www.elsevier.com / locate / bres
Research report
Regulation of
a
2A-adrenoceptor expression by chronic stress in
neurons of the brain stem
a a b a ,
*
¨
Heiko Meyer , Monika Palchaudhuri , Mika Scheinin , Gabriele Flugge
a
¨
Division of Neurobiology, German Primate Center, Kellnerweg 4, D-37077 Gottingen, Germany
b
Department of Pharmacology and Clinical Pharmacology, University of Turku, FIN-20520 Turku, Finland
Accepted 2 August 2000
Abstract
a2-Adrenoceptors are supposed to be important regulatory elements in responses to stress. Previous receptor binding studies in male tree shrews have shown that chronic psychosocial stress down-regulates binding sites for a2-adrenergic ligands in several brain stem nuclei. The aim of the present study was to quantify effects of chronic subordination stress on expression of thea2-adrenoceptor subtype A gene in identified neurons of the brain stem. We partially cloned thea2A-adrenoceptor cDNA of the tree shrew (1.22 kb) and localized
35
receptor RNA expression in brain stem neurons by in situ hybridization using a S-labeled cRNA probe (1.06 kb). To identify neurons expressing receptor mRNA, brain sections were first immunocytochemically stained with antibodies against tyrosine hydroxylase, phenylethanolamine-N-methyltransferase, or glutamate, and then processed for in situ hybridization. Furthermore, expression of receptor-specific RNA was quantified in single neurons of animals which had been psychosocially stressed during 4 weeks and in unstressed controls. We found strong in situ hybridization in the noradrenergic neurons of the locus coeruleus, but only weak labeling of A2 neurons in the solitary tract nucleus and no labeling of A1 neurons in the caudal ventrolateral medulla. Adrenergic neurons in the solitary tract nucleus (group C2) did not express thea2A-adrenoceptor, and C1 neurons in the rostral ventrolateral medulla showed only a minor labeling by the in situ probe. In contrast, large glutamatergic neurons in the lateral reticular nucleus were strongly labeled by the probe. Chronic psychosocial stress reduceda2A-adrenoceptor RNA expression in locus coeruleus neurons (224.0%), in solitary tract neurons (231.0%), and in neurons of the lateral reticular nucleus (218.8%). These findings show that stress not only decreases the expression of thea2A-adrenergic autoreceptor in the locus coeruleus but also ofa2A-heteroreceptors in glutamatergic neurons. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behaviour
Topic: Stress
Keywords: a2A-Adrenoceptor; Chronic stress; Tree shrew; Locus coeruleus; Lateral reticular nucleus
1. Introduction tigation was to determine whether chronic psychosocial
stress alters expression of thea2-adrenoceptor subtype A. Previous work from our group has shown that chronic Several pieces of experimental evidence indicate that this subordination stress down-regulates a2-adrenoceptors in subtype is important in the locus coeruleus (LC), where it selected brain nuclei [6]. While these receptor binding regulates neuronal activity and therefore also noradrenaline studies demonstrated a decrease in numbers of binding release in the projection areas of the LC [2,4,17,32]. sites for radioligands, the experiments could not show The distribution of a2A-adrenoceptor expression has whether the effects were due to reduced expression of been studied in rat and mouse brain [25,33,35,40]. Al-receptor genes and which of thea2-adrenoceptor subtypes though it was demonstrated that the receptor is strongly was affected. Therefore, the aim of the present inves- expressed in the noradrenergic LC neurons, it is not entirely clear whether also other noradrenergic or adren-ergic cells express it. Electron microscopic studies re-*Corresponding author. Tel.:149-551-3851-133: fax:1
49-551-3851-vealed that most of the a -adrenoceptor immunoreactive
228. 2A
¨
E-mail address: [email protected] (G. Flugge). structures in the rostral ventrolateral medulla do not
contain tyrosine hydroxylase, indicating that they are not 2.2. a2 a-Adrenoceptor cloning and cRNA probe noradrenergic [23]. We therefore identified neurons in the
tree shrew brain stem expressing thea2A-adrenoceptor by PolyA1-RNA was extracted from the tree shrew brain combining in situ hybridization and immunocytochemical stem with the Micro Fast Tract kit (Invitrogen, Leek, techniques. Expression of mRNA for the receptor was Netherlands) and cDNA was generated using the cDNA-quantified in neurons of male tree shrews that had been Cycle kit (Invitrogen). Published a2A-AR cDNA se-psychosocially stressed for four weeks and in unstressed quences of human, rat and mouse [15,16,18], EMBL / controls. GenBank data bank (EMBL, Heidelberg, Germany) and Male tree shrews (Tupaia belangeri ) provide an animal IntelliGenetics (Mountain View, CA, USA) were used to model to study the effects of chronic psychosocial stress design two oligonucleotide primers from the conserved [9]. When two males are kept together in one cage, there elements of the coding regions of the a2A-adrenoceptor are short social encounters whereupon a clear dominant- gene (size of coding region, 1.35 kb). The sequence 59 -subordinate hierarchy is established. During such periods CGCGGCGGAAATCGTGGTTGAAGAT-3 served as re-of stress, the subordinate shows persistent activation re-of the verse, and 59-CTGCAGGTGACGCTGACGCTGGTGT-3 sympathetic–adrenomedullary system and the hypo- as forward primer (KEBO Lab, Espoo, Finland). The PCR thalamic–pituitary–adrenal (HPA) axis. In the brains of was performed at 96, 67 and 728C, each for 60 s (35 subordinates, the noradrenergic system is chronically acti- cycles) in the presence of 10 mM Tris–HCl (pH 8.8), 50 vated, as reflected by a high noradrenaline turnover rate mM KCl, 0.5 mM MgCl , 0.1% Triton X-100 and 2002 mM [30]. dNTP-mix with 100 ng cDNA, 2 units DynaZyme DNA In the present study,a2A-adrenoceptor expression in the polymerase (Finnzymes, Espoo, Finland), and 50 pmol of tree shrew brain stem was visualized by in situ hybridiza- each primer. The amplification reaction was terminated by tion. To obtain probes for the experiments, a 1.22-kb primer extension at 728C for 25 min. Three PCR-products cDNA-fragment of the tree shrew a2A-adrenoceptor was of approximately 1.2 kb were subcloned in pGEM-T
35
cloned using RT-PCR techniques. A [ S]UTP labeled (Promega, Madison, WI, USA), and characterized by run-off cRNA transcript was generated to visualize the manual sequence analysis using the Sequenase 2.0 kit distribution of receptor mRNA on autoradiography films. (Pharmacia, Biotech, Uppsala, Sweden). For cRNA pro-In brain sections coated with photographic emulsion, the duction, the plasmid was cut with BglII (Pharmacia receptor mRNA expression was visualized in single neu- Biotech, Uppsala, Sweden) to generate a 1057-nucleotide rons that were identified with antibodies against catechol- fragment complementary to positions 238–1219 of the amine-synthesizing enzymes and against glutamate. For cloned cDNA fragment and 75 nucleotides of the vector semi-quantitative analysis of receptor expression in chroni- (antisense