Impact of wet deposited nickel on the cation content of a
mat-forming lichen
Cladina stellaris
M. Hyva¨rinen *, M. Roitto, R. Ohtonen, A. Markkola
Department of Biology,Uni6ersity of Oulu,PO Box3000,90014Oulu,Finland
Received 7 September 1999; received in revised form 5 November 1999; accepted 5 November 1999
Abstract
The impact of experimentally sprayed aqueous nickel solution on the concentrations of potassium, calcium, magnesium and nickel in three horizontal strata (top, 0 – 20 mm; middle, 20 – 40 mm; and base, 40 – 60 mm) of the cushion-forming lichenCladina stellariswas investigated. The experimental nickel deposition range used corresponded with that from the pristine forests of the Finnish border to polluted industrial sites of Russian Kola Peninsula (0 – 1000 mg Ni2+ m−2year−1). The lichen mat retained ca. 31 – 66% of the nickel deposited during two growing
seasons and the relative retention efficiency was highest at the low deposition end. The concentrations of cations in lichen thalli were significantly reduced only after the highest nickel deposition. Furthermore, the separate horizontal strata responded differently to nickel exposure indicating that the cation exchange sites of the top stratum were not completely saturated by nickel even after the most severe treatment. However, nickel deposited in high doses caused considerable reduction in potassium concentration indicating damage to cell membranes. Episodically deposited high concentrations of nickel can probably affect membrane integrity before detectable changes in total concentrations of cations in the lichen thallus take place. Thus, ratios of total concentrations of cations in the lichen thallus are fairly insensitive to nickel deposition, which reduces the risk of compounding effects when the ratios are used to indicate long-term acid deposition in areas with multiple pollution problems such as Kola Peninsula. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Boreal forests; Cation exchange; Cladina stellaris; Lichen; Nickel
www.elsevier.com/locate/envexpbot
1. Introduction
Mat-forming lichens (i.e.Cladina, Cetrariaand
Sterocaulon) are major vegetation components in northern boreal forests and subarctic heathlands
(e.g. Larsen, 1980; Longton, 1988; Ahti and Ok-sanen, 1990). Since higher plants are distributed very sparsely in lichen-dominated ecosystems, lichens receive atmospheric deposits that are largely unmodified by the plant canopy. Therefore mat-forming lichens may be of great help in as-sessing the impact of airborne pollutants on nutri-ent-poor northern ecosystems. One such area is the Russian Kola Peninsula where both acid rain
* Corresponding author. Tel.:+358-8-5531510; fax:+ 358-8-5531061.
E-mail address:[email protected] (M. Hyva¨rinen)
and heavy metal deposition from metal smelters have caused serious damage to vast areas and greatly increased the susceptibility of local ecosys-tems to natural stresses such as harsh winters, forest pathogens and reindeer grazing (e.g. Tik-kanen and Niemela¨, 1995).
Lichens possess considerable passive ion ex-change capability which is based on negatively charged binding sites located in the cell wall and on the outer surface of the cell membrane (Brown, 1976; Nieboer et al., 1978; Burton et al., 1981). The uptake of Ni2+ by lichens, for instance, is
purely a physicochemical process and not metabolically regulated (Nieboer et al., 1976). Nieboer and Richardson (1980) presented the fol-lowing sequence of metal cations according to their affinity to cation exchange sites based on competition experiments: monovalent class A (e.g. K+)Bdivalent class A (e.g. Mg2+, Ca2+)B
bor-derline divalent (e.g. Ni2+)Bdivalent class B (e.g.
Cu2+). As a general rule of thumb, two
monova-lent cations are needed to replace one divamonova-lent cation (Nieboer et al., 1976). The chemical nature of the binding sites, however, is still subject to speculation as well as the contribution of the algal symbiont in extracellular binding (e.g. Brown and Beckett, 1984; Richardson et al., 1985; Brown 1991). Even though cation uptake and exchange in the lichen thallus has been the focus of a number of studies during recent decades (see above) there are only a few reports where the potential of lichen cation exchange as an environ-mental biomarker has been explicitly addressed. Hyva¨rinen and Crittenden (1996) investigated the use of lichen cation content as a bioindicator of acid deposition insufficient to produce adverse effects either on physiological processes or growth. Their results showed that the ratio of K+/Mg2+, for instance, had a clear positive
cor-relation with concentration of H+ in
precipita-tion. This phenomenon was interpreted as an outcome of enhanced displacement of extracellu-lar Mg2+ by H+whereas K+, as a predominantly
intracellular cation, remained intact. However, it is not clear whether deposition of heavy metals produces comparable shifts in cation content in natural environments and thus enhance the influ-ence of acid rain when co-deposited.
