Solid state fermentation of agricultural wastes for
endoglucanase production
Luiza Jecu
Department of Biotechnology,Research Institute for Chemistry,Str.Pictor Bancila8,76327,Bucharest,Romania
Received 11 February 1999; received in revised form 23 March 1999; accepted 20 April 1999
Abstract
The lignocellulosic biomass (especially agricultural wastes) is known to be an excellent carbon source for microbial enzyme production. In this paper, the cellulase production from lignocellulosic materials under solid state fermenta-tion (SSF) was investigated. The effects of fermentafermenta-tion condifermenta-tions, such as moisture content, initial pH, temperature, and composition of mixed substrate (wheat straw and wheat bran) on endoglucanase production byAspergillus niger
38 were studied. With a moisture content of 74% a pH range of 4.5 – 5.5 on mixed substrate containing wheat straw:wheat bran of 9:1, 14.80 international units (UI) endoglucanase activity/ml were obtained in 96 h. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Solid state fermentation; Cellulases;Aspergillus niger
www.elsevier.com/locate/indcrop
1. Introduction
Hydrolases are produced by submerged cultiva-tion or by solid state cultivacultiva-tion using moistened cereals and agricultural wastes. As the microor-ganisms in solid state fermentation (SSF) grow under conditions closer to their natural habitats, they may be more capable of producing certain enzymes and metabolites which usually will not be produced or will be produced only with low yield in a submerged culture. Taking into consideration the simplicity of the cultivation equipment and lower expense for operation, wider application of this traditional method is expected with the ad-vanced knowledge and approaches in biochemical engineering.
Various agricultural substrates/byproducts and microbial cultures have been used successfully in solid state fermentation for cellulase production (Chahal, 1985; Madamwar et al., 1989; Duenas et al., 1995).
Cellulase is a complex mixture of enzyme proteins with different specificities to hydrolyze glycosidic bonds. The three major cellulase en-zyme activities are: endocellulase (1,4-beta-D
-glu-can-4-glucanohydrolase, endoglucanase, EG, EC 3.2.1.4.), exocellulase (1,4-beta-D
-glucan-cellobio-hydrolase, CBH, EC 3.2.1.91) and beta-glucosi-dase (beta-D-glucosido-glucohydrolase, cellobiose,
EC 3.2.1.21).
The microorganisms which appear to be most promising at present are the Trichoderma reesei
mutants. However, it is of an interest to us to examine a new microorganism to improve the cellulase production.Aspergillusspecies are major agents of decomposition and decay and thus pos-sess the capability to produce a broad range of enzymes. The genus is known to be a good pro-ducer of cellulases (Berka et al., 1992; Oxenboll, 1994).
In this paper, the experiments were conducted to obtain optimum levels of cellulase by Asper
-gillus nigerstrain using easily available substrates like wheat straw and wheat bran on solid state fermentation. The effects of the different propor-tions in mixed substrates, moisture levels, initial pH values, and growth temperature were investigated.
2. Materials and methods
2.1. Organism
The organism used in this study, A. niger 38 was obtained from the microbial collection of Research Institute for Chemistry. The stock cul-ture was maintained on potato – glucose – agar.
2.2. Substrates
Air-dried and milled wheat straw (WS) and wheat bran (WB) were utilized as substrates for solid state fermentation.
2.3. Culture media
The composition of mineral culture medium was (g/l): 10 (NH4)SO4; 3 KH2PO4; 0.5 MgSO47
H2O and 0.5 CaCl2 H2O (Toyama and Ogawa,
1977). Wheat straw and wheat bran in different proportions (9:1 to 1:9) and controls (WS and WB) were dispersed.
2.4. Growth conditions
Following heat sterilization (121°C for 30 min) the medium was inoculated with 0.5 ml fungal spores suspended in sterile water containing a drop of Tween 20. The volume of inoculum
con-tributed to a maximum variation of only 2.5% in moisture level. The moisture content was varied by adding distilled water to obtain a humidity range of 55 – 80% (w/w). The cultures were incu-bated at different temperatures: 25; 28; 30; 34 and 37°C. Static cultivation were performed at various initial pH values: 3.5; 4.0; 4.5; 5.0; 5.5 and 6.0. The incubation time was limited to 96 h after which mouldy substrates were extracted without drying.
2.5. Enzyme extraction
Fresh mouldy substrates were soaked in dis-tilled water (1:20, w/v), the mixtures were allowed to stand for 1 h and extracts were obtained by filtering the mixtures through nylon cloth. The clarified filtrates were checked for endoglucanase activity.
2.6. Enzyme acti6ity
The endoglucanase activity was determined ac-cording to Mandels (Mandels et al., 1976), using 1% carboximethylcellulose. The amount of reduc-ing sugars released was determined usreduc-ing dini-trosalicylic reagent (Miller, 1959). The activity was expressed in international units (IU), defined as the amount of enzyme required to produce 1
mmol glucose/min.
3. Results and discussion
In a previous paper (Jecu, 1996) we have stud-ied the use of the cellulolytic fungus A. niger 38 for cellulase production in submerged cultures. The strain was able to produce in submerged culture a cellulase complex comprising endoglu-canase, exoglucanase and cellobiase.
other aspergilli show little capacity to cleave highly ordered forms of cellulose (Berka et al., 1992).
