Taq1B CETP polymorphism, plasma CETP, lipoproteins,
apolipoproteins and sex differences in a Jewish population sample
characterized by low HDL-cholesterol
J.D. Kark
a,*, R. Sinnreich
a, E. Leitersdorf
b, Y. Friedlander
a, S. Shpitzen
b, G. Luc
caEpidemiology Unit,Department of Social Medicine,
Hadassah Medical Organization and Hebrew Uni6ersity–Hadassah School of Public Health and Community Medicine, Hadassah Uni6ersity Hospital,P.O. Box12000,Ein Kerem,Jerusalem, Israel
bDi6ision of Internal Medicine,Hadassah Uni6ersity Hospital,Ein Kerem,Jerusalem, Israel cDepartment of Atherosclerosis–Inserm U325,Pasteur Institute,Lille, France
Received 27 April 1999; received in revised form 1 September 1999; accepted 28 September 1999
Abstract
Mean high-density lipoprotein cholesterol (HDL-C) concentrations are low in the Jewish population of Israel. With this in mind we assessed the association of the Taq1B CETP polymorphism, plasma CETP mass and plasma lipid, lipoprotein and apolipoprotein concentrations in a sample of 884 Jerusalem residents aged 28 – 32. The allele frequency (0.43590.017(S.E.)) is similar to that reported elsewhere. There was a strong (apparently codominant) association of the Taq1 B allele with plasma CETP in both sexes, and an inverse association with HDL-C and apo A-1, significant in women and undiminished upon adjustment for plasma CETP. There was evidence in this population for an admixture of two plasma CETP distributions, with 9% belonging to a distribution with the higher mean, pointing to a possible major gene effect. Mean plasma CETP was higher in women than men. Plasma CETP was inversely associated with HDL-C in men but not in women (PB0.05 for the sex difference, multivariate analysis), inversely related to the HDL-C/apo A-1 ratio in men and positively related in women (PB0.005 for the sex difference), and was positively associated with total cholesterol (TC) and low-density lipoprotein cholesterol in both sexes, and with the TC/HDL-C ratio and apo B in men alone. The sex differences may reflect dissimilarities in the regulatory function of CETP in lipid exchange. The absence of an unusual allele frequency of the Taq1B CETP polymorphism and its relatively modest association with HDL-C argue against an important role for this or strongly linked sites in determining the low population levels of HDL-C in Israel. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Apolipoproteins; CETP; Genetic polymorphisms; HDL cholesterol; Jews; LDL cholesterol; Sex differences; Taq1B
www.elsevier.com/locate/atherosclerosis
1. Introduction
Plasma high-density lipoprotein cholesterol (HDL-C) concentrations are low in the Jewish population of Israel in both men and women compared with North American and European populations [1,2]. The consis-tent inverse association between HDL-C and coronary heart disease (CHD) evident elsewhere [3,4] is apparent also in the Israeli population [5 – 9]. In light of this, elucidation of the environmental and genetic
determi-nants of HDL-C may be especially informative in the Jewish population of Israel. Among the environmental attributes in this population relevant to low HDL-C levels are its dietary characteristics including a high polyunsaturated fatty acid and relatively low saturated fatty acid intake [10], and an extraordinarily small alcohol consumption [11].
Regulation of HDL-C concentration has a substan-tial genetic component with an estimated genetic herita-bility of 0.55 in the Jewish population of Jerusalem, similar to that in the US [12]. Segregation analysis pointed to a major recessive gene for depressed HDL-C with an allele frequency of 0.06 and a polygenic compo-nent of 0.45 [13]. A recently reported association of * Corresponding author. Tel.:+972-2-677-7113; fax:+
972-2-642-7652.
E-mail address:[email protected] (J.D. Kark)
heterozygous Gaucher mutations, and consequent ele-vated levels of glucocerebrosidase, with depressed HDL-C concentrations is a step in identifying major gene involvement [14]. The allele frequency, although relatively high in Ashkenazi Jews [15,16], is far too low to explain the low HDL-C levels in Israeli Jews of European origin, and cannot explain the low levels evident also among the other ethnic origin groups comprising the Jewish population. Given that some of the ethnic groups had been separated geographically in the diaspora for 2000 – 2500 years, any influential muta-tions affecting HDL-C are likely to be of biblical or pre-biblical vintage.
The function of HDL in reverse transport of choles-terol from the periphery to the liver is believed to play a key role in its protective effect [17]. Cholesterol ester transfer protein (CETP) partakes in the process of reverse transport, by transferring esterified cholesterol from the HDL molecule to triglyceride-rich lipo-proteins, in exchange for triglycerides [18,19]. However, the implications of the action of CETP are not well understood. There are apparently conflicting reports as to the association of CETP mass or activity with the risk of coronary heart disease [20 – 24].
Some studies [25 – 27] have shown an inverse associa-tion of plasma CETP with HDL-C, but this is not consistent [22,28]. There is some evidence that HDL-C regulation by CETP is dependent on plasma triglyce-ride (TG) levels [29,30]. Polymorphisms of the CETP
gene, in particular a frequent Taq1B RFLP
[22,25,27,31 – 36], but also others such as the Msp 1 and Rsa 1 RFLPs [27,37], are associated with HDL-C, but this too is not consistent [34,38,39]. These genetic poly-morphisms are associated in some studies [22,25 – 27], but not all [33], with altered plasma CETP activity or mass, which may mediate the relation with HDL-C.
We studied the interrelationship of the Taq1B RFLP, plasma CETP levels, plasma HDL-C and low density lipoprotein cholesterol (LDL-C) and the A-1 and B apolipoproteins in a population sample of young Jew-ish adults of differing ethnic origin in Jerusalem.
2. Methods
2.1. Sample
The Jerusalem Lipid Research Clinic (LRC) initially examined 8646 17-year-olds between 1976 – 1979 and a sample comprising 6950 of their parents. The young-sters were sequentially sampled at their induction medi-cal examinations for military service which is obligatory in Israel for the Jewish population except for female religious objectors. The study design, response rates and characteristics have been described previously [40].