Further-more, in experimental studies relatively extreme acid treatments (pH 2 – 3; i.e. 10 – 1.0 mM) have usually been found necessary in order to bring about significant effects on photosynthesis and growth (e.g. Hutchinson et al., 1986; Sigal and Johnston, 1986; Roy-Arcand et al., 1989; Scott et al., 1989; Hallingba¨ck and Kellner, 1992) whereas considerably lower concentrations (e.g. 0.01 – 1 mM) of heavy metals may cause adverse changes in lichen physiology (Brown and Beckett, 1984; Branquinho et al., 1997). Therefore the feasibility of changes in thallus cation content as a pre-dam-age bioindicator of heavy metals may be limited. On the other hand, Buck (1980), who studied the influence of nickel on the cation content ofClado
-nia rangiformis, observed a significant reduction in concentration of potassium indicating membrane damage only after treatment with concentrations higher than 0.2 M Ni2+. In this paper we
investi-gate the changes in cation concentration of C.
stellaris as a response to experimentally applied aqueous nickel solution. The experimental nickel deposition ranges from amounts that correspond to the most polluted areas of the Kola Peninsula to the virtually pristine forests of the Finnish border. We hypothesise that reduction in total concentrations of mainly or partially extracellu-larly bound cations (Ca2+, Mg2+) occurs in
lower concentrations than that of an intracellular one (K+) indicating changes in extracellular
bind-ing sites before membrane damage takes place.
2. Materials and methods
2.1. Design and execution of the experiment
The experiment was set up in a dry heath forest dominated by Scots pine (Pinus syl6estrisL.) and
a mat-forming lichen, C. stellaris (Opiz) Brodo, on the island of Hailuoto (65°01%N, 24°47%E) in
one as an intact dry control (subsequently referred to as DC), one was treated with deionized distilled water (Ni0), and the remaining three with 0.06, 0.6 and 6 mM NiCl2.6H2O (aq.) solutions yielding ca
10, 100 and 1000 mg Ni m−2depositions during a
growing season (referred to as Ni1, Ni2, and Ni3, respectively). All quadrats except DC were sprayed six (in 1992) to seven (in 1993) times with 50 ml of NiCl2solution during the 3 – month periods (June –
August). Spraying took place only during natural rainfall events or early in the morning when lichens were naturally moist after morning dew. Average natural rainfall in the area during the three summer months is ca 160 mm (Alalammi, 1988), and thus watering increased the amount of water received by the experimental plots by ca 2%.
2.2. Total concentrations of Ni2+, K+, Mg2+ and Ca2+ in lichen thalli
After treatments, five replicate lichen thalli were collected from each quadrat and rehydrated overnight at 4°C and 100% relative humidity (over water in a desiccator), then sprayed twice with deionized water to ensure full saturation and agi-tated briefly in deionized water to remove potential surface contaminants. Thalli were then cut horizon-tally with a razor blade into three strata at 20-mm intervals from the apex downwards (top, 0 – 20 mm; middle, 20 – 40 mm; and basal, 40 – 60 mm, seg-ments). These segments represent the living green top, intermediary pale zone and greyish necromass. These samples were then oven-dried at 80°C for 6 h, weighed, powdered and pressed into pellets and measured for Ni2+, K+, Mg2+and Ca2+by X-ray
fluorescence (Siemens SRS 303 AS with an AG-66 Rh-anode). A lithium fluoride (LiF100) crystal was used for K+, Ca2+ and Ni2+ and a OVO 55
layered crystal for Mg2+. Both international (SRM
1575) and ad hoc standards were used in the analysis. Ad hoc standards were prepared by adding known amounts of salts in solution that was absorbed by ground C. stellaris collected from a background area.