Taking count of the advantages of the solid state fermentation, we have investigated some fac-tors affecting cellulase production, such as: water content of fermenting substrates, growth tempera-ture, initial pH values. Moreover we have investi-gated the optimal composition for cellulase production of the mixed substrates.
3.1. Effect of carbon source composition
Cellulase production was found to be depen-dent upon the nature of the carbon source used in the culture media. Various ratio of wheat straw and wheat bran were tested for their effect on cellulase production. The influence of the mixed substrate composition upon the cellulase biosyn-thesis at A. niger 38 is recorded in Table 1. One part of wheat bran and nine parts of wheat straw showed the best activities.
3.2. Effect of moisture le6el
The influence of the substrate humidity is pre-sented in Table 2. An increase in the initial mois-ture content of the substrate from 55 to 74% greatly enhanced the enzyme activity of the broth. However, a further increase to 80% had a nega-tive effect on the production of cellulolytic en-zyme. The experiments were done with mixed substrate WB:WS, 1:9, as determined above.
Table 2
Influence of initial moisture content on endoglucanase produc-tion byAspergillus niger
Growth time (h) Endoglucanase activity (IU/ml)
55% 60% 74% 80%
96 6.20 8.90 11.25 9.45
3.3. Effect of culture initial pH
Continuing consecutive optimization of cellu-lase production by A. niger, the effects of initial pH of culture medium was investigated. Produc-tion of cellulase did not vary appreciably within an initial pH range of 4.5 – 5.5 (Table 3). It was found that, in the course of time, maximum activ-ity was obtained when the initial pH was adjusted to 4.5 – 5.0. The above results are similar to those reported for other fungal cellulases (Okada, 1985; Acebal et al., 1986; Murao et al., 1988).
3.4. Effect of growth temperature
The results of the tests made at different tem-perature values showed that the optimum for endoglucanase production by A. niger 38 was 28 – 34°C (Table 4). The temperature did not af-fect strongly the enzyme production, this behavior being favorable for stabile cultivation in solid medium. Szewczyk and Myszka (1994) have also reported a similar phenomenon in an experiment with A. niger. For temperatures above 34°C, the
Table 1
Effect of mixed substrate composition on endoglucanase pro-duction byAspergillus niger
Substrate ratio WB:WS Endoglucanase activity (IU/ml)
1:9 9.30
Effect of culture initial pH on endoglucanase production by
Aspergillus niger
Table 4
Effect of growth temperature on endoglucanase production by
Aspergillus niger
Temperature (°C) Endoglucanase activity (IU/ml)
25 6.40
plex) to the enzymologist, which are rarely encountered in the study of other enzymes. In the face of these difficulties it is understandable that the comparison of cellulase activities produced by different laboratories is not readily made in a quantitative manner. On the other hand, the en-doglucanase measurements do not reflect the true activity for hydrolyzing crystalline cellulose. The ability of Trichoderma reesei and its mutants to produce cellulases is considered in measuring the enzyme concentration FPU (filter paper activity)/
ml (Esterbauer et al., 1991).
4. Conclusions
The results reported in this paper indicate that
A. niger 38 can be cultivated under solid state fermentation for the production of cellulolytic enzyme using as carbon source available agricul-tural wastes. Under mixed solid state fermenta-tion condifermenta-tions, the cellulolytic fungus produces 14.80 IU endoglucanase activity/ml in 96 h. The fungus performances could be improved by fur-ther investigation in larger scale operation and by mutagenic methods. The technique of solid state fermentation would help in producing enzyme solutions of any desired concentration in a shorter time and consequently may help in reducing the cost of enzyme production.
enzyme biosynthesis decreases as temperature in-creases. This fact is probably due to temperature deactivation.
From the above experiments, the optimized medium and conditions in solid state fermentation were: mixed substrate (WS:WB, 9:1); growth tem-perature 28 – 43°C; initial culture pH 4.5 – 5.0 and moisture level 74% (w/w). Under optimal condi-tion for the enzyme produccondi-tion in solid state fermentation byA.niger strain the endoglucanase activity was 14.80 UI/ml. The comparison of maximal endoglucanase activity produced by A.
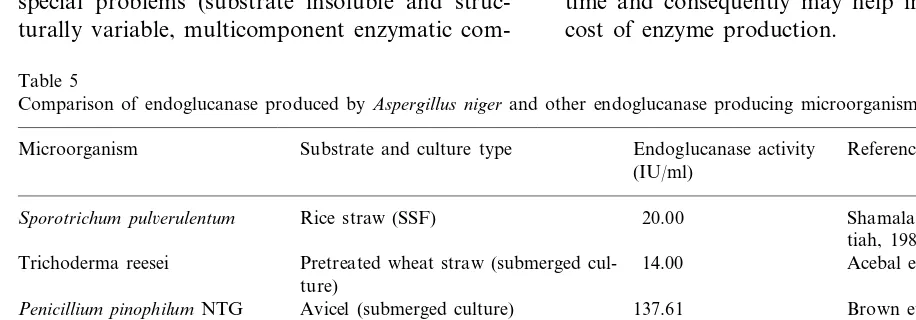
niger in SSF with those reported for other cellu-lase producing microorganisms (Table 5) showed that the fungus investigated in this work com-pared favourably with most of those microorganisms.