For the offspring follow-up study we drew a sex-stratified random sample of 1093 men and 753 women from the original 8646 participants. Non-Jerusalem res-idents according to the national population registry, women who were pregnant or up to 3 months after giving birth or were breastfeeding up to 6 months after birth, and institutionalized people or those unable to provide informed consent were excluded. A total of 786 men (71.9%) and 469 women (62.3%) were eligible for the study; 9.2% of eligible men and 9.4% of women could not be located, 17.8% of men and 23.0% of women refused to participate, and 570 men (72.5%) and 314 women (67.0%) were examined in 1989 – 1991 at ages 28 – 32 years.
Ethical approval for the study was granted by the Hadassah University Hospital Helsinki Committee.
2.2. Data collection
Blood was drawn with minimal venous constriction after an overnight fast. Information on sociodemo-graphic characteristics, personal and family medical history, reproductive history, and health-relevant be-haviors including smoking, alcohol intake, exercise and diet was obtained by a standardized interview. Trained technicians made standardized measurements of blood pressure, heart rate and anthropometric variables (weight, height, waist, hip and arm circumference, and subscapular, triceps and supra-iliac skinfold thickness). Red blood cells were separated for erythrocyte mem-brane fatty acid analysis by gas chromatography and the buffy coat was separated for DNA preparation. Serum and EDTA plasma samples were aliquotted into
cryotubes and stored at −70 to −80°C until
examination.
2.3. Laboratory methods
Genomic DNA was prepared from separated buffy coat by a salting out procedure. Determination of the Taq1B polymorphism located on intron 1, using PCR, was performed as previously described [22] for 819 individuals (527 men and 292 women), comprising 93% of the total sample examined.
Since plasma CETP concentration and CETP activity
are well correlated, r0.75 – 0.85 [41,42], it is
Plasma total cholesterol, HDL-C, and TG concentra-tions were determined by an enzymatic method on a Cobas bio autoanalyzer. HDL-C was measured after precipitation of apo B containing lipoproteins by phos-photungstic acid and magnesium chloride, and LDL-C was calculated by the Friedewald method. Apo A-I and apo B were determined by an immunoturbidi-metric assay read on a Cobas Bio autoanalyzer. Corr-ection was made for laboratory drift in the measure-ment of the apolipoproteins over the duration of the study.
2.4. Statistical methods
The allele frequency was determined by direct count-ing. Conformity with Hardy – Weinberg equilibrium was checked. Plasma CETP was log transformed to reduce skewness in its distribution and geometric means and their approximate S.D. or 95% confidence intervals (CI) are presented. Plasma TG was also log
trans-formed. Plasma lipoproteins and apolipoproteins and ratio measures were adjusted by regression or ANOVA for known determinants such as body mass index
(BMI; kg/m2), smoking, alcohol intake, exercise and
oral contraceptive use. Interactions of sex, BMI, smok-ing or alcohol with the Taq1B genotype on plasma CETP levels, and of sex, BMI, or TG with plasma CETP on HDL-C levels were tested in ANOVA or
regression models.P-values were not corrected for
mul-tiple comparisons. Genes with a major effect on pheno-types may produce bimodality that can be detected using admixture (commingling) analysis. In order to distinguish between skewness and the possibility of admixture in the distribution of plasma CETP, we applied MacLean’s method of transformation [44,45] on standardized CETP data. A maximum likelihood procedure was used to determine whether a single dis-tribution fits the data as well as a mixture of two or three normal distributions.
3. Results
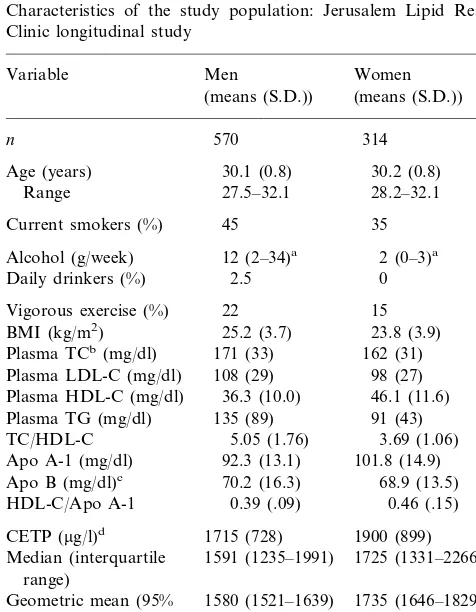
3.1. Population characteristics (Table 1)
A third of the women and almost half the men smoked regularly. Alcohol intake was extremely low in both sexes (as has been noted among Jewish popula-tions), more so in women. Men were more overweight than women at this age. Only 22% of the men and 15% of the women reported vigorous leisure-time exercise at least once weekly. Of the women 21% were using an oral contraceptive.
Mean plasma total cholesterol (TC), LDL-C and TG levels were significantly higher in men than women, apo B levels were similar, and as expected HDL-C was higher in women. A salient characteristic of this study population is the low level of plasma HDL-C in both sexes, irrespective of whether of European (‘Ashke-nazi’), North African or Asian origin. This is reflected also in the relatively high ratio of TC to HDL-C, notable in men. Mean apo A-1 levels were low, consis-tent with the low HDL-C levels, and were as expected significantly higher in women than in men. The ratio of HDL-C to apo A-1 was higher in women than men, indicating a higher cholesterol content per molecule of protein in HDL among women.
Mean plasma CETP was significantly lower in men (geometric mean 1580 mg/l (95% CI 1521 – 1639 mg/l))
than women (1735 mg/l (1646 – 1829 mg/l), (P=.004)).
3.2. Taq1B polymorphism
3.2.1. Allele frequency
The frequency of the B2 allele, representing the
absence of the cutting site, was 0.43590.017(S.E.).
Table 1
Characteristics of the study population: Jerusalem Lipid Research Clinic longitudinal study
Age (years) 30.1 (0.8) 30.2 (0.8) 27.5–32.1
Daily drinkers (%) 0
15
1715 (728) 1900 (899) CETP (mg/l)d
1591 (1235–1991)
Median (interquartile 1725 (1331–2266) range)
1580 (1521–1639)
Geometric mean (95% 1735 (1646–1829) CI)
0.42 0.44
Taq1B2 allele frequencye
aMedian and interquartile range. bPlasma total cholesterol.
Table 2
Plasma CETP concentration (mg/l) within Taq1B genotypes by sex
Women Men
% Geometric mean S.D. n
n % Geometric mean S.D.
