 47) and the ischemic event is moderately severe (53). They decrease the mortality rate by 28% in patients with a reperfused myocardial infarction, particularly in diabetics (44). The beneficial effect of GIK is also observed in animal models (2, 4, 36). Several proposals were made to explain this effect (6): activation of ATP-sensitive potassium (KATP) channels
(34), restoration of the metabolic pools resulting from glucose oxidation (50), reduced reactive oxygen species (ROS) produc-tion (10, 27), and insulin-mediated antiapoptotic activity (21, 28). Except for the restoration of the metabolic pools, the other potential metabolic effects of GIK solutions have not been well studied. Several investigators have shown that GIK solutions shift myocardial energy metabolism from long-chain fatty acids toward glucose as a fuel (18, 33, 41, 48). This could increase the cytosolic NADH/NAD⫹ ratio, since complete glucose degradation to CO2leads to the production of NADH
in the cytosolic space (glycolysis). The high amount of cyto-solic reduced equivalent could then enter the mitochondria and favor the reduction of oxidized glutathione to its reduced form through glutathione reductase. This could thus increase the activity of glutathione peroxidase and improve the ROS scav-enging activity of the mitochondria. The role of a high cyto-solic NADH/NAD⫹ ratio on the recovery of mechanical ac-tivity during postischemic reperfusion has not been fully in-vestigated yet.
The aim of this study was to examine first whether the cardioprotective effect of GIK solutions during reperfusion is associated with an increase in the cytosolic NADH/NAD⫹ ratio, then if this beneficial action is associated with a reduction of the mitochondrial ROS production, and, finally, if altera-tions of the cytosolic NADH/NAD⫹ratio lead to the expected changes in the severity of reperfusion. To answer these ques-tions, we first had to show that the cardioprotective effect of GIK solutions was associated with an increased cytosolic NADH/NAD⫹ ratio. This was performed in the isolated per-fused rat heart subjected to global low-flow ischemia (0.5% of the preischemic coronary flow for 20 min) followed by reper-fusion (45 min). The cytosolic NADH/NAD⫹ ratio was as-sessed by determining the lactate/pyruvate ratio of the coronary effluent (43). Second, we had to show that the GIK solution-induced increase in the cytosolic NADH/NAD⫹ ratio was associated with a decrease in the mitochondrial ROS produc-tion. This was carried out in isolated mitochondria purified from the postischemic myocardium of hearts perfused with or without a GIK solution. Third, we had to demonstrate that * I. W. Suranadi and L. Demaison contributed equally to this work.
† Deceased 8 November, 2010.
Address for reprint requests and other correspondence: L. Demaison, Labo-ratoire de Bioénergétique Fondamentale et Appliquée, INSERM, U1055, Université Joseph Fourier, BP 53, 38041 Grenoble cedex 09, France (e-mail: [email protected]).
J Appl Physiol113: 775–784, 2012. First published July 12, 2012; doi:10.1152/japplphysiol.01153.2011.
8750-7587/12 Copyright©2012 the American Physiological Society
http://www.jappl.org 775
at INSERM on September 26, 2012
http://jap.physiology.org/
different substrates known to modulate the cytosolic NADH/ NAD⫹ratio triggered the expected changes in the recovery of mechanical function during reperfusion. On one hand, the addition of pyruvate (2 mM) to the basal perfusion medium containing glucose (5.5 mM) and hexanoate (0.5 mM) could allow the reduction of the cytosolic NADH/NAD⫹ratio with-out decreasing glucose oxidation and the mitochondrial pro-duction of NADH. If so, this was expected to be associated with a worse mechanical recovery compared with the control group. On the other hand, the addition of lactate (2 mM) or pyruvate (2 mM) plus ethanol (2 mM) to the basal medium could allow the increase in the cytosolic NADH/NAD⫹ratio, perhaps favoring the recovery of mechanical function during reperfusion. Because the cytosolic NADH/NAD⫹ratio mainly depends on the glycolysis rate, the fructose-6-phosphate/dihy-droxyacetone-phosphate (F6P/DHAP) ratio was estimated in the five experimental groups.
MATERIALS AND METHODS
Animal care.The experiments followed the European recommen-dation guidelines for the use of laboratory animals and were approved by the local ethics review board (authorization number: 380537). Seventy-two 3-mo-old male Wistar rats (250 –300 g) were housed in an animal facility with controlled temperature (24°C), hygrometry (60%), and 12:12-h light-dark cycle. They were fed a standard commercial diet (A04; Safe, Gannat, France) and received water ad libitum.
Heart perfusion. All the hearts were perfused during the active phase of the rats (from 12:00 PM to 8:00 AM). Animals were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg) and heparinized (1,000 IU/kg) through the saphenous vein. After a rapid thoracotomy, the heart was quickly collected and immediately immersed in a large volume of cold (4°C) Krebs-Heinselet buffer. The heart was then perfused according to the Langendorff mode (35) with a control Krebs-Heinselet buffer con-taining (in mM) 129 NaCl, 2.4 MgCl2·6H2O, 21 NaHCO3, 4.5 KCl,
2.5 CaCl2·2H2O, and various substrates according to the experiments
performed. The time between chest opening and heart mounting on the Langendorff apparatus never exceeded 1 min, thus limiting the risk of cellular damage or preconditioning. During the perfusion, the buffer was maintained at 37°C and continuously gassed with 95% oxygen-5% CO2 to obtain a stable pH value of 7.4. The perfusion
pressure was maintained at 59 mmHg to ensure a sufficient supply of perfusion medium to the coronary network. A latex balloon connected to a pressure gauge (PowerLab, ADInstruments) was introduced in the left ventricle to allow the estimation of the left ventricle pressure throughout the cardiac cycle. The diastolic pressure was fixed at a value close to 10 mmHg. The heart was then put in a thermostatized chamber (37°C) to allow warming of the organ. The pulmonary artery was cannulated to anaerobically channel the coronary effluent to a chamber in which a Clarke oxygen electrode connected to an oxy-graph (model 203; Instech) allowed measurement of the venous oxygen concentration. The arterial oxygen concentration was mea-sured by collecting the arterial solution in a second chamber. All the parameters of the cardiac function were recorded throughout the experiment and calculated with specific software (PowerLab). The left ventricular developed pressure (LVDP) was calculated as the differ-ence between the systolic and diastolic pressures. The rate-pressure product (RPP) was the product of the LVDP and heart rate. When severe arrhythmia occurred, the heart rate and RPP were considered to be equal to zero. The oxygen consumption was calculated as follows: JO2⫽[(CaO2⫺CvO2)⫻CF]/HW, whereJO2is the oxygen consump-tion (nmol·min⫺1
·g wet wt⫺1
), CaO2 and CvO2 are the arterial and
venous oxygen concentrations (nmol/ml), CF is the coronary flow (ml/min), and HW is the cardiac wet weight (g).