2.3. Statistical analysis
Relationships between treatment and lichen
cation contents were analysed using univariate ANOVA for repeated measures (ANOVAR). Op-tions for the ANOVAR analysis were chosen according to Potvin et al. (1990). If variance homogeneity or distribution normality was not met, log10(x+1) or (x+0.5) transformations
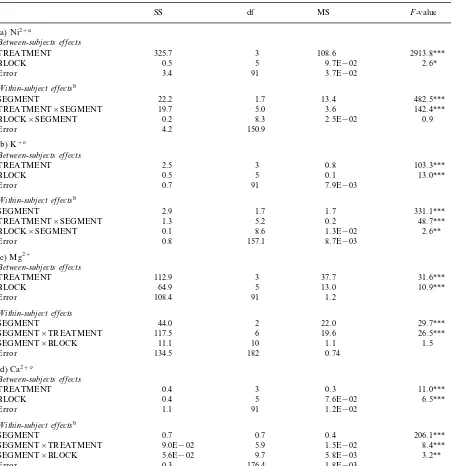
were applied to original concentration data in order to achieve the necessary prerequisites for analysis. A main effects model (BLOCK+TREATMENT) was fitted for between-subjects effects and the following one for within-subjects effects: SEG-MENT+TREATMENT*SEGMENT+BLOCK *SEGMENT. Thus it was assumed, as customary in randomised block designs (see e.g. Sokal and Rohlf, 1995), that the block factor had not inter-acted with the treatment.
3. Results
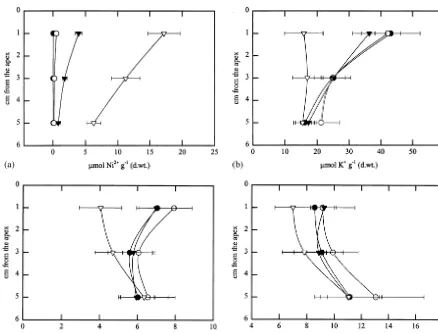
Concentrations of exchangeable cations in lichen tissue varied markedly between the treatments and showed altered patterns of vertical distribution in the lichen mat (Fig. 1). Since there were no signifi-cant differences in concentrations of any cations between Ni0 and DC treatments, only the former values were used in the subsequent statistical analy-ses.
that may be based on spatial variation in cation exchange capability or cation content in the ex-perimental area. It can be estimated on the basis of the known lichen biomass per unit area and the thickness of the lichen mat in the experimental area (ca 930 g dry wt m−2
and 6 cm) that lichens contained at the harvest approximately 66, 49 and 31% of the total nickel applied in Ni1, Ni2 and Ni3, respectively.
The most severe nickel treatment (Ni3) had a negative impact on the potassium content of the two uppermost strata whereas the influence of Ni2 treatment could only be seen in the top stratum (Fig. 1b). Potassium concentration also varied between the blocks. The divergent influence of nickel on different strata was evident on the basis
of strong interaction between treatment and seg-ment (Table 1b) but somewhat surprisingly the block-factor also had a significant interaction with the segment. The concentration of magnesium, the most uniformly distributed cation between the horizontal strata in intact lichens (Fig. 1c), showed parallel changes to those of potassium after Ni3 treatment. However, the concentration of magnesium seemed to be fairly insensitive to more moderate nickel treatments. Changes in the vertical distribution of calcium were perhaps less evident than in other cations, but still the strongest nickel treatment brought about signifi-cant changes in calcium concentrations (Fig. 1d;Table 1d). Furthermore, the block factor had a clear influence on overall concentration of
cal-Fig. 1. Vertical distribution of (a) Ni2+, (b) K+, (c) Mg2+ and (d) Ca2+ concentrations (
mmol g−1dry wt) in lichen thalli after
Table 1
Univariate analysis of variance with repeated measures (ANOVAR) for (a) Ni2+, (b) K+, (c) Mg2+and (d) Ca2+concentrations
(mmol g−1dry wt) in the three segments (0–20, 20–40 and 40–60 mm) in lichen thalli from six separate blocks after Ni2+treatment
df MS
SS F-value
(a) Ni2+a
Between-subjects effects
3
TREATMENT 325.7 108.6 2913.8***
5 9.7E−02
0.5 2.6*
BLOCK
3.4
Error 91 3.7E−02
Within-subject effectsb
1.7 13.4
22.2 482.5***
SEGMENT
19.7
TREATMENT×SEGMENT 5.0 3.6 142.4***
BLOCK×SEGMENT 0.2 8.3 2.5E−02 0.9
150.9 4.2
Error
(b) K+c
Between-subjects effects
3 0.8
2.5 103.3***
TREATMENT
0.5
BLOCK 5 0.1 13.0***
0.7
Error 91 7.9E−03
Within-subject effectsb
SEGMENT 2.9 1.7 1.7 331.1***
TREATMENT×SEGMENT 1.3 5.2 0.2 48.7***
8.6 1.3E−02
0.1 2.6**
BLOCK×SEGMENT
Error 0.8 157.1 8.7E−03
(c) Mg2+
Between-subjects effects
3 37.7
TREATMENT 112.9 31.6***
5 13.0
64.9 10.9***
BLOCK
108.4
Error 91 1.2
Within-subject effects
2 22.0
44.0 29.7***
SEGMENT
117.5
SEGMENT×TREATMENT 6 19.6 26.5***
11.1
SEGMENT×BLOCK 10 1.1 1.5
182 0.74
134.5 Error
(d) Ca2+c
Between-subjects effects
0.4
TREATMENT 3 0.3 11.0***
5 7.6E−02
