Study catalytic oxidation of
α
-pinene
using hydrogen peroxide-iron(III) chloride
Merry Pradhita, Masruri*, Mohammad Farid Rahman
aDepartment of Chemistry, Brawijaya University, Jl. Veteran Malang 65145, Indonesia
Abstract
Indonesia has abundance source of alpha-pinene, and mainly can be isolated from turpentine oils. This oils itself, was harvested from pine tree (Pinus merkusiiJunh. & de Vr). This paper reveals the recent investigation on oxidation of -pinena using hydrogen peroxide catalyzed by iron(III) chloride. Meanwhile the end objective of this study basically was derivatization of -pinene to provide basic and fine chemicals for industry and pharmaceuticals. A green oxidator, hydrogen peroxide, commonly provided epoxide group to the alkene double bond of -pinene. However, it was found a diverse oxidation product was observed during reaction catalyzed by iron(III) chloride. Analysis of the oxidation product was performed by using gas chromatography-mass spectrometry and infrared spectrophotometry.
Keywords -pinene, carvone, catalytic, oxidation, turpentine oil
1. Introduction
Indonesia as a tropical country has wide area for forestry. And also, its natural products and natural oils harvested from forestry is an indispensable product including the essential oils. Pine forestry generally grows species of Pine merkusii Junh. & de Vr, along island of Indonesia and produces turpentine oils beside its gum rosin. It was reported in 2013, total production was recorded about 11,851 ton (Perum Perhutani, 2014). It was predicted increase by ten during a decade. However, this production was exported directly as raw turpentine oils.
-pinene contains in turpentine oils as major component. Recent investigation reported that -pinene composed turpentine oil harvested from local forestry in average 88% from total component (Amini et al., 2014). This high composition leads to purification of -pinene of turpentine oil for further application (Masruri et al., 2007). Previous result described -pinena has activity to inhibit bacterial growth and also possible for antiseptic (Masruri et al., 2007; Leite et al., 2007), bioactivity as anti-inflammation in neuronal cell (Khotimah et al., 2006). Besides, it was also reported application -pinene as starting material in organic synthetic for adhesive, finishing product, ink, and perfumery (Sarwar, 2012).
Transformation of -pinene into fine chemicals by oxidation reaction was also reported by other researcher. It applied various different oxidation agents. For example, hydrogen peroxide (H2O2) using some catalyst such as titanium(VI), iron(III), zirconium(IV), titanium-supported silica, and solid acid (H5PW11TiO40). Some products were reported such as verbenol (4-9%), verbenone (2-10%), dan campholenic aldehyde (2-9%) (Maksimchuket
al., 2005). Reaction was generally undertaken in room temperature until 50oC (Maksimchuk et al., 2005). However, low selectivity and yield was always reported (see Figure 1).
Figure 1.Scheme of oxidation reaction of -pinene.
Oxidation using hydrogen peroxide is interesting process since the by-product resulted is water. It is known as a green oxidator. It has efficiency of oxygen atom to convert organic substances by 47% (Noyori et al., 2003). According to Hasan and co-worker, oxidation using hydrogen peroxide is economically and ecologically advantageous. It was easily to manage, handle, low cost, and only provided water as by product (Hasan et al., 2011). Thus, applying hydrogen peroxide basically follows the principles of green oxidation. This study will report oxidation of -pinene using hydrogen proxide and catalyzed by iron(III) chloride. The result was expected can open the way to control selectivity and further application of Indonesian turpentine oils.
2. Materials and methods
2.1
Materials
The materials used for research including turpentine oils (given from PT Perhutani Anugerah Kimia, Trenggalek, East Java). Procedure and method for -pinene purification was undertaken following Masruri et al. (2007). Other materials was some chemicals used as bought from the manufacturer or as mentioned, including hydrogen peroxide 30% (Merck), iron(III) chloride (Merck), ethyl acetate (Smart Lab), n-hexane (Smart Lab), magnesium sulfate anhydrate (Merck), pre-coated silica TLC F60 (Merck), and aquadest.
2.2
Instrumentation and glassware
Some instrumentation for analysis was used such as ultraviolet-visible spectrophotometer (Shimadzu UV-1601), infrared spectrophotometer (Shimadzu FTIR-8400S), and gas chromatography-mass spectrometry (Shimadzu GCMS-QP2010S), refractometer (Abbe), analytical balance (Ohaus), and magnetic stirrer-heater. Meanwhile some glassware applied such as a set of vacuum fractional distillation, pycnometer, TLC development glass, and other minor glassware.