Experimental protocol.In a first set of experiments, 50 rats were randomly divided into 5 groups of 10 animals, and their hearts were perfused with the control Krebs-Heinselet buffer containing glucose (5.5 mM) and hexanoate (0.5 mM) as a hydrosoluble fatty acid for 30 min. After that period, the thermostatized chamber was filled with control Krebs-Heinselet buffer maintained at 37°C, and the hearts were subjected to a 20-min global low-flow ischemia at 0.5% of the preischemic coronary flow. It is known that this level of residual coronary flow is adequate to produce an ischemia of low severity in our experimental conditions. A 45-min reperfusion then followed. At the beginning of the reperfusion, the thermostatized chamber sur-rounding the heart was emptied. During the ischemic and reperfusion periods, the hearts were perfused with one of the five following buffers: 1) control Krebs-Heinselet buffer (C group), 2) control Krebs-Heinselet buffer supplemented with lactate (2 mM; L group), 3) control Krebs-Heinselet buffer supplemented with pyruvate (2 mM; P group),4) control Krebs-Heinselet buffer supplemented with pyru-vate (2 mM) and ethanol (2 mM) (PE group), and5) GIK-like buffer similar to the control buffer except that the KCl and glucose concen-trations were adjusted to 5.6 and 11 mM, respectively, and insulin (10 IU/l) was added.
Aliquots of the coronary effluent were collected throughout (just before ischemia and after 5, 15, 30, and 45 min of reperfusion). They were then stored at ⫺80°C for determination of the lactate and pyruvate concentrations. At the end of the reperfusion period, the hearts were freeze-clamped at the temperature of liquid nitrogen and stored at⫺80°C until the biochemical analyses were performed.
In a second set of experiments, 12 rats were randomly divided into 2 groups of 6 animals, and their hearts were subjected to the same ischemia-reperfusion protocol except that the reperfusion duration was limited to 30 min. The two media used during the ischemia and reperfusion periods were the control medium with glucose (5.5 mM) and hexanoate (0.5 mM) and the GIK-like buffer previously de-scribed. At the end of the 30th minute of reperfusion, the hearts were collected and mitochondria were immediately prepared to determine their oxidative properties and capacities of ROS production.
Finally, in a third set of experiments, 10 hearts were perfused with the control medium with glucose (11 mM) for 20 min. Thereafter, the medium was changed three times by three other media containing one of the following substrates: hexanoate (0.5 mM), lactate (2 mM), or pyruvate (2 mM). Each substrate was perfused for a period of 10 min. The rank in which the substrates were perfused was changed regularly to allow good comparison of the effect of each substrate on the cardiac function. At the end of each period, the heart rate, LVDP, RPP, coronary flow, oxygen consumption, and cardiac metabolic efficiency (RPP-to-oxygen consumption ratio) were evaluated.
Mitochondria preparation.After the 30-min reperfusion, the atria and remaining aorta were cut off from the heart. Myocardium was minced with scissors in a cold isolation buffer composed of (in mM) 150 sucrose, 75 KCl, 50 Tris·HCl, 1 KH2PO4, 5 MgCl2, 1 EGTA, pH
7.4, and fatty acid-free serum albumin (0.2%). The pieces of myocar-dium were rinsed several times on a filter and put in a Potter-Elvehjem homogenizer containing 15 ml of isolation buffer. A protease (0.02% subtilisin) was added to digest myofibrils for 1 min at ice-cold temperature, and the totality was then homogenized (300 rpm, 3– 4 transitions). Subtilisin action was stopped by the addition of isolation buffer (30 ml). The homogenate was then centrifuged (800g, 10 min, 4°C), and the resulting supernatant was collected and filtered. Mito-chondria were then washed through two series of centrifugation (8,000 g, 10 min, 4°C). The last pellet of mitochondria was resus-pended in sucrose (250 mM), Tris·HCl (10 mM), and EGTA (1 mM), pH 7.4, at the approximate concentration of 20 mg/ml.
Respiration measurements.The mitochondrial oxidative phosphor-ylation was determined by evaluating the rate of mitochondrial oxy-gen consumption at 30°C with a Clarke-type O2 electrode. The
at INSERM on September 26, 2012
http://jap.physiology.org/
respiration medium contained (in mM) 125 KCl, 20 Tris·HCl, 3 KH2PO4, 0.05 EDTA, 0.01 CaCl2, pH 7.2, and fatty acid-free bovine
serum albumin (0.15%). All measurements were performed on mito-chondria (0.2 mg mitomito-chondrial protein/ml) incubated with freshly prepared pyruvate (5.5 mM)/malate (2.5 mM) or DL
-palmitoylcarni-tine (50M)/malate (2.5 mM), without or with ADP (100 mM; state III) and then oligomycin (1M; state IV). The incubation medium was constantly stirred with a built-in electromagnetic stirrer and bar flea. Coupling of the mitochondrial oxidative phosphorylation was assessed by the state III/state IV ratio, which measures the degree of
control imposed on oxidation by phosphorylation (respiratory control ratio, RCR).
Mitochondrial ROS release.The rate of mitochondrial production of H2O2 was measured at 30°C following the linear increase in
fluorescence (excitation at 560 nm, emission at 584 nm) due to enzymatic oxidation of Amplex red by H2O2 in the presence of
horseradish peroxidase on a F-2500 computer-controlled Hitachi flu-orometer. Reaction conditions were 0.25 mg/ml mitochondrial pro-tein, 5 U/ml horseradish peroxidase, and 1 M Amplex red, with pyruvate (5.5 mM)/malate (2.5 mM) and/orDL-palmitoylcarnitine (50
Table 1. Evaluation of cardiac function during the preischemic period
C L G P PE ANOVA
Systolic pressure, mmHg 155⫾8 150⫾11 149⫾9 153⫾7 144⫾8 NS
Diastolic pressure, mmHg 6.4⫾0.8 6.5⫾0.6 7.8⫾0.7 7.2⫾0.8 6.9⫾0.3 NS
LVDP, mmHg 148⫾8 144⫾11 139⫾9 146⫾5 138⫾7 NS
Heart rate, beats/min 266⫾10 269⫾8 273⫾11 273⫾9 279⫾7 NS
RPP, mmHg/min 39,110⫾3,077 38,518⫾3,529 37,832⫾1,964 39,624⫾2,190 38,432⫾1,356 NS CF, ml䡠min⫺1䡠g wet wt⫺1 9.7⫾0.5 10.4⫾0.4 9.6⫾0.1 9.8⫾0.3 10.5⫾0.4 NS
Values are means⫾SE. The number of experiments was 10 per group: C, control group; L, lactate group; G, glucose-insulin-potassium (GIK) group; P, pyruvate group; PE, pyruvate⫹ethanol group. LVDP, left ventricular developed pressure; heart rate; RPP, rate-pressure product; CF, coronary flow; ANOVA, analysis of variance; NS, not significant.