BLOCK 0.4 6.5***
91 1.2E−02
1.1 Error
Within-subject effectsb
SEGMENT 0.7 0.7 0.4 206.1***
5.9 1.5E−02
9.0E−02 8.4***
SEGMENT×TREATMENT
5.6E−02
SEGMENT×BLOCK 9.7 5.8E−03 3.2**
176.4
Error 0.3 1.8E−03
aConcentration values(x+0.5) transformed. bDegrees of freedom Huynh–Feldtocorrected. cConcentration values log
cium in the three strata studied just as with the other cations and the responses of different hori-zontal strata to nickel treatment were divergent (Table 1d).
4. Discussion
The present results demonstrate that the distri-bution of cations in the lichen thallus is altered by moderate additions of nickel in aqueous solution. The vertical distribution of nickel clearly indi-cated that much of the element taken up by the thallus was retained in the top part. It was clear that the ion exchange capacity of the top stratum was not saturated by nickel on the day of the harvest since calcium concentration in the top segment was still more than half that of intact thalli. On the other hand, this does not exclude the possibility that ion exchange sites that were closest to the lichen surface may have become at least temporally saturated during the nickel expo-sures. As the lichens contained only up to two-thirds of the amount applied, and also the proportion of nickel captured was lowest after the most severe treatment, a considerable proportion of the nickel might not have entered the thallus at all and rather leached to the soil as surface flow. Furthermore, Hyva¨rinen and Crittenden (1996) suggested that because calcium is located predom-inantly in the extracellular space it is possible that its vertical distribution in lichen mats varied to a greater extent than those of other cations in re-sponse to short-term fluctuations in thallus water status. This was actually reflected in higher ran-dom variation and between-block variation shown in the present results. High mobility of calcium in thallus could effectively lead to reoccupation of some exchange sites loaded by nickel cations and perhaps lead to changes in the vertical distribu-tion of nickel. Hence it is quesdistribu-tionable whether changes in the calcium content are suitable for bioindication purposes.
The impact of the highest nickel treatment on the concentration of potassium, which is located predominantly in protoplasts in lichens (e.g. Brown and Beckett, 1984; Hyva¨rinen and Critten-den, 1996), indicated damage to cell membranes
rather than cation exchange. This could also be confirmed by casual observation since the apices of C. stellaris were clearly more brownish after Ni3 treatment compared to other treatments. In Ni2 treatment the concentration of potassium was slightly lower than in the control but this may well be due to extracellular replacement of potas-sium rather than changes in the intracellular pool. According to Puckett (1976), dosage of an ele-ment supplied (i.e. product of concentration and time) has a correlation with the degree of mem-brane damage. In the present experiment the de-position was delivered in a relatively few episodes and direct toxic effects may have emerged in lichens soon after being exposed to the relatively high nickel concentration of Ni3 treatment. One can only guess whether deposition distributed more evenly in time would have caused less dam-age to cell membranes.
The effect on magnesium concentration may be largely explained by loss of permeability of cells as well but it cannot be excluded that replacement of magnesium from extracellular binding sites might have contributed in the results. In fact, this cannot satisfactorily be answered because magne-sium has typically a ‘fifty-fifty’ distribution be-tween the two cellular compartments (Hyva¨rinen and Crittenden, 1996). However, in contrast to the results of Hyva¨rinen and Crittenden (1996) on the influence of acid rain on lichen cation content, the concentration of magnesium was insensitive to nickel treatments other than the most severe one (Ni3 in Fig. 1c).