The characterization of cellulase enzymes poses special problems (substrate insoluble and struc-turally variable, multicomponent enzymatic
com-Table 5
Comparison of endoglucanase produced byAspergillus nigerand other endoglucanase producing microorganisms
Substrate and culture type Endoglucanase activity
Microorganism Reference
(IU/ml)
Sporotrichum pul6erulentum Rice straw (SSF) 20.00 Shamala and
Sreekan-tiah, 1986 Pretreated wheat straw (submerged cul- Acebal et al., 1986
Trichoderma reesei 14.00
ture)
Avicel (submerged culture) Brown et al., 1987
Penicillium pinophilumNTG 137.61
(mutant)
65.00 Stewart and Parry, 1981
Aspergillus fumigatus Cellulose Whatman (submerged cul-ture)
Szczodrak et al., 1986 Pretreated sawdust (submerged culture)
Aspergillus terreus 23.28
14.00
Rice straw and wheat bran (SSF) Shamala and Sreekan-Aspergillus ustus
tiah (1986)
14.80 This work
References
Acebal, C., Castillon, M., Estrada, P., Mata, I., Costa, E., 1986. Enhanced cellulase production from Trichoderma reesei QM 9414 on physically treated wheat straw. Appl. Microbiol. Biotechnol. 24, 218 – 223.
Berka, R., Coleman, N., Ward, M., 1992. Industrial enzymes from Aspergillus species. In: Bennett, J., Klich, M.A. (Eds.),Aspergillus. Butterworth – Heinemann, pp. 155 – 202. Brown, A.J., Collin, S.A., Wood, T.M., 1987. Development of a medium for high cellulase, xylanase and beta-glucosidase production by a mutant strain (NTG III/6) of the cellu-lolytic fungus Penicillium pinophilum. Enzyme Microb. Technol. 9, 355 – 360.
Chahal, D.S., 1985. Solid-state fermentation withTrichoderma reeseifor cellulase production. Appl. Environ. Microbiol. 49, 205 – 210.
Duenas, R., Tengerdy, R.P., Gutierrez-Correa, M., 1995. Cel-lulase production by mixed fungi in solid state fermenta-tion of bagasse. World J. Microbiol. Biotechnol. 11, 333 – 337.
Esterbauer, H., Steiner, W., Labudova, I., Herman, A., Hayn, M., 1991. Production of Trichodermacellulase in labora-tory and pilot scale. Biores. Technol. 36, 51 – 65. Jecu, L., 1996. The use of lignocellulosic waste for production
of cellulase by an Aspergillus niger strain. In: Sand, W. (Ed.), Dechema Monographs Biodeterioration and Biodegradation. Hamburg, 133, 689 – 694.
Madamwar, D., Patel, S., Parekh, M., 1989. Solid state fer-mentation for cellulase and beta-glucosidase production by
Aspergillus niger. J. Ferment. Bioeng. 67, 424 – 426.
Mandels, M., Andreotti, R., Roche, C., 1976. Measurement of saccharifying cellulose. Biotechnol. Bioeng. Symp. 6, 21 – 33.
Miller, G.L., 1959. Use of dinitrosalicylic as reagent for the determination of reducing sugars. Anal. Chem. 31, 426 – 428.
Murao, S., Sakamoto, R., Arai, M., 1988. Cellulase ofAsper
-gillus aculeatus. In: Methods in Enzymology. Academic Press, 160 pp. 275 – 284.
Okada, G., 1985. Cellulase of Aspergillus niger. Agric. Biol. Chem. 49, 1259 – 1264.
Oxenboll, K., 1994. Aspergillusenzymes and industrial uses, In: Powell, K.A. (Ed.), The Genus Aspergillus. Plenum Press, New York, pp. 147 – 158.
Shamala, T.R., Sreekantiah, K.R., 1986. Production of cellu-lases and D-xylanase by some selected fungal isolates. Enzyme Microb. Technol. 8, 178 – 182.
Stewart, C., Parry, J.B., 1981. Factors influencing the produc-tion of cellulase by Aspergillus fumigatus (Fresenius). J. Gen. Microb. 125, 33 – 39.
Szczodrak, J., Ilczuk, Z., Rogalski, J., Leonowicz, A., 1986. Intensification of oak sawdust enzymatic hydrolysis by chemical or hydrothermal pretreatment. Biotechnol. Bio-eng. 28, 504 – 510.
Szewczyk, K.W., Myszka, L., 1994. The effect of the tempera-ture on the growth of Aspergillus niger in solid state fermentation. Bioproc. Eng. 10, 123 – 126.
Toyama, N., Ogawa, K., 1977. In: Ghose, T.K. (Ed.), Interna-tional Course on Biochemical Engineering Bioconversion. Biochem. Eng. Research Centre, IIT, New Delhi (India), pp. 182 – 207.