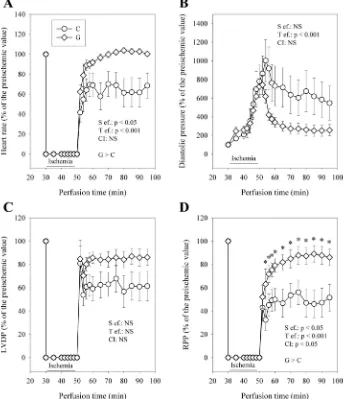
Fig. 1. Influence of glucose-insulin-potassium (GIK) solution on heart rate (A), diastolic pressure (B), left ventricular developed pressure (LVDP;
C), and rate-pressure product (RPP; D) during perfusion with control medium (C) or medium enriched with GIK solution (G). The number of experiments was 10 per group. S ef., substrate effect; T ef., effect of reperfusion duration; CI, cross-interaction between those 2 factors; NS, not significant.
777 GIK Solutions, Cardioprotection, and Redox State • Suranadi IW et al.
J Appl Physiol•doi:10.1152/japplphysiol.01153.2011•www.jappl.org
at INSERM on September 26, 2012
http://jap.physiology.org/
M)/malate (2.5 mM) added to start the reaction in the same incuba-tion media used for measurements of mitochondrial oxygen consump-tion. Mitochondrial ROS were measured in the absence of ADP (state II respiration rate). Rotenone (1M) and antimycin A (0.5M) were sequentially added to determine the maximum rate of H2O2
produc-tion of complexes I and III, respectively, of the respiratory chain. Biochemical analysis.Lactate and pyruvate in the coronary effluent were evaluated spectrophotometrically according to the method of Bergmeyer (5) by using the transformation of each compound in the other by lactate dehydrogenase and estimating the appearance or disappearance of NADH.
F6P and DHAP were assayed in the heart after perchloric acid extraction. The two compounds were evaluated fluorimetrically ac-cording to the methods described by Bergmeyer (5).
Statistical analysis.Results are means⫾SE. The data describing the cardiac functioning and those representing the fluxes of lactate and pyruvate in the coronary effluent were subjected to a repeated-measures analysis of variance with the composition of the ischemic and reperfusion buffer as external factor and the reperfusion time as internal factor. This analysis described the effect of the composition of the perfusion buffer (group effect), that of the reperfusion time (time effect), and the cross-interaction between these two factors. When necessary, the means were compared using a two-way Fisher’s least significant difference test. The results of the myocardial F6P and DHAP contents, mitochondrial function, and perfusion in aerobic conditions with the different substrates were submitted to one-way analysis of variance, describing the effect of the composition of the perfusion buffer (group effect). The means were compared using a one-way Fisher’s least significance difference test. P ⬍ 0.05 was considered significant. All the calculations were performed using NCSS 2007 software.
RESULTS
Cardiac function during the stabilization period.In preisch-emic conditions, all the hearts were perfused with the control buffer. Since the cardiac weight and the temperature of the perfusion fluid were similar in the different groups (general mean ⫽ 1.17 ⫾0.02 g), the cardiac mechanical activity was similar in all the groups (Table 1). Because slight differences existed, however, the results describing the cardiac function
during the reperfusion are expressed as percentages of the preischemic value.
Effect of GIK solution on ischemia and reperfusion dysfunctions. During ischemia, the systolic pressure rapidly dropped to the level of the diastolic pressure until the end of ischemia. From the 5th minute of ischemia, the diastolic pressure then progres-sively increased to a value that was maximal at the end of the
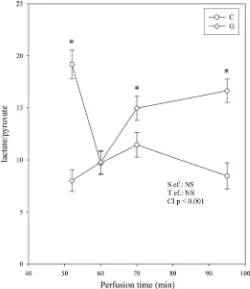
Fig. 3. Influence of GIK solution on the lactate/pyruvate ratio of the coronary effluent. This parameter reflects the cytosolic NADH/NAD⫹ratio. The number
of experiments was 10 per group. *P⬍0.05 indicates a significant difference. Fig. 2. Influence of GIK solution on coronary
flow (A) and myocardial oxygen consump-tion (B) during perfusion. The number of experiments was 10 per group. *P⬍ 0.05 indicates a significant difference.
at INSERM on September 26, 2012
http://jap.physiology.org/
ischemic period (Fig. 1B). The ischemia-induced increase in diastolic pressure was similar in the G and C groups. Con-versely, during reperfusion, the diastolic pressure of the G group was lower compared with that of the C group. This was true from the beginning to the end of the reperfusion with a 53% decrease at the 45th minute of reperfusion. Despite this difference, the recovery of the LVDP did not significantly differ in the two groups, although it tended to be enhanced in the G group (Fig. 1C). The recovery of the heart rate was improved in the G group whatever the duration of reperfusion (⫹46% at the 45th minute of reperfusion, Fig. 1A). Conse-quently, the RPP (Fig. 1D) was significantly higher in the G group throughout the duration of reperfusion (⫹67% at the 45th minute of reperfusion compared with the C group). This improved recovery was not associated with a better coronary flow (Fig. 2A). Instead, this parameter was higher in the C group during the first 6 min of reperfusion (⫹20% compared with the G group). Thereafter, the difference in coronary flow progressively diminished until it became nil. The oxygen consumption behaved differently (Fig. 2B). Although it was lower in the G group during the first 4 min of reflow, it became higher from the 15th minute until the end of reperfusion (⫹16% at the 45th minute of reperfusion). Interestingly, the lactate/pyruvate ratio, reflecting the cytosolic NADH/NAD⫹ ratio, followed roughly the progression of oxygen consumption (Fig. 3). It was lower in the G group at the 5th minute of reperfusion but progressively increased thereafter to reach a significantly higher value from the 15th minute of reperfusion (⫹97% at the 45th minute of reperfusion compared with the C group).
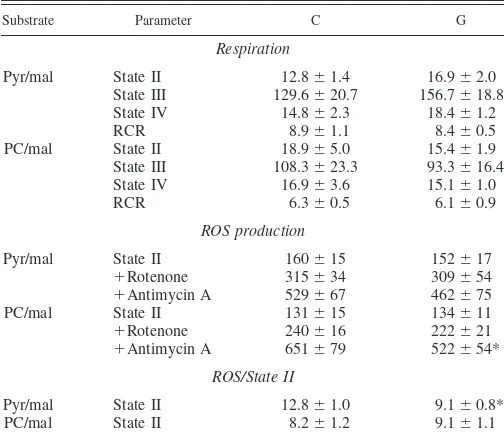
Effect of GIK solution on the function of mitochondria purified from reperfused myocardium.Mitochondria were col-lected from the myocardium of the C and G groups after 30 min of postischemic reperfusion. Their capacities to consume oxygen and to produce ROS are presented in Table 2. As shown by the high RCR value observed with pyruvate (⬃8.5), they were perfectly functioning. The GIK solution did not alter their respiratory parameters. ROS production was also gener-ally unaltered, but a GIK solution-induced decrease in the maximal rate of ROS production at the level of complex III of the respiratory chain (⫺20%) was observed when palmitoyl-carnitine was used as a substrate. Furthermore, the yield of ROS production normalized to the oxygen consumption during state II respiration rate was interestingly reduced by 29% when pyruvate was used as a substrate. This was not the case with palmitoylcarnitine. Similar results were obtained when the yield of ROS production was calculated with state IV respira-tion rate (data not shown).