One feature that should not pass unnoticed was the relatively large variation in the concentrations of all cations, except nickel, between the blocks (Table 1). The reason for these differences may be genetic or perhaps due to minor differences in shading or inclination which have lead to differen-tial growth rates and growth dilution. Neverthe-less, the observed variation emphasises the importance of blocking as a useful tool for trolling spatial variation in analysing element con-tents of mat-forming lichens.
ombro-trophic bogs. Typically, potassium is highest in the living top part and calcium is highest in the necromass. One should note that the necromass may have a cation exchange capacity equivalent to, or even greater than, the living thallus (Pakari-nen, 1985). Pakarinen (1981, 1985) suggested that potassium as well as phosphorus and nitrogen are actively scavenged and recycled from the old parts of thallus in order to fund the growth of apices. In fact, the experimental data on the uptake of nitrate, ammonium and phosphate by lichens sug-gest that they are actively absorbed (Smith, 1960; Farrar, 1976; Crittenden, 1996; Hyva¨rinen and Crittenden, 1998a,b). It has also been shown re-cently by Hyva¨rinen and Crittenden (1999) that
Cladina portentosais capable of actively recycling phosphorus from decaying lower strata to the top. However, the fate of potassium in the lichen thallus has largely been unexplored but experi-mental studies have shown that when rainfall percolates through lichen mats it becomes en-riched in calcium, magnesium and sodium but not in potassium (Kyto¨viita, 1993).
5. Conclusion
The present results show that the impact of long-term deposition of cationic elements with high phytotoxicity on lichens may cause damage to protoplast membranes at the level of deposition that is insufficient to cause any changes in extra-cellular binding sites. Therefore the use of the ratios of total concentrations of intra- and extra-cellular cations is not a feasible method for biomonitoring pollution of heavy metals. This seems to be due to the insensitivity of total cation concentration in lichen thallus to episodic nickel deposition rather than the susceptibility of proto-plast membranes to it. As the influence of nickel deposition to lichen cation exchange system seems negligible at moderate levels of deposition, the use of lichen cation ratios as indicators of precipita-tion acidity as presented by Hyva¨rinen and Crit-tenden (1996) is a feasible method in all but the most polluted areas. It is hoped that these findings may have wider relevance in pollution assessment in nutrient-poor ecosystems of polar regions
where mat-forming lichens are prominent vegeta-tion components.
Acknowledgements
We thank U. Ahonen-Jonnarth, V.-P. Pelkonen, A. Jumpponen, H. Va¨re and J. Lamppu for technical assistance and P. Rautio for useful discussions. Access to the study area was kindly granted by E. Merila¨. The research was supported by the Jarna Foundation.
References
Ahti, T., Oksanen, J., 1990. Epigeic lichen communities of taiga and tundra regions. Vegetatio 86, 39 – 70.
Alalammi, P. (Ed.), 1988. Atlas of Finland, Folio 131: Cli-mate, 5th edn. Publications Division of the Natural Board of Survey, Helsinki.
Branquinho, C., Brown, D.H., Catarino, F., 1997. The cellular location of Cu in lichens and its effects on membrane integrity and chlorophyll fluorescence. Environ. Exp. Bot. 38, 165 – 179.
Brown, D.H., 1976. Mineral uptake by lichens. In: Brown, D.H., Hawksworth, D.L., Bailey, R.H. (Eds.), Lichenol-ogy: Progress and Problems. Academic Press, London, pp. 419 – 439.
Brown, D.H., 1991. Lichen mineral studies — currently clarified or confused. Symbiosis 11, 207 – 223.
Brown, D.H., Beckett, R.P., 1984. Uptake and effects of cations on lichen metabolism. Lichenologist 16, 173 – 188. Buck, G.W., 1980. The cellular location of physiological and heavy metal cations in lichens and bryophytes under nor-mal and stressed conditions. Ph.D. thesis, University of Bristol, UK.
Burton, M.A.S., LeSueur, P., Puckett, K.J., 1981. Copper, nickel, and thallium uptake by the lichen Cladina rangiferina. Can. J. Bot. 59, 91 – 100.
Crittenden, P.D., 1996. The effect of oxygen deprivation on inorganic nitrogen uptake in an Antarctic macrolichen. Lichenologist 28, 347 – 354.
Farrar, J.F., 1976. The uptake and metabolism of phosphate by the lichenHypogymnia physodes. New Phytol. 77, 127 – 134.