2.3
Procedure for distillation of turpentine oils
2.4
Procedure for study oxidation reactions
A pure of -pinene (4.85 mL; 30.0 mmol) in a 100-mL of round bottom flask was added iron(III) chloride (8.11 g; 30.0 mmol). This mixture was setting up on the reflux apparatus, and further added dropwise of with hydrogen peroxide 30% (30.67 mL; 300 mmol). This was undertaken during 10 min, and it was further stirring at 80 oC for 2 h. Reaction progress was monitor by spotting the reaction sampling on TLC. Disappearing of -pinene on TLC plate as indication that reaction completed. Then, the reaction mixture was washed with water (2 x 10 mL), and was extracted with ethyl acetate (3 x 10 mL). Combined the ethyl acetate layers was dried under magnesium sulfate anhydrate and decanted for further concentrated using rotary evaporator. The product was further analyzed and haracterized by using GCMS and FTIR.
3. Results and discussion
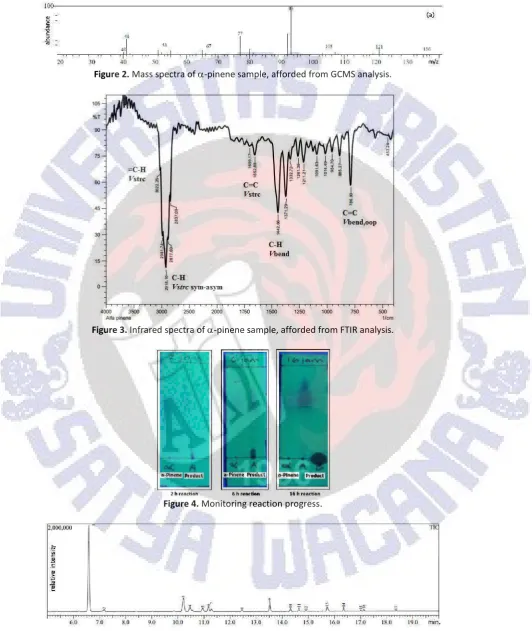
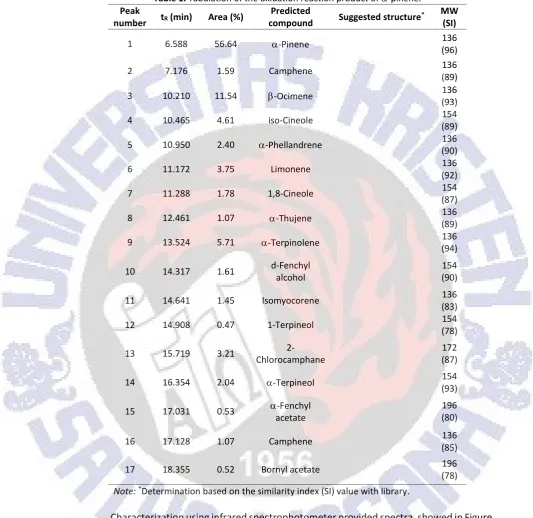
Isolation of-pinene from turputine oils was undertaken under vacuum condition. It was recorded the pressure scale at 0 mmHg, and first fraction boiled at 30oC, and-pinene was detected in this fraction as major component. It was a clear oils, fresh turpentine oil aroma, density 0.8400 mL/g (28oC), index refractive 1.4635 (26.3oC). It was also found that characteristic both GCMS (Figure 2) and FTIR (Figure 3) spectra data very similar to that reported by Masruri et al. (2007) and Amini et al. (2014). Mass spectra detected m/z 136 for molecule ion of-pinene. Further fragmentation pattern provided mass fragments m/z 121, 105, 93 (base peak), 77, 67, 53, and 41, respectively. This pattern as indication for defragmented of alkyl group from molecular ion of -pinene. Meanwhile from the chromatogram, it was found the -pinene had 91.01% purity (chromatogram did not reported, see Masruri et al., 2007). In addition, the infrared spectra gave important bands for functional group in-pinene such as alkene (=C-Hstretching, C=Cstretching, C=Cbendingvibration) and alkyl group (C-Hstretchingand C-Hbendingvibration). By this result, the research applied-pinene isolated from turpentine as starting material for study oxidation reaction using hydrogen peroxide catalyzed by iron(III) chloride.
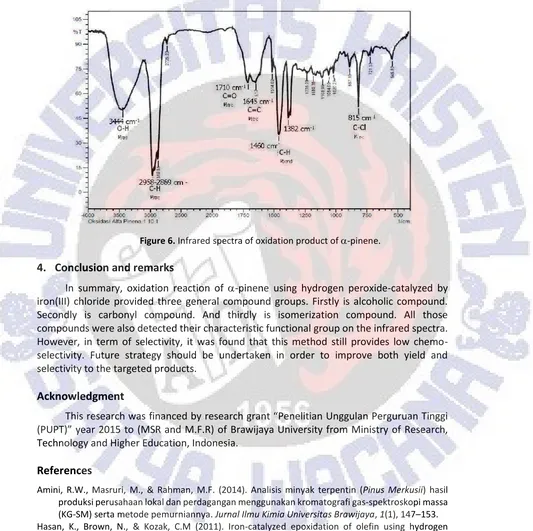
Oxidation of -pinene using hydrogen peroxide, as previously reported by Maksimchuk et al. (2005), provided verbenol, verbenone, and camphonelic aldehyde (Figure 1). Reaction was undertaken at 50oC for 5 h reaction. In the investigation, oxidation of -pinene used mol equivalence of hydrogen peroxide 1.0, and applied reaction in reflux condition. Iron(III) chloride was mixed first before addition of oxidant. In general, reaction completed after 16 h stirring (Figure 4).