Effect of changes in the cytosolic redox state on ischemic contracture. At the end of ischemia, the diastolic pressure reached levels ranging from ⬃600 to 1,200% of the preisch-emic value, depending on the group studied (Fig. 4). Interest-ingly, the composition of the perfusion buffer modulated the ischemic contracture. As soon as the 5th minute of ischemia, the P and PE groups displayed higher diastolic pressures than that measured in the C group. This difference was roughly maintained during all the ischemic period. The diastolic pres-sure of the L group was close to that evaluated in the C group and was generally lower than those of the P and PE groups.
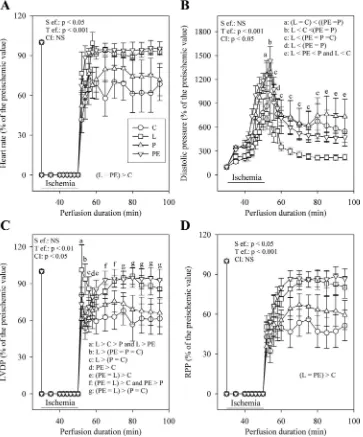
Effect of changes in the cytosolic redox state on reperfusion dysfunctions. As shown in Fig. 5, the cardiac mechanical function partly recovered during reperfusion. The recovery of RPP (Fig. 5D) was different according to the group studied. In the L and PE groups, the RPP was almost as high after stabilization during reperfusion as at the end of the stabilization period. This was not the case for the C group, which displayed a RPP during reperfusion never exceeding 60% of the preische-mic value. Thus the recovery of the RPP in the L and PE Table 2. Function of the mitochondria extracted from
30-min-reperfused myocardium
Values are means⫾SE. The number of experiments was 6 per group: C, control; G, hearts perfused with GIK solution during ischemia and reperfusion. The results of state II, III, and IV respiration rates are expressed in ng atoms O2䡠min⫺1䡠mg mitochondrial protein⫺1, reactive oxygen species (ROS)
pro-duction is expressed in pmol䡠min⫺1䡠mg mitochondrial protein⫺1, and the
ROS/state II ratio is expressed in pmol/ng atoms O2. Pyr/mal, pyruvate/malate;
PC/mal, palmitoylcarnitine/malate; RCR, respiratory control ratio. *P⬍0.05 indicates a significant difference.
Fig. 4. Progression of the diastolic pressure during low-flow ischemia in the control, lactate, pyruvate, and pyruvate plus ethanol groups. The number of experiments was 10 per group. L, lactate-rich medium; P, pyruvate-rich medium; PE, medium enriched with pyruvate plus ethanol.a,b,c,d,eP⬍0.05,
different letters indicate significant differences as represented.
779 GIK Solutions, Cardioprotection, and Redox State • Suranadi IW et al.
J Appl Physiol•doi:10.1152/japplphysiol.01153.2011•www.jappl.org
at INSERM on September 26, 2012
http://jap.physiology.org/
groups was higher than that measured in the C group. The RPP of the P group ranged between those of the three other groups but never differed significantly from them.
The better recovery of the RPP in the L and PE groups compared with the C group was due to an enhancement of recovery of the heart rate (Fig. 5A) and LVDP (Fig. 5C), but also to a reduced diastolic pressure (Fig. 5B), which was particularly low in the L group. As for the RPP, the recovery of the heart rate and LVDP of the P group was intermediary compared with those of the PE, L, and C groups.
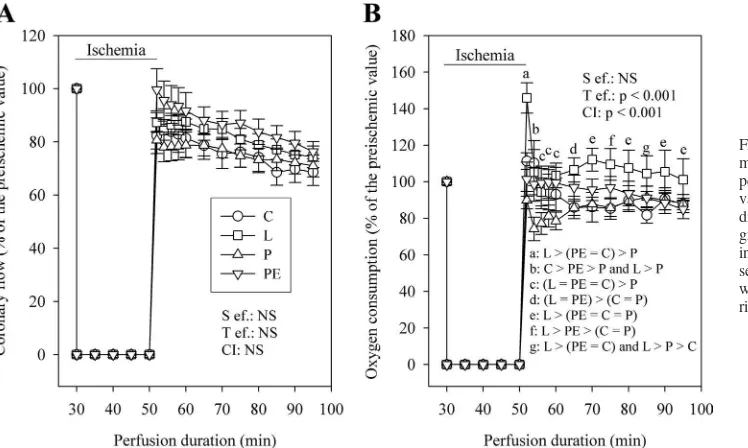
Figure 6Ashows that the recovery of the coronary flow was not responsible for the effect of the perfusion conditions on the restoration of mechanical function. Indeed, the coronary flow was close to the values measured before ischemia in the four groups and never differed from one group to another during postischemic reperfusion. Conversely, the oxygen consump-tion of the reperfused hearts (Fig. 6B) was different in the four groups. At the 2nd minute of reperfusion, the oxygen consump-tion was higher in the L group and lower in the P group
compared with those determined in the PE and C groups. Between the 6th and 10th minutes of reperfusion, the high oxygen consumption of the L group normalized compared with that of the PE and C groups, but that of the P group remained lower. Thereafter, for the remaining duration of reperfusion, the oxygen consumption of the L group became higher again and that of the P group remained normal.
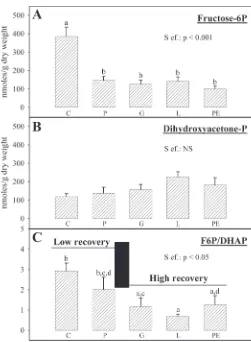
Effects of various interventions on glycolytic intermediates. The levels of F6P and DHAP were evaluated in the myocardial tissue frozen at the end of the reperfusion period (Fig. 7,AandB). F6P levels were similar in the P, PE, L, and G groups and lower compared with that measured in the C group (⫺62, ⫺74,⫺63, and ⫺68% in the P, PE, L, and G groups, respectively). Con-versely, DHAP levels were similar in the five groups studied (Fig. 7B). Interestingly, the F6P/DHAP ratio (Fig. 7C) was high in the C and P groups and low in the L, PE, and G groups.