Hallingba¨ck, T., Kellner, O., 1992. Effects of simulated nitro-gen rich and acid rain on the nitronitro-gen-fixing lichen Peltig-era aphthosa(L.) Willd. New Phytol. 120, 99 – 103. Hutchinson, T.C., Dixon, M., Scott, M., 1986. The effect of
simulated acid rain on feather mosses and lichens of the boreal forest. Water Air Soil Pollut. 31, 409 – 416. Hyva¨rinen, M., Crittenden, P.D., 1996. Cation ratios in
Hyva¨rinen, M., Crittenden, P.D., 1998a. Phosphate uptake in Cladonia portentosa. Lichenologist 30, 297 – 301.
Hyva¨rinen, M., Crittenden, P.D., 1998b. Relationships be-tween atmospheric nitrogen inputs and the vertical nitro-gen and phosphorus concentration gradients in the lichen Cladonia portentosa. New Phytol. 140, 519 – 530.
Hyva¨rinen, M., Crittenden, P.D., 2000. 33P translocation in the thallus of the mat-forming lichenCladonia portentosa. New Phytol., 142 in press.
Kyto¨viita, M.-M., 1993. Effects of acid rain on growth and nutrient relations in mat-forming lichens. Ph.D. thesis, University of Nottingham, UK.
Larsen, J.A., 1980. The Boreal Ecosystem. Academic Press, New York.
Longton, R.E., 1988. Biology of Polar Bryophytes and Lichens. Cambridge University Press, Cambridge. Nieboer, E., Richardson, D.H.S., 1980. The replacement of
the nonscript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut. 1, 3 – 26.
Nieboer, E., Puckett, K.J., Grace, B., 1976. The uptake of nickel byUmbilicaria muhlenbergii: a physicochemical pro-cess. Can. J. Bot. 54, 724 – 733.
Nieboer, E., Richardson, D.H.S., Tomassini, F.D., 1978. Min-eral uptake and release by lichens: an overview. Bryologist 81, 226 – 246.
Pakarinen, P., 1981. Nutrient and trace metal content and retention in reindeer lichen carpets of Finnish ombro-trophic bogs. Ann. Bot. Fenn. 18, 265 – 274.
Pakarinen, P., 1985. Mineral element accumulation in bog lichens. In: Brown, D.H. (Ed.), Lichen Physiology and Cell Biology. Plenum Press, New York, pp. 185 – 192.
Potvin, C., Lechowicz, M.J., Tardif, S., 1990. The statistical analysis of ecophysiological response curves obtained from experiments involving repeated measures. Ecology 71, 1389 – 1400.
Puckett, K.J., 1976. The effect of the heavy metals on some aspects of lichen physiology. Can. J. Bot. 54, 2695 – 2703. Richardson, D.H.S., Kiang, S., Ahmadjian, V., Nieboer, E., 1985. Lead and uranium uptake by lichens. In: Brown, D.H. (Ed.), Lichen Physiology and Cell Biology. Plenum Press, New York, pp. 227 – 246.
Roy-Arcand, L., Delisle, C.E., Brie`re, F.G., 1989. Effects of simulated acid precipitation on the metabolic activity of Cladina stellaris. Can. J. Bot. 67, 1796 – 1802.
Scott, M.G., Hutchinson, T.C., Feth, M.J., 1989. Contrasting responses of lichens andVaccinium angustifoliumto long-term acidification of a boreal forest ecosystem. Can. J. Bot. 67, 579 – 588.
Sigal, L.L., Johnston, J.W., 1986. Effects of simulated acidic rain on one species each of Pseudoparmelia, Usnea, and Umbilicaria. Water Air Soil Pollut. 27, 315 – 322. Smith, D.C., 1960. Studies in the physiology of lichens 1. The
effects of starvation and ammonia absorption upon the nitrogen content of Peltigera polydactyla. Ann. Bot. 24, 52 – 62.
Sokal, R.R., Rohlf, F.J., 1995. Biometry: the principles and practice of statistics in biological research, 3rd edn. W.H. Freeman & Co., New York.
Tikkanen, E., Niemela¨, I. (Eds.), 1995. Kola Peninsula Pollu-tants and Forest Ecosystems in Lapland. Final Report of The Lapland Forest Damage Project. Gummerus, Jy-va¨skyla¨.