B2B2 97 22.4 1390 578 42 18.4 1347 586
44.2 1572 616 105
192 46.1
B1B2 1723 674
145
B1B1 33.4 1693 660 81 35.5 1914 810
100.0
Totala 434 228 100.0
0.001
PANOVA 2 d.f. B0.001
Plinear trend 0.0002 B0.0001
aCETP genotype and phenotype available for 434 men and 228 women.
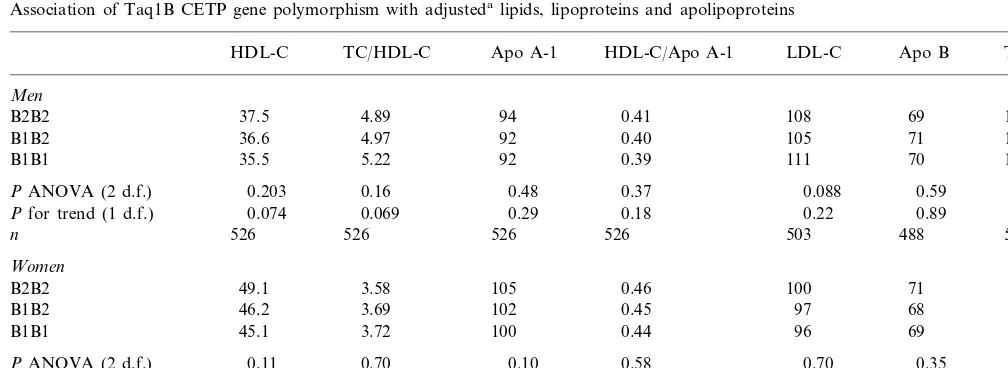
Table 3
Association of Taq1B CETP gene polymorphism with adjustedalipids, lipoproteins and apolipoproteins
TC/HDL-C
HDL-C Apo A-1 HDL-C/Apo A-1 LDL-C Apo B TGb
Men
4.89 94 0.41 108 69 107
B2B2 37.5
4.97 92 0.40
36.6 105
B1B2 71 120
5.22 92 0.39 111 70
B1B1 35.5 114
0.16 0.48 0.37
PANOVA (2 d.f.) 0.203 0.088 0.59 0.073
0.069 0.29 0.18
Pfor trend (1 d.f.) 0.074 0.22 0.89 0.31
526 526 526 503
526 488
n 526
Women
3.58 105 0.46
49.1 100
B2B2 71 84
3.69 102 0.45 97
B1B2 46.2 68 78
3.72 100 0.44 96
45.1 69
B1B1 87
0.70 0.10 0.58 0.70 0.35
PANOVA (2 d.f.) 0.11 0.14
0.44 0.036 0.40
0.042 0.34
Pfor trend (1 d.f.) 0.33 0.61
292 292 292 291 276 292
n 292
aAdjusted for BMI, smoking, alcohol intake, exercise and oral contraceptive use. bGeometric mean.
(There was a modest deviation from Hardy – Weinberg
equilibrium with a relative deficiency of B1B2
heterozygotes x2=4.71, P=0.03). The allele
frequencies by country of origin groups were 0.40 (0.033) for Israel, 0.388 (0.039) for Europe and countries of emigration from Europe, 0.441 (0.031)
for Asia and 0.508 (0.036) for North Africa (P=0.08
for the ethnic difference).
3.2.2. Association with plasma CETP
In this population, the Taq1B genotype was associ-ated with plasma CETP levels in an apparently codom-inant mode with the B2 homozygote showing the lowest
levels and the B1B2 heterozygote intermediate (P5
0.0002 for trend) (Table 2). The geometric mean CETP of the B1B1 compared with the B2B2 homozygotes was 22% higher in men and 42% higher in women, there being no sex difference in CETP levels among B2B2 homozygotes and an increasing female – male difference through B1B2 heterozygotes to B1B1 homozygotes.
However, although the effect appeared to be larger in women, a formal test for a sex – genotype interaction was not statistically significant (P=0.14).
3.2.3. Association with lipoproteins and apolipoproteins The Taq1B RFLP was associated with multivariable adjusted plasma HDL-C and apo A-1 levels (Table 3). The B1 allele was associated with lower HDL-C and with lower apo A-I, both statistically significant only in women. The associations in women were undimin-ished upon control for plasma CETP, indicating that
they are not mediated by CETP. The ratios of HDL-C/
apo A-I and TC/HDL-C, and the levels of LDL-C, TG
or apo B did not differ significantly between the geno-types.
We assessed the association of HDL-C with TG within genotype and sex categories (Table 4). In men the correlation in the B2B2 and B1B2 groups was high
Table 4
Pearson correlations of triglycerides (ln transformed) and HDL-Ca
within CETP Taq1B genotypes
Correlation x2For heterogeneity n
Men
−0.45 B2B2 116
−0.51
232 x2=9.82, d.f.=2 B1B2
B1B1 179 −0.25 P=0.007
−0.41 527
Allb
Women
−0.20 54
B2B2
B1B2 140 −0.37 x2=1.50, d.f.=2
−0.28
98 P=0.47
B1B1
292
Allb −0.31
aBoth unadjusted for covariates. b
x2 for heterogeneity between the sexes=2.49, 1 d.f., P=0.12.
Following Hannuksela et al. [26], we determined whether smoking modifies the association of the Taq1B polymorphism with plasma CETP. In both sex-specific and sex-pooled analyses there was no evidence for a smoking – genotype interaction. There was no associa-tion of plasma CETP with BMI, alcohol intake or exercise in either sex, nor was there evidence for a genotype – BMI interaction on CETP levels.