Quality of various substrates to sustain cardiac mechanical activity. The effects of perfusion with glucose (11 mM), hexanoate (0.5 mM), lactate (2 mM), or pyruvate (2 mM) on Fig. 5. Progression of heart rate (A), diastolic
pressure (B), LVDP (C), and RPP (D) during perfusion with control, lactate-rich, pyruvate-rich, and pyruvate plus ethanol-rich media. The number of experiments was 10 per group.
a,b,c,d,e,f,gP ⬍ 0.05, different letters indicate
significant differences as represented in each panel. Statistical analyses were performed only for the reperfusion period.
at INSERM on September 26, 2012
http://jap.physiology.org/
cardiac function are presented in Table 3. Each substrate similarly sustained the cardiac mechanical activity. The coro-nary flow and myocardial oxygen consumption were also similar in general, except for hexanoate perfusion, which slightly increased these parameters. However, the cardiac met-abolic efficiency was never significantly altered by the various substrates.
DISCUSSION
The aim of this study was to determine whether an increase in the cytosolic NADH/NAD⫹ratio contributed to the benefi-cial action of the GIK solutions during ischemia-reperfusion. The beneficial action of GIK solutions is supposed to occur through different mechanisms, including activation of KATP
channels, restoration of the metabolic pools resulting from glucose oxidation, reduced ROS production, and insulin-me-diated antiapoptotic activity. In the present study, we showed that our GIK solution increased the cytosolic NADH/NAD⫹ ratio during postischemic perfusion, leading to cardioprotec-tion through reduced mitochondrial ROS produccardioprotec-tion.
Strengths and limitations of the study. In our study, we performed 20-min low-flow ischemia. According to the litera-ture and our own experience, this leads, through ischemia and reperfusion, to cellular damage of low severity. This is prob-ably a requirement to observe the influence of GIK solutions and that of changes in the cytosolic NADH/NAD⫹ratio on the severity of ischemia-reperfusion. Indeed, with acute levels of severity, the oxidative stress and cellular death (13) would be high and changes in the cytosolic redox potential would not be able to alter the resulting low cardiac function, a fact that constitutes a limitation of our study.
The experiments performed in this study were carried out in rat hearts collected during the active phase of the animals (dark period). In humans, myocardial ischemia, myocardial infarc-tion, and cardiac death mainly occur during the first hours of the light period (45, 55), when heart rate, blood pressure, and myocardial oxygen consumption are increased compared with what can be measured at nighttime (46). This is explained by
the disequilibrium between a restricted blood supply and the increased myocardial oxygen demand. In the rat, the rhythm is different. Because of the nocturnal activity of this animal, the increase in the heart rate and blood pressure occurs during the dark period (3) and seems related to changes in endocrine and neural influences. The heart itself has an internal circadian clock that is associated with modifications of gene expression, metabolism, and contractile performance (56). We therefore decided to perform our animal study during the dark period, which has the advantage of mimicking the situation occurring during the active phase of humans.
Biochemical assessments of cardiac NADH and NAD⫹are possible, but these two molecules are mainly located in the mitochondria. Estimation of NADH and NAD⫹ thus reflects mainly the mitochondrial pools. To estimate the cytosolic NADH/NAD⫹ ratio, we therefore had to use the lactate/ pyruvate ratio of the coronary effluent. This last parameter reflects the functional equilibrium of lactate dehydrogenase, which depends on the cytosolic redox potential. That is why it is usually used as a marker of the cytosolic NADH/NAD⫹ratio in different organs, including the heart (43). However, it can be estimated only when lactate and pyruvate are not added to the perfusion fluid. In the present study, this was the case for the C and G groups. However, the L, P, and PE groups did contain lactate or pyruvate, making the estimation of the cytosolic NADH/NAD⫹ ratio impossible through the measurement of the lactate/pyruvate ratio. To discuss the effects of the enrich-ment with lactate, pyruvate, or pyruvate plus ethanol, we were only able to hypothesize the expected effects of each substrate. This is a limitation of our study.
Effects of the GIK solution. We showed that the GIK solution is cardioprotective during ischemia-reperfusion. Its effect was characterized by an improved recovery of the RPP, mainly due to an enhanced heart rate. Indeed, in the C group, but not in the G group, severe arrhythmias occurred and estimation of the heart rate was impossible. In this situation when the developed pressure was almost nil, the heart rate and RPP were considered to be zero. Severe arrhythmias are known Fig. 6. Progression of coronary flow (A) and myocardial oxygen consumption (B) during perfusion with control, lactate-rich, pyru-vate-rich, and pyruvate plus ethanol-rich me-dia. The number of experiments was 10 per group. a,b,c,d,e,f,gP ⬍ 0.05, different letters
indicate significant differences as repre-sented in each panel. Statistical analyses were performed only for the reperfusion pe-riod.
781 GIK Solutions, Cardioprotection, and Redox State • Suranadi IW et al.
J Appl Physiol•doi:10.1152/japplphysiol.01153.2011•www.jappl.org
at INSERM on September 26, 2012
http://jap.physiology.org/
to result from heterogeneity between the different cardiomyo-cytes, which can be healthy or severely damaged. The effect was associated with increased oxygen consumption and re-duced F6P/DHAP ratio. This strongly suggests that the con-version of glucose to CO2was increased, perhaps through the
effect of insulin. This reaction is able to produce NADH in the mitochondria through the conversion of pyruvate to CO2, but
also in the cytosol through the glycolytic pathway. As a matter of fact, the lactate/pyruvate ratio of the coronary effluent was progressively increased during reperfusion until it reached values much higher than those measured in the C group. This strongly suggests that the GIK solution increased the cytosolic NADH/NAD⫹ ratio (43). Such an improvement could act
locally at the level of key enzymes such as creatine kinases, favoring the energy transfer and restoration of the adequate ionic equilibrium. Moreover, cytosolic NADH can enter the mitochondria, where it can be converted into NADPH via several enzymes (49). The surplus mitochondrial NADPH can then improve the detoxification of ROS, particularly those produced during fatty acid oxidation, by increasing the reduced glutathione pool. Indeed, we reported that our GIK solution reduced ROS production by mitochondria purified from reper-fused myocardium. This was true for palmitoylcarnitine as an oxidative phosphorylation substrate for the maximal rate of ROS production at the level of complex III of the respiratory chain. This was also true for pyruvate during state II respiration rate, since the yield of ROS production normalized to the oxygen consumption was reduced. This GIK solution-induced low mitochondrial ROS production could prevent the opening of the PTP (permeability transition pore) and could account for an antiapoptotic effect previously attributed to the effect of insulin (21, 28).