Figure 2.Mass spectra of-pinene sample, afforded from GCMS analysis.
Figure 3.Infrared spectra of-pinene sample, afforded from FTIR analysis.
Figure 4.Monitoring reaction progress.
Table 1.Tabulation of the oxidation reaction product of-pinene.
Peak
number tR(min) Area (%) compoundPredicted Suggested structure* MW(SI)
1 6.588 56.64 -Pinene (96)136
2 7.176 1.59 Camphene (89)136
3 10.210 11.54 -Ocimene (93)136
4 10.465 4.61 iso-Cineole (89)154
5 10.950 2.40 -Phellandrene (90)136
6 11.172 3.75 Limonene (92)136
7 11.288 1.78 1,8-Cineole (87)154
8 12.461 1.07 -Thujene (89)136
9 13.524 5.71 -Terpinolene (94)136
10 14.317 1.61 d-Fenchylalcohol (90)154
11 14.641 1.45 Isomyocorene (83)136
12 14.908 0.47 1-Terpineol (78)154
13 15.719 3.21 Chlorocamphane2- (87)172
14 16.354 2.04 -Terpineol (93)154
15 17.031 0.53 acetate-Fenchyl (80)196
16 17.128 1.07 Camphene (85)136
17 18.355 0.52 Bornyl acetate (78)196
Note:*Determination based on the similarity index (SI) value with library.
isomyocorene. Besides that, infrared spectra also recorded the the presence of alkyl group existed in every molecules. Bands recorded in between 2958 and 2869 cm-1characteristic for stretching vibration symmetry and asymmetry of C-H alkyl. And also, their bending vibration in 1460 and 1382 cm-1was clearly come up.
Figure 6.Infrared spectra of oxidation product of-pinene.
4. Conclusion and remarks
In summary, oxidation reaction of -pinene using hydrogen peroxide-catalyzed by iron(III) chloride provided three general compound groups. Firstly is alcoholic compound. Secondly is carbonyl compound. And thirdly is isomerization compound. All those compounds were also detected their characteristic functional group on the infrared spectra. However, in term of selectivity, it was found that this method still provides low chemo-selectivity. Future strategy should be undertaken in order to improve both yield and selectivity to the targeted products.
Acknowledgment
This research was financed by research grant Penelitian Unggulan Perguruan Tinggi (PUPT) year 2015 to (MSR and M.F.R) of Brawijaya University from Ministry of Research, Technology and Higher Education, Indonesia.
References
Amini, R.W., Masruri, M., & Rahman, M.F. (2014). Analisis minyak terpentin (Pinus Merkusii) hasil produksi perusahaan lokal dan perdagangan menggunakan kromatografi gas-spektroskopi massa (KG-SM) serta metode pemurniannya.Jurnal Ilmu Kimia Universitas Brawijaya,1(1), 147 153. Hasan, K., Brown, N., & Kozak, C.M (2011). Iron-catalyzed epoxidation of olefin using hydrogen
peroxide.Green Chemistry,13(5), 1230 1237.
Khotimah, H., Lyrawati, D., & Masruri (2006). Antiinflammatory effects of alpha-pinene extracted from pinus mercusii on levels of TNF-alpha signaling, iNOS, and apoptosis of neuronal cells.Proceeding
Leite, A.M., Lima, E.D.O., Souza, E.L.D., Diniz, M.D.F.F.M., Trajano, V.N., & Medeiros, I.A. D (2007). Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing gram-positive bacteria.Revista Brasileira de Ciências Farmacêuticas,43(1), 121 126.
Maksimchuk, N.V., Melgunov, M.S., Mrowiec-Bialon, J., Jarzebski, A.B., & Kholdeeva, O.A. (2005). H2O2-based allylic oxidation of -pinene over different single site catalysts.Journal of Catalysis,
235, 175 183.
Masruri, Rahman, M.F., & Prasodjo, T.I. (2007). Identifikasi dan uji aktifitas antibakteri senyawa volatil terpenoid minyak terpentin.Jurnal Ilmu-Ilmu Hayati (Life Sciences),19(1), 32 35.
Noyori, R., Aoki, M., & Kazuhito, S. (2003). Green oxidation with aqueous hydrogen peroxide.Chemical
Communication, 1977 1986.
Perum Perhutani (2014). Buku statistik Perum Perhutani Tahun 2009 2013. Retrieved from http://bumn.go.id/perhutani/halaman/199