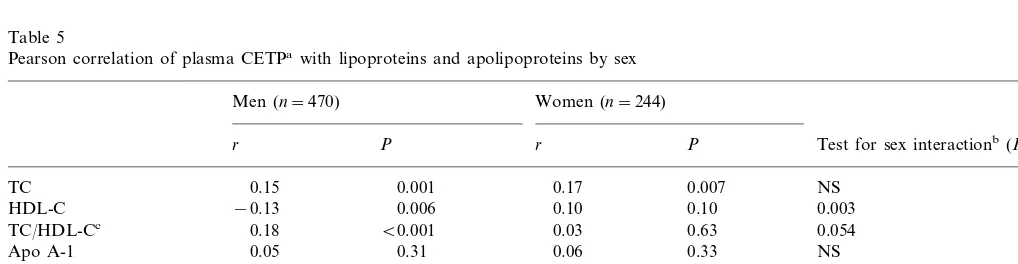
3.3.2. Association with lipoproteins and apolipoproteins In unadjusted correlation analysis, plasma CETP was significantly related to lipoprotein and apolipoprotein levels (Table 5). In men plasma CETP was inversely
correlated with HDL cholesterol (r= −0.13, P=
0.006), in contrast with women in whom the association
was non-significantly positive (r=0.10,P=0.10). The
sex difference in association was significant (P=0.003). There was no significant correlation with apo A-I in either sex. Consequently, the ratio of HDL-C to apo
A-I was inversely related to plasma CETP (r= −0.18)
in men and positively related in women (r=0.15) (PB
0.0001 for sex difference). For TC and LDL-C the correlations were significantly positive in both sexes,
and for apo B only in men (P=0.05 for sex difference).
For the ratio of TC to HDL-C the correlation was
correspondingly positive in men (r=0.18) and almost
zero in women (P=0.054 for sex difference). There was
no association with TG. To assess whether the sex difference in the CETP – HDL-C association might be determined in part by male versus female patterns of fat distribution, we tested for an interaction with waist to hip ratio in each sex. There was no evidence for effect modification.
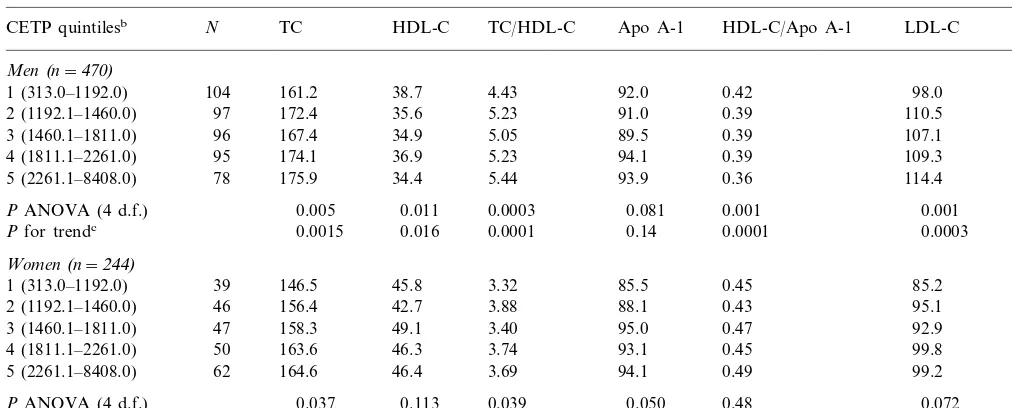
We further explored these associations in an analysis of quintiles of plasma CETP, adjusting for BMI, smok-ing, alcohol intake, exercise and oral contraceptive use (Table 6). In men HDL-C was highest in the bottom
d.f., P=0.007), who also have the highest plasma
CETP levels. In women there was no genotype effect; the correlations were non-significantly lower overall than in men and were similar to that in B1B1 men.
No interaction was found between genotype and either alcohol intake, BMI, or smoking in determining HDL-C levels.
3.3. Plasma CETP
3.3.1. Association with beha6ioral risk factors
Cigarette smoking was associated with significantly lower plasma CETP levels in women (geometric mean
1571 vs. 1845 mg/l in nonsmokers, P=0.0035). Male
smokers had non-significantly lower plasma CETP than
nonsmokers (1534 vs. 1612 mg/l, P=0.19), with the
association evident only in the B2B2 genotype (nominal borderline significance). Consequently the sex difference in plasma CETP was smaller in smokers.
Table 5
Pearson correlation of plasma CETPawith lipoproteins and apolipoproteins by sex
Women (n=244) Men (n=470)
r P r P Test for sex interactionb(P)
0.007 0.17
0.001 NS
0.15 TC
−0.13 0.006
HDL-C 0.10 0.10 0.003
0.054
TC/HDL-Cc 0.18 B0.001 0.03 0.63
0.05 0.31
Apo A-1 0.06 0.33 NS
HDL-C/Apo A-1 −0.18 B0.001 0.15 0.02 0.00002
0.19 0.04
LDL-Cd B0.001 0.13 NS
−0.03
0.008 0.6
0.13
Apo Be 0.050
0.06 0.23 0.03 0.66
ln TG NS
aln transformed. b
x2 test for heterogeneity in correlations between men and women.
cTC/HDL-C.
Table 6
Plasma lipids, lipoproteins and apolipoproteins by CETP quintiles and by sexa
N
CETP quintilesb TC HDL-C TC/HDL-C Apo A-1 HDL-C/Apo A-1 LDL-C Apo B
Men(n=470)
161.2
1 (313.0–1192.0) 104 38.7 4.43 92.0 0.42 98.0 67.1
172.4 35.6 5.23 91.0
97 0.39
2 (1192.1–1460.0) 110.5 70.6
96
3 (1460.1–1811.0) 167.4 34.9 5.05 89.5 0.39 107.1 69.9
174.1 36.9 5.23
4 (1811.1–2261.0) 95 94.1 0.39 109.3 71.7
175.9 34.4 5.44 93.9
78 0.36
5 (2261.1–8408.0) 114.4 73.4
PANOVA (4 d.f.) 0.005 0.011 0.0003 0.081 0.001 0.001 0.11
0.0015 0.016 0.0001 0.14 0.0001
Pfor trendc 0.0003 0.01
Women(n=244)
146.5 45.8 3.32 85.5 0.45 85.2 68.2
1 (313.0–1192.0) 39
156.4 42.7 3.88 88.1
46 0.43
2 (1192.1–1460.0) 95.1 71.0
47
3 (1460.1–1811.0) 158.3 49.1 3.40 95.0 0.47 92.9 65.9
163.6 46.3 3.74 93.1
50 0.45
4 (1811.1–2261.0) 99.8 70.2
62
5 (2261.1–8408.0) 164.6 46.4 3.69 94.1 0.49 99.2 67.6
0.037 0.113 0.039 0.050 0.48 0.072
PANOVA (4 d.f.) 0.39
0.003 0.36 0.26 0.011 0.19 0.011
Pfor trendc 0.70
aAll lipids variables adjusted for BMI, smoking, alcohol intake, exercise and oral contraceptive use. bSex-pooled.