Effects of lactate addition in the control medium.We tested the effects of a medium expected to increase the cytosolic NADH/NAD⫹ ratio. This medium contained the same oxida-tive substrates as the control medium (5.5 mM glucose and 0.5 mM hexanoate), but it was also enriched with 2 mM lactate. It was expected to increase the cytosolic NADH/NAD⫹ ratio through the conversion of lactate to pyruvate by cytosolic lactate dehydrogenase. Unfortunately, the high level of lactate added to the perfusion fluid prevented the estimation of that ratio through the assessment of the lactate/pyruvate ratio. Lactate addition to the medium significantly improved the recovery of the heart rate, diastolic pressure, LVDP, and RPP compared with the control group. Yet, lactate does not seem to have had a direct effect on the myocardium, since lactate per se in the perfusion fluid sustains the cardiac function in aerobic conditions as well as glucose. The effect of that substrate on the NADH/NAD⫹ ratio was thus rather involved in the car-dioprotection.
The literature reports interesting information concerning the action of lactate. In fact, the beneficial effect of lactate ob-served in our study was surprising compared to what has been published in the literature. Indeed, lactate has a reputation for being either inefficient (22, 54) or even deleterious (9, 11, 12, 14) during ischemia-reperfusion. Only one study has reported the beneficial effect of lactate during postischemic reperfusion in the isolated perfused heart (23). However, all the studies available in the literature have considered the effect of lactate in the absence of free fatty acids in the perfusion buffer, which does not constitute a physiological situation. In vivo studies testing the effect of lactate during reperfusion were in favor of a positive action of this substrate (51, 52). In our study, we Fig. 7. Influence of the different media on the contents of fructose-6-phosphate
(fructose-6P;A) and dihydroxyacetone-phosphate (dihydroxyacetone-P;B) as well as the fructose-6P/dihydroxyacetone-P ratio (F6P/DHAP; C) in the myocardium at the end of the reperfusion period. The number of experiments was 10 per group.a,b,c,dP⬍0.05, different letters indicate significant
differ-ences as represented in each panel.
Table 3. Myocardial function during heart perfusion with various substrates
Glucose Lactate Pyruvate Hexanoate
Heart rate, beats/min 274⫾14 282⫾15 280⫾7 270⫾7
LVDP, mmHg 129⫾6 130⫾6 130⫾6 136⫾6
RPP, mmHg/min 35,602⫾2,741 36,846⫾2,764 36,225⫾1,423 36,618⫾1,654
CF, ml䡠min⫺1䡠g wet wt⫺1 12.1⫾0.9 12.3⫾0.6 12.0⫾0.7 13.5⫾0.6*
O2consumption,mol䡠min⫺1䡠g wet wt⫺1 8.3⫾0.9 8.9⫾0.8 9.0⫾0.6 9.8⫾0.6*
Metabolic efficiency, mmHg/mol 4,098⫾503 3,876⫾390 3,953⫾328 3,534⫾275
Values are means⫾SE. The number of experiments was 10 per group. *P⬍0.05 indicates a significant difference.
at INSERM on September 26, 2012
http://jap.physiology.org/
added hexanoate, a short-chain fatty acid, as a substrate for myocardial energy production. We believe that the presence of hexanoate in the perfusion buffer was essential in the observed beneficial effect of lactate. Excessive lipid oxidation has ad-verse effects during reperfusion (1, 7, 24, 32, 39, 40, 42, 57). Lactate could partly suppress the deleterious effect of exces-sive fatty acid oxidation. In favor of this theory, we observed that the F6P/DHAP ratio was reduced in the lactate group compared with the control group. This suggests an acceleration of the glycolysis and, perhaps, an activation of the glucose oxidation. Perfusion of the lactate-rich medium also increased the myocardial oxygen consumption compared with that ob-tained with the control buffer. This is in agreement with the increased recovery of the RPP observed in that group during reperfusion and also with a higher glucose oxidation rate, since increasing the cardiac mechanical work is known to boost the rate of glucose oxidation (31).
Effects of a solution expected to decrease the cytosolic NADH/NAD⫹ ratio.We also tested the supplementation with pyruvate (2 mM). The addition of that substrate could increase the supply of substrate directly oxidizable by the mitochondrial pyruvate dehydrogenase. Yet, pyruvate per se sustained cardiac function similarly to lactate, indicating comparable energizing capacities. However, it could decrease the cytosolic NADH/ NAD⫹ ratio through the action of lactate dehydrogenase. Compared with the control buffer, the supplementation with 2 mM pyruvate did not improve the recovery of the diastolic pressure, heart rate, LVDP, and RPP during reperfusion. This confirms that the extra carbon moieties supplied by the addition of pyruvate had no effect. Furthermore, in contrast with the media expected to increase the cytosolic NADH/NAD⫹ratio, the buffer containing pyruvate augmented the F6P/DHAP ratio, suggesting that the glycolytic pathway was not activated. In past years, pyruvate was considered to be beneficial during ischemia-reperfusion (8, 14, 16, 19), although some investiga-tors reported no effect of this substrate (9, 22, 25). In the present study, we did not see any beneficial action of pyruvate addition. As mentioned by Johnston and Lewandowski (30), the presence of hexanoate, a lipid substrate directly utilizable by the mitochondrial -oxidation pathway (38), in the perfu-sion media may have contributed to attenuate the beneficial effect of pyruvate.
Effect of ethanol addition in the pyruvate-rich medium.We finally increased the cytosolic NADH/NAD⫹ ratio by supple-menting the pyruvate-rich medium with ethanol (2 mM). Eth-anol is known to be oxidized by the cytosolic alcohol dehy-drogenase and to produce reduced equivalents through that reaction. The beneficial effect of pyruvate plus ethanol was slightly different from that observed with lactate. Indeed, the increased recovery of the RPP was also associated with a reduction of the F6P/DHAP ratio, but the diastolic pressure and oxygen consumption were not improved. The difference might be due to the type of reduced equivalent produced. In the heart, it seems that the alcohol dehydrogenase forms NADPH rather than NADH (20). That reduced equivalent could also enter the mitochondria and reinforce the enzymatic antioxidative prop-erties. However, it could also have a local action by preventing the oxidative stress-induced inhibition of key enzymes in the cytosol. For example, creatine kinase is known to be reversibly inhibited when the redox ratio is low. Since this enzyme plays a crucial role in the energy transfer, the restoration of its
activity through an adequate redox potential could favor the recovery of the cardiac cells to a normal physiology.
Conclusion. Our study indicates that the GIK solutions increased the cytosolic NADH/NAD⫹ ratio during postisch-emic reperfusion. As indicated by the addition of various substrates modulating this ratio, this effect could be partly responsible for the cardioprotective action of GIK solutions. The high amount of NADH produced could enter the mito-chondria and stimulate the enzymatic defenses against ROS overproduction, leading to an antiapoptotic effect. However, other mechanisms including stimulation of KATPchannels and
recovery of the metabolic pools could also be involved.
ACKNOWLEDGMENTS
We thank Christophe Cottet for accurate reading of the manuscript and correction of the English language.
GRANTS
This work was financially supported by Institut National de la Recherche Agronomique (INRA), National Institute of Health and Medical Research (INSERM), and the Joseph Fourier University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
I.W.S., L.D., V.C., and S.P. performed experiments; L.D. and X.M.L. conception and design of research; L.D. analyzed data; L.D. and M.R. interpreted results of experiments; L.D. prepared figures; L.D. drafted manu-script; L.D. edited and revised manumanu-script; L.D. and M.R. approved final version of manuscript.
REFERENCES
1. Allely MC, Alps BJ.Prevention of myocardial enzyme release by ranolazine in a primate model of ischaemia with reperfusion.Br J Pharmacol99: 5–6, 1990.
2. Angelos MG, Murray HN, Gorsline RT, Klawitter PF.Glucose, insulin and potassium (GIK) during reperfusion mediates improved myocardial bioenergetics.Resuscitation55: 329 –336, 2002.
3. Anigbogu CN, Williams DT, Brown DR, Silcox DL, Speakman RO, Brown LC, Karounos DG, Randall DC.Circadian variations in blood pressure, heart rate, and HR-BP cross-correlation coefficient during pro-gression of diabetes mellitus in rat.Int J Hypertens2011: 738689, 2011. 4. Bellows SD, Kloner RA. Glucose-insulin-potassium does not reduce myocardial infarct size in an ischemic/reperfusion rabbit model.J Thromb Thrombolysis5: 25–27, 1998.
5. Bergmeyer HU.(editor).Methods of Enzymatic Analysis. London: Aca-demic, 1974.
6. Bertuglia S, Giusti A, Fedele S, Picano E.Glucose-insulin-potassium treatment in combination with dipyridamole inhibits ischaemia-reperfu-sion-induced damage.Diabetologia44: 2165–2170, 2001.
7. Black SC, Gralinski MR, McCormack JG, Driscoll EM, Lucchesi BR. Effect of ranolazine on infarct size in a canine model of regional myo-cardial ischemia/reperfusion.J Cardiovasc Pharmacol24: 921–928, 1994. 8. Bunger R, Mallet RT, Hartman DA.Pyruvate-enhanced phosphoryla-tion potential and inotropism in normoxic and postischemic isolated working heart. Near-complete prevention of reperfusion contractile fail-ure.Eur J Biochem180: 221–233, 1989.
9. Cross HR, Clarke K, Opie LH, Radda GK.Is lactate-induced myocar-dial ischaemic injury mediated by decreased pH or increased intracellular lactate?J Mol Cell Cardiol27: 1369 –1381, 1995.
10. Das UN.Insulin: an endogenous cardioprotector.Curr Opin Crit Care9: 375–383, 2003.
11. De Groot MJ, Coumans WA, Van Der Vusse GJ. The nucleotide metabolism in lactate perfused hearts under ischaemic and reperfused conditions.Mol Cell Biochem118: 1–14, 1992.
12. De Groot MJ, Van Der Vusse GJ.The effects of exogenous lactate and pyruvate on the recovery of coronary flow in the rat heart after ischaemia.
Cardiovasc Res27: 1088 –1093, 1993.
783 GIK Solutions, Cardioprotection, and Redox State • Suranadi IW et al.
J Appl Physiol•doi:10.1152/japplphysiol.01153.2011•www.jappl.org
at INSERM on September 26, 2012
http://jap.physiology.org/
13. Demaison L, Moreau D, Vergely-Vandriesse C, Grégoire S, Degois M, Rochette L.Effects of dietary polyunsaturated fatty acids and hepatic steatosis on the functioning of isolated working rat heart under normoxic conditions and during post-ischemic reperfusion.Mol Cell Biochem224: 103–116, 2001.
14. Dennis SC, Hearse DJ, Coltart DJ.A model for the generation of sponta-neous yet predictable ventricular arrhythmias.Eur J Cardiol12: 377–389, 1981.
15. Díaz R, Paolasso EA, Piegas LS, Tajer CD, Moreno MG, Corvalán R, Isea JE, Romero G.Metabolic modulation of acute myocardial infarc-tion. The ECLA (Estudios Cardiologicos LatinoAmerica) Collaborative Group.Circulation98: 2227–2234, 1998.
16. Dobsak P, Courderot-Masuyer C, Zeller M, Vergely C, Laubriet A, Assem M, Eicher JC, Teyssier JR, Wolf JE, Rochette L.Antioxidative properties of pyruvate and protection of the ischemic rat heart during cardioplegia.J Cardiovasc Pharmacol34: 651–659, 1999.
17. Doenst T, Bothe W, Beyersdorf F.Therapy with insulin in cardiac surgery: controversies and possible solutions.Ann Thorac Surg75: S721–S728, 2003. 18. Elvenes OP, Korvald C, Irtun O, Larsen T, Sorlie D.Both glucose-insulin-potassium and glutamine in warm blood cardioplegia increase the rates of myocardial glucose and free fatty acid oxidation.Scand Cardio-vasc J36: 19 –26, 2002.
19. Flood A, Hack BD, Headrick JP.Pyruvate-dependent preconditioning and cardioprotection in murine myocardium.Clin Exp Pharmacol Physiol
30: 145–152, 2003.
20. Forsyth GW, Nagasawa HT, Alexander CS.Ethanol metabolism by the rat heart and alcohol dehydrogenase activity.Can J Biochem54: 539 –545, 1976. 21. Gao F, Shi DW, Wang XM, Dong L, Wang YM, Ma XL.Effects of glucose-insulin-potassium cocktail on cardiac myocyte death and post-ischemic recovery cardiac functional recovery: the critical role of insulin.
Zhonghua Nei Ke Za Zhi42: 148 –152, 2003.
22. Griffin JL, White LT, Lewandowski ED.Substrate-dependent proton load and recovery of stunned hearts during pyruvate dehydrogenase stimulation.Am J Physiol Heart Circ Physiol279: H361–H367, 2000. 23. Goodwin GW, Taegtmeyer H.Metabolic recovery of isolated working
rat heart after brief global ischemia.Am J Physiol Heart Circ Physiol267: H462–H470, 1994.
24. Gralinski MR, Black SC, Kilgore KS, Chou AY, Mccormack JG, Lucchesi BR.Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart.Cardiovasc Res28: 1231–1237, 1994. 25. Gutterman DD, Chilian WM, Eastham CL, Inou T, White CW,
Marcus ML.Failure of pyruvate to salvage myocardium after prolonged ischemia.Am J Physiol Heart Circ Physiol250: H114 –H120, 1986. 26. Hachida M, Ookado A, Nonoyama M, Koyanagi H.Effect of HTK
solution for myocardial preservation. J Cardiovasc Surg (Torino) 37: 269 –274, 1996.