cWith quintiles coded as 1–5 in a linear regression.
fifth of CETP and lowest in the top fifth. The relation-ship was not monotonic. In women the relation differed and was not statistically significant. In a linear regres-sion including terms for sex, alcohol intake, current cigarette smoking and BMI, CETP quintiles(coded 1 – 5 as a linear term), and a sex by CETP interaction, the
sex difference in association remained significant (P=
0.04). There was a positive association of plasma CETP with apo A-I in women. The ratio of HDL-C to apo A-I decreased in men from a mean of 0.42 in the lowest fifth of the CETP distribution to 0.36 in the upper fifth
(PB0.001), whereas in women there was no association
(P=0.0048 for sex difference in association). The ratio
of TC to HDL-C increased in men from 4.4 to 5.4 from the lowest to the highest quintile of plasma CETP
(PB0.001). In women the relation was inconsistent
(P=0.14 for sex difference). There was a positive
asso-ciation with TC and LDL-C in both sexes, and with apo B in men alone.
3.3.3. Admixture (commingling) of the plasma CETP distribution
Admixture was assessed for men and women with
low HDL-C levels (below 35 and 45 mg/dl,
respec-tively), and for the whole population without regard for HDL-C levels. There was no evidence for more than one distribution fitting the low HDL-C population. However, in the total study population two distribu-tions fitted significantly better than one (PB0.05). Of the population 91% were included in a lower distribu-tion and 9% in an upper distribudistribu-tion with a mean 2.3 S.D. above the major mode.
3.3.4. Effect modification by plasma TG
We tested a hypothesis [29,30] that regulation of plasma HDL-C levels by CETP is dependent on TG levels (i.e. an inverse association is present only if TG is elevated), using ANOVA with tertile cutoff points for CETP and TG. There was no significant interaction. In men, the inverse association with HDL-C was slightly
attenuated (P=0.062) upon adjustment for TG, and
the direct relation with TC/HDL-C and with HDL-C/
apo A-1 remained strong and independent of TG, and
was not limited to the upper quintile of TG (]178
mg/dl). In women the positive CETP – HDL-C
relation-ship was enhanced (P=0.014) and was not dependent
on TG level (however, the upper quintile cut-off point
was only 118 mg/dl). The absence of effect modification
was reexamined treating ln TG as a continuous vari-able; again there was no support for the hypothesis. Similarly, the association of CETP with HDL-C was also independent of BMI levels.
4. Discussion
The low mean HDL-C observed in this sample con-firms previous reports [1,2] and is characteristic of the Jewish population of Israel. Apo A-1 levels were
corre-spondingly low. The TC/HDL-C ratio was high in men.
dif-ferences in HDL-C, averaging 9 – 10 mg/dl lower in
Jerusalem, and in parallel the TC/HDL-C ratio was
higher. Prominent environmental differences between these populations, other than those associated with geographic location, are the exceedingly low alcohol intake in Israel [11], confirmed in our study, and the unusual dietary fatty acid intake. In the EURAMIC
study polyunsaturated fatty acids, predominantly
linoleic acid, comprised 25% of adipose tissue in a Jerusalem population sample, a considerably larger proportion than in eight European countries including Germany [46]. It is in this context that we assessed the associations of the Taq1B CETP polymorphism and plasma CETP concentration with regulation of HDL-C levels.
4.1. Taq1B allele frequencies
The allele frequencies in the ethnic groups comprising the Jewish population were relatively homogenous and differed little from those reported in European popula-tions with higher mean HDL-C levels. For example, in the ECTIM study that included samples from Northern Ireland and France [22], and in a Dutch sample [27], the B2 allele frequency was 0.40 – 0.41, in a Scottish study 0.46 [33], and in Italian and Greek migrants to Australia 0.43 and 0.48, respectively [34]. In Sinhalese Sri Lankans the frequency was 0.49 [32]. The similarity across populations suggests that this polymorphic marker is ancient.
4.2. Taq1B association with HDL-C
Our finding of an association of the B1 allele with lower HDL-C accords with studies in Dutch [27], Nor-wegian (also with A-1) [31], Scottish (no association with apo A-1) [25,33], Sinhalese Sri Lankan [32] and in Greek migrant samples [34], but differs from studies in Italian migrants [34] and Finns [39] which suggests differing linkage disequilibrium in different ethnic groups, differing gene – environment interactions, or the effects of chance or selection. Sib-pair linkage studies were inconsistent, one showing an association of the CETP gene locus with HDL-C [35] and one not [38]. We did not confirm the sex interaction reported by Kauma et al. [36], who showed that the association of the B1 allele with lower HDL-C was restricted to women in a Finnish population sample, although in our data and those reported by Mitchell et al. [34] the relationship appeared to be stronger in women, and in our study was significant only in women. Sex modifica-tion with the Taq1A RFLP was observed in a
French-Canadian population where the association was
restricted to females [47].
Our findings differ somewhat from the ECTIM study [22] where no relationship was observed in men who
drank B25 g/day alcohol (which would include almost
our entire study population), whereas among heavier drinkers a strong positive association with HDL-C was evident in B2B2 homozygous men. We were unable to adequately assess effect modification by alcohol, al-though alcohol intake even at the low levels prevalent in the Israeli population is positively associated with HDL-C and inversely related to CHD (Kark, J.D., unpublished data).
A stronger effect of the Taq1B RFLP on plasma HDL-C or apo A-1 and on CETP in nonsmokers reported in several studies [26,31,33] was not confirmed by our study, the ECTIM study [22] or a Finnish study [39].
The Taq1B RFLP is unlikely to be functional, does not affect coding, and is probably a marker for func-tional variants [22]. Kuivenhoven et al. [27] infer that the B1 allele serves as a marker for specific CETP haplotypes, such as Taq1B-Msp1-Rsa1, that may affect HDL metabolism. In that study the M1 allele (Msp1 RFLP) was also strongly associated with low HDL-C. However, these variants do not affect the CETP struc-ture in contrast with the Rsa 1 RFLP (Ile405val) shown by Funke et al. [37] to be associated with increased HDL-C. This was confirmed by Kuivenhoven et al. [27] who showed, however, that this locus was responsible for only 3.6% of the variance in CETP concentration in their population. In our population as in ECTIM [22] there was little effect of the Taq1B polymorphism on the apo B-containing lipoproteins.
Therefore, taking account of both the absence of an unusual allele frequency of the Taq1B RFLP in the Jerusalem population and its weak association with HDL-C in men and albeit stronger association in women, it is unlikely that this site, either functionally or by linkage disequilibrium with functional sites, is an important determinant of the low HDL-C levels in our population. Consequently, other gene loci must exert a strong influence, or strong and highly prevalent envi-ronmental determinants, such as common dietary prac-tices, should play a dominant role. Gene – environment interactions are likely to be important.
We further explored the association of the Taq1B CETP polymorphism with plasma CETP, and the rela-tionships of CETP with plasma lipoproteins not so much as in a quest to explain the low HDL-C levels in Israel, but rather to assess the relationships and func-tions of CETP within this context.
4.3. Taq1B and plasma CETP concentration
suggesting that the association of Taq1B with plasma CETP activity is affected by smoking [26]. Further-more, there was no evidence for an effect modification by BMI, another important determinant of HDL-C. Although the Taq1B polymorphism explained only 3.1% (men) and 7.6% (women) of the variance in plasma ln CETP in our population, it has been esti-mated that at least 20% of the between-individual vari-ation in CETP is attributable to the CETP gene; this leaves a large proportion of variance due to other factors [28]. These may include other regulatory en-zymes such as LCAT [41].
4.4. Admixture analysis
Tato et al. [21] reported a bimodal distribution for CETP among a sample with low HDL-C, suggesting heterogeneous determination of low HDL-C levels. This was not confirmed by Kuivenhoven et al. [27] or in our study. We did observe, however, an admixture of two plasma CETP distributions in the overall popula-tion, pointing to the likelihood of a major gene effect on CETP in this population, with about 10% belonging to a distribution characterized by a high mean CETP; nonetheless, an environmental factor could produce this result.
4.5. Plasma CETP and plasma lipid 6ariables
It is believed that elevated CETP activity may be an important determinant of low HDL-C [21]. However, the functional relationship between CETP activity and HDL-C is not well understood. An inverse relationship between plasma CETP and HDL-C has been described
in some studies [21,26,27] but not in others
[22,28,33,48]. Freeman et al. [33] postulated that differ-ences in CETP activity may be seen only in extreme conditions such as hyperalphalipoproteinemia (which is not consistent with our findings or those of others [21,27]) and that, in the general population, gene effects on CETP activity are not responsible for the relation between the CETP gene polymorphism and HDL-C. This latter interpretation is supported by our findings and by McPherson et al. [28] and Fumeron et al. [22] who concluded that the effects of the CETP gene on plasma CETP and HDL-C are independent, but not by the inference of Kuivenhoven et al. [27].
A notable finding in our study was a significant effect modification by sex of the association of plasma CETP with HDL-C, in men the association being inverse whereas in women it tended to be positive. Although the apo A-1 containing lipoproteins are the main car-rier of plasma CETP, a positive association in the multivariate-adjusted analysis was evident only in women. These findings are consistent with a different effect of CETP in men and women on cholesterol
ester – TG exchange. This could alter the cholesterol content of HDL as reflected by the significantly declin-ing ratio of HDL-C to apo A-1 with increasdeclin-ing CETP evident in men, but not in women. This interpretation is consistent also with an apparent unequal correlation of TG with HDL-C between the sexes in those with the B2 allele, which although suggestive was not statisti-cally significant (Table 4).
Several studies [29,30] indicate that regulation of HDL-C by CETP depends on TG levels, with an inverse relationship with HDL-C and apo A-1 evident only in the presence of high TG. In men with normal TG, CETP was positively associated with apo A-1 and HDL-C [29,48] (in contrast to our findings for HDL-C in men), but adjustment for LPL, which is strongly correlated with CETP, eliminated the association [29]. We did not confirm findings of differing CETP – HDL-C relationships by level of TG in this population sam-ple of young Jewish adults in Jerusalem in which the
proportion with elevated TG (\200 mg/dl) was small.
The associations were largely independent of TG levels. Some authors have concluded that the association of plasma CETP with lipoproteins is stronger for the apo B-containing lipoproteins than for HDL cholesterol [18], as seen in ECTIM [22] and a Japanese study [41], where an association was reported with LDL-C, but not with HDL-C. In our data there was a consistent association of CETP with LDL-C in both sexes, in contrast with a significant interaction of sex with CETP on HDL-C.
4.6. Sex differences in association
We summarize a number of differences observed between the sexes: (1) Plasma CETP levels were signifi-cantly higher among women, consistent for example with studies in Japanese [41]. (2) The association of the Taq1B1 allele with plasma CETP and the inverse B1 allele associations with HDL-C and apo A-1 appeared to be stronger in women than men, but these differ-ences were not statistically significant. (3) A significant inverse association of smoking with plasma CETP was restricted to women; the female excess in plasma CETP was consequently reduced in smokers. (4) There were statistically significant sex differences in the association of plasma CETP with HDL-C and with the ratio of HDL-C to apo A-1, but not with TC or LDL-C. These differences suggest different mechanisms regulating the content of cholesterol in HDL in women and men. A significant multivariate-adjusted positive association of CETP with apo A-1 was apparent only in women, is consistent with an inference of a sex difference in the affinity of CETP for apo A-1 [49].
that the CETP Taq1B polymorphism or strongly linked loci play an important role in setting the low HDL-C levels in the Jewish population of Israel. Low HDL-C is almost certainly a heterogeneous combination of condi-tions with different metabolic determinants, some asso-ciated with increased risk of CHD and some not [50,51]. Currently there are no simple available labora-tory measures to readily define and quantify these components of low HDL-C at the population level. Individuals with low plasma HDL-C and normal TG often have an increased fractional catabolic rate for apo A-1, reflecting abnormal HDL metabolism [52,53], and which may explain the low apo A-1 levels in our population. Recently, hepatic triglyceride lipase activity was found to be elevated in Turkish men and women, who represented another population characterized by low HDL-C levels [54]. Comparative studies of apo A-1 metabolism and of hepatic lipase activity in Jewish and non-Jewish populations are warranted. A continued search for major genes and major environmental effects regulating HDL-C in this population and for gene – en-vironment interactions should be rewarding.