27. Hess ML, Okabe E, Poland J, Warner M, Stewart JR, Greenfield LJ. Glucose, insulin, potassium protection during the course of hypothermic global ischemia and reperfusion: a new proposed mechanism by the scavenging of free radicals.J Cardiovasc Pharmacol5: 35–43, 1983. 28. Jonassen AK, Brar BK, Mjos OD, Sack MN, Latchman DS, Yellon
DM.Insulin administered at reoxygenation exerts a cardioprotective effect in myocytes by a possible anti-apoptotic mechanism.J Mol Cell Cardiol
32: 757–764, 2000.
29. Jonassen AK, Aasum E, Riemersma RA, Mjos OD, Larsen TS. Glucose-insulin-potassium reduces infarct size when administered during reperfusion.Cardiovasc Drugs Ther14: 615–623, 2000.
30. Johnston DL, Lewandowski ED.Fatty acid metabolism and contractile function in the reperfused myocardium. Multinuclear NMR studies of isolated rabbit hearts.Circ Res68: 714 –725, 1991.
31. Kobayashi K, Neely JR.Mechanism of pyruvate dehydrogenase activa-tion by increased cardiac work.J Mol Cell Cardiol15: 369 –382, 1983. 32. Korb H, Hoeft A, Hunneman DH, Schraeder R, Wolpers HG, Wober
W, Hellige G.Changes in myocardial substrate utilisation and protection of ischemic stressed myocardium by oxfenicine [(S )-4-hydroxyphenylg-lycine].Naunyn Schmiedebergs Arch Pharmacol327: 70 –74, 1984. 33. Korvald C, Elvenes OP, Myrmel T.Myocardial substrate metabolism
influences left ventricular energetics in vivo.Am J Physiol Heart Circ Physiol278: H1345–H1351, 2000.
34. Ladisa JF, Krolikowski JG, Pagel PS, Warltier DC, Kersten JR. Cardioprotection by glucose-insulin-potassium: dependence on K-ATP channel opening and blood glucose concentration before ischemia.Am J Physiol Heart Circ Physiol287: H601–H607, 2004.
35. Langendorff O. Untersuchungen am überlebenden Saügetierherzen.
Pflügers Arch61: 291–332, 1895.
36. Lazar HL, Zhang X, Rivers S, Bernard S, Shemin RJ. Limiting ischemic myocardial damage using glucose-insulin-potassium solutions.
Ann Thorac Surg60: 411–416, 1995.
37. Lazar HL.Enhanced preservation of acutely ischemic myocardium and improved clinical outcomes using glucose-insulin-potassium (GIK) solu-tions.Am J Cardiol80: 90A–93A, 1997.
38. Longnus SL, Wambolt RB, Barr RL, Lopaschuk GD, Allard MF. Regulation of myocardial fatty acid oxidation by substrate supply.Am J Physiol Heart Circ Physiol281: H1561–H1567, 2001.
39. Lopaschuk GD, McNeil GF, McVeigh JJ.Glucose oxidation is stimu-lated in reperfused ischemic hearts with the carnitine palmitoyltransferase 1 inhibitor, etomoxir.Mol Cell Biochem88: 175–179, 1989.
40. Lopaschuk GD, Barr R, Thomas PD, Dyck JR.Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme A thiolase.Circ Res93: 33–37, 2003.
41. Marzilli M.Management of ischaemic heart disease in diabetic patients. Is there a role for cardiac metabolic agents?Curr Med Res Opin17: 153–158, 2001.
42. McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused isch-emic rat hearts.Circulation93: 135–142, 1996.
43. Nuutinen EM.Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol79: 49 –58, 1984.
44. Pache J, Kastrati A, Mehilli J, Bollwein H, Ndrepepa G, Schuhlen H, Martinoff S, Seyfarth M, Nekolla S, Dirschinger J, Schwaiger M, Schömig A.A randomized evaluation of the effects of glucose-insulin-potassium infusion on myocardial salvage in patients with acute myocar-dial infarction treated with reperfusion therapy.Am Heart J148: e3, 2004. 45. Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Circadian variations in the occurrence of cardiac arrests: initial and repeat episodes.
Circulation98: 31–39, 1998.
46. Pepine CJ.Circadian variations in myocardial ischemia. Implications for management.JAMA265: 386 –390, 1991.
47. Satler LF, Green CE, Kent KM, Pallas RS, Pearle DL, Rackley CE. Metabolic support during coronary reperfusion.Am Heart J114: 54 –58, 1987.
48. Schofield RS, Hill JA.Role of metabolically active drugs in the manage-ment of ischemic heart disease.Am J Cardiovasc Drugs1: 23–35, 2001. 49. Singh R, Lemire J, Mailloux RJ, Appana VD.A novel strategy involved anti-oxidative defense: the conversion of NADH into NADPH by a metabolic network.PLoS One3: 1–7, 2008.
50. Taegtmeyer H, Goodwin GW, Doenst T, Frazier OH.Substrate me-tabolism as a determinant for postischemic functional recovery of the heart.Am J Cardiol80: 3A–10A, 1997.
51. Teoh KH, Mickle DA, Weisel RD, Madonik MM, Ivanov J, Harding RD, Romaschin AD, Mullen JC.Improving myocardial metabolic and functional recovery after cardioplegic arrest.J Thorac Cardiovasc Surg
95: 788 –798, 1988.
52. Teoh KH, Mickle DA, Weisel RD, Madonik MM, Ivanov J, Harding RD, Romaschin AD, Wilson GJ, Mullen JC. The effect of lactate infusion on myocardial metabolism and ventricular function following ischemia and cardioplegia.Can J Cardiol6: 38 –46, 1990.
53. Van Der Horst IC, Zijlstra F, Van’t Hof AW, Doggen CJ, De Boer MJ, Suryapranata H, Hoorntje JC, Dambrink JH, Gans RO, Bilo HJ. Glucose-insulin-potassium infusion in patients treated with primary an-gioplasty for acute myocardial infarction: the glucose-insulin-potassium study: a randomized trial.J Am Coll Cardiol42: 784 –791, 2003. 54. White LT, O’Donnell JM, Griffin J, Lewandowski ED.Cytosolic redox
state mediates postischemic response to pyruvate dehydrogenase stimula-tion.Am J Physiol Heart Circ Physiol277: H626 –H634, 1999. 55. Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE.
In-creased onset of sudden cardiac death in the first three hours after awakening.Am J Cardiol70: 1, 1992.
56. Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes.J Mol Cell Cardiol34: 223–231, 2002.
57. Zacharowski K, Blackburn B, Thiemermann C.Ranolazine, a partial fatty acid oxidation inhibitor, reduces myocardial infarct size and cardiac troponin T release in the rat.Eur J Pharmacol418: 105–110, 2001.
at INSERM on September 26, 2012
http://jap.physiology.org/