Acknowledgements
The authors thank Philippe Hauw for his technical assistance. This study was supported by the United States – Israel Binational Scientific Foundation and by the Israel Ministry of Health.
References
[1] Eisenberg S, Heiss G, Friedlander Y, et al. Comparison of plasma lipids, lipoproteins, and dyslipoproteinemia in Israel and the United States. Atherosclerosis 1986;59:63 – 74.
[2] Bobak M, Hense HW, Kark JD, et al. Explaining cardiovascular disease rate differences: risk factors in Czech, Bavarian and Israeli men. Int J Epidemiol 1999;28:437 – 44.
[3] Miller GJ, Miller NE. Plasma high density lipoprotein choles-terol concentration in development of ischaemic heart disease. Lancet 1975;1:16 – 9.
[4] Gordon DJ, Rifkind BM. High-density lipoprotein: the clinical implications of recent studies. New Engl J Med 1989;321:1311 – 6.
[5] Brunner D, Loeb K. Serum cholesterol, electrophoretic lipid pattern, diet and coronary heart disease: a study in coronary patients and in healthy men of different origin and occupation in Israel. Ann Intern Med 1958;49:732 – 50.
[6] Brunner D, Altman S, Loebl K, Schwartz S. Cholesterol percent-ages in coronary patients with and without increased total serum cholesterol levels and in healthy controls. J Atheroscler Res 1962;2:424 – 37.
[7] Goldbourt U, Medalie JH. High density lipoprotein cholesterol and coronary heart disease incidence — The Israeli Ischemic Heart Disease Study. Am J Epidemiol 1979;109:296 – 308. [8] Goldbourt U, Kark JD. The epidemiology of coronary heart
disease in the ethnically and culturally diverse population of Israel. Isr J Med Sci 1982;18:1077 – 97.
[9] Friedlander Y, Lev-Merom D, Kark JD. A comparison of different matching designs in case-control studies: an empirical example using continuous exposures, continuous confounders and incidence of myocardial infarction. Stat Med 1993;12:993 – 1004.
[10] Kaufmann NA, Dennis BH, Heiss G, Friedlander Y, Kark JD, Stein Y. Comparison of nutrient intake of selected populations in the United States and Israel: The Lipid Research Clinics Prevalence Study. Am J Clin Nutr 1986;43:604 – 20.
[11] World Health Organization. Highlights on Health in Israel. WHO Regional Office for Europe, Copenhagen 1996.
[12] Bucher KD, Friedlander Y, Kaplan EB, et al. Biological and cultural sources of familial resemblance in plasma lipids: a comparison between North America and Israel — the Lipid Research Clinics Program. Genet Epidemiol 1988;5:17 – 33. [13] Friedlander Y, Kark JD, Stein Y. Complex segregation analysis
of low levels of plasma high-density lipoprotein cholesterol in a sample of nuclear families in Jerusalem. Genet Epidemiol 1986;3:285 – 97.
[14] Pocovi M, Cenarro A, Civeira F, et al. B-glucocerebrosidase gene locus as a link for Gaucher’s disease and familial hypo-a-lipoproteinaemia. Lancet 1998;352:1919 – 23.
[15] Zimran A, Gelbart T, Westwood B, Grabowski GA, Beutler E. High frequency of the Gaucher disease mutation at nucleotide 1226 among Ashkenazi Jews. Am J Hum Gen 1991;49:855 – 9. [16] Beutler E, Nguyen NJ, Henneberger MW, et al. Gaucher disease:
gene frequencies in the Ashkenazi Jewish population. Am J Hum Genet 1993;52:85 – 8.
[17] Tall AR. Plasma high density lipoproteins: metabolism and relation to atherogenesis. J Clin Invest 1990;86:379 – 84. [18] Tall AR. Plasma cholesterol ester transfer protein. J Lipid Res
1993;34:1255 – 74.
[19] Bruce C, Tall AR. Cholesterol ester transfer proteins, reverse cholesterol transport, and atherosclerosis. Curr Opin Lipidol 1995;6:306 – 11.
[20] Bhatnagar D, Durrington PN, Channon KM, Prais H, Mack-ness MI. Increased transfer of cholesterol esters from high density lipoproteins to low density and very low density lipo-proteins in patients with angiographic evidence of coronary heart disease. Atherosclerosis 1993;98:25 – 32.
[21] Tato F, Vega GL, Grundy SM. Bimodal distribution of choles-terol ester transfer protein activities in normotriglyceridemic men with low HDL cholesterol concentrations. Arterioscler Thromb Vasc Biol 1995;15:446 – 51.
[22] Fumeron F, Betoulle D, Luc G, et al. Alcohol intake modulates the effect of a polymorphism of the cholesterol ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J Clin Invest 1995;96:1664 – 71.
[23] Kuivenhoven JA, Jukema JW, Zwinderman AH, et al. The role of a common variant of the cholesterol ester transfer protein gene in the progression of coronary atherosclerosis. New Engl J Med 1998;338:86 – 93.
[24] Zhong S, Sharp DS, Grove JS, et al. Increased coronary heart disease in Japanese-American men with mutation in the choles-terol ester transfer protein despite increased HDL levels. J Clin Invest 1996;97:2917 – 23.
[25] Freeman DJ, Packard CJ, Sherperd J, Gaffney D. Polymor-phisms in the gene coding for cholesterol ester transfer protein are related to plasma high-density lipoprotein cholesterol and transfer protein activity. Clin Sci 1990;79:575 – 81.
[26] Hannuksela ML, Liinamaa MJ, Kesaniemi YA, Savolainen MJ. Relation of polymorphisms in the cholesterol ester transfer protein gene to tranfer protein activity and plasma lipoprotein levels in alcohol drinkers. Atherosclerosis 1994;110:35 – 44. [27] Kuivenhoven JA, de Knijff P, Boer JMA, et al. Heterogeneity at
[28] McPherson R, Grundy S, Guerra R, Cohen JC. Allelic variation in the gene encoding the cholesterol ester transfer protein is associated with variation in the plasma concentrations of choles-terol ester transfer protein. J Lipid Res 1996;37:1743 – 8. [29] Foger B, Ritsch A, Doblinger A, Wessels H, Patsch JR.
Relation-ship of plasma cholesterol ester transfer protein to HDL choles-terol. Arterioscler Thromb Vasc Biol 1996;16:1430 – 6.
[30] Mann CJ, Yen FT, Grant AM, Bihain BE. Mechanism of plasma cholesterol ester transfer in hypertriglyceridemia. J Clin Invest 1991;88:2059 – 66.
[31] Kondo I, Berg K, Drayna D, Lawn R. DNA polymorphism at the locus for human cholesterol ester transfer protein (CETP) is associated with high density lipoprotein cholesterol and apolipo-protein levels. Clin Genet 1989;35:49 – 56.
[32] Mendis S, Sherperd J, Packard CJ, Gaffney D. Genetic variation in the cholesterol ester transfer protein and apolipoprotein A-1 genes and its relation to coronary heart disease in a Sri Lankan population. Atherosclerosis 1990;83:21 – 7.
[33] Freeman DJ, Griffin BA, Holmes AP, et al. Regulation of plasma HDL cholesterol and subfraction distribution by genetic and environmental factors: associations between the Taq1 B RFLP in the CETP gene and smoking and obesity. Arterioscler Thromb 1994;14:336 – 44.
[34] Mitchell RJ, Earl L, Williams J, Bisucci T, Gasiamis H. Polymor-phisms of the gene coding for the cholesterol ester transfer protein and plasma lipid levels in Italian and Greek migrants to Australia. Hum Biol 1994;66:13 – 25.
[35] Bu X, Warden CH, Xia YR, et al. Linkage analysis of the genetic determinants of high density lipoprotein concentrations and composition: evidence for involvement of the apolipoprotein A-II and cholesterol ester transfer protein. Hum Genet 1994;93:639 – 48. [36] Kauma H, Savolainen MJ, Heikkila R, et al. Sex difference in the regulation of plasma high density lipoprotein cholesterol by genetic and environmental factors. Hum Genet 1996;97:156 – 62. [37] Funke H, Wiebusch H, Fuer L, Muntoni S, Schulte H, Assmann G. Identification of mutations in the cholesterol ester transfer proteins in Europeans with elevated high density lipoprotein cholesterol. Circulation 1994;90(2):241 Abstract.
[38] Cohen JC, Wang Z, Grundy SM, Stoesz MR, Guerra R. Variation at the hepatic lipase and apolipoprotein AI/CIII/AIV loci is a major cause of genetically determined variation in plasma HDL cholesterol levels. J Clin Invest 1994;94:2377 – 84.
[39] Tenkanen H, Koskinen P, Kontula K, et al. Polymorphisms of the gene encoding for cholesterol ester transfer protein and serum lipoprotein levels in subjects with and without coronary heart disease. Hum Genet 1991;87:574 – 8.
[40] Slater PE, Friedlander Y, Baras M, et al. The Jerusalem Lipid Research Clinic: sampling, response and selected methodological issues. Isr J Med Sci 1982;18:1106 – 12.
[41] Kinoshita M, Teramoto T, Shimazu N, et al. CETP is a determi-nant of serum LDL-cholesterol but not HDL-cholesterol in healthy Japanese. Atherosclerosis 1996;120:75 – 82.
[42] McPherson R, Mann CJ, Tall AR, et al. Plasma concentrations of cholesterol ester transfer protein in hyperproteinemia. Rela-tion to cholesterol ester transfer protein activity and other lipoprotein variables. Arterioscler Thromb 1991;11:797 – 804. [43] Mezdour H, Kora I, Parra HJ, Tartar A, Marcel YA, Fruchard
JC. Two-site enzyme immunoassay of cholesterol ester transfer protein with monoclonal and polyclonal antibodies. Clin Chem 1994;40:593 – 7.
[44] MacLean J, Morton NE, Elston RC, Yee S. Skewness in com-mingled distributions. Biometrics 1976;32:695 – 9.
[45] Friedlander Y, Kark JD, Cohen T, Eisenberg S, Stein Y. Ad-mixture analysis of high density lipoprotein cholesterol distribu-tion in a Jerusalem population sample. Clin Genet 1983;24:117 – 27.
[46] Kardinaal AFM, Aro A, Kark JD, et al. Association between B-carotene and acute myocardial infarction depends on polyun-saturated fatty acid status. The EURAMIC study. Arterioscler Thromb Vasc Biol 1995;15:726 – 32.
[47] Kessling A, Ouellette S, Bouffard O, et al. Patterns of associa-tion between genetic variability in apolipoprotein (apo)B, apo AI-CIII-AIV, and cholesterol ester transfer protein gene regions and quantitative variation in lipid and lipoprotein traits: influ-ence of gender and exogenous hormones. Am J Hum Genet 1992;50:92 – 106.
[48] Marcel YL, McPherson R, Hogue M, et al. Distribution and concentration of cholesterol ester transfer protein in plasma of normolipidemic subjects. J Clin Invest 1990;85:10 – 7.
[49] Moulin P, Cheung MC, Austin AA, et al. Gender effect on the distribution of the cholesterol ester transfer protein in apolipo-protein A-1-defined lipoapolipo-protein subpopulations. J Lipid Res 1994;35:793 – 802.
[50] Roma P, Gregg RE, Meng MS, et al. In vivo metabolism of a mutant form of apolipoprotein A-1, apo A-1 Milano associated with familial hypoalphalipoproteinemia. J Clin Invest 1993;91:1445 – 52.
[51] Betteridge DJ, Kahn M. Genetic influence on HDL cholesterol. Lancet 1998;351:1903 – 4.
[52] Gylling H, Vega GL, Grundy SM. Physiologic mechanisms for reduced apolipoprotein A-1 concentrations associated with low levels of high density lipoprotein cholesterol in patients with normal plasma lipids. J Lipid Res 1992;33:1527 – 39.
[53] Brinton EA, Eisenberg S, Breslow JL. Human HDL cholesterol levels are determined by apo A-1 fractional catabolic rate, which correlates inversely with estimates of HDL particle size. Effects of gender, hepatic and lipoprotein lipases, triglyceride and insulin levels, and body fat distribution. Arterioscler Thromb 1994;14:707 – 20.
[54] Bersot TP, Vega GL, Grundy SM, et al. Elevated hepatic lipase activity and low levels of high density lipoprotein in a nor-motriglyceridemic, nonobese Turkish population. J Lipid Res 1999;40:432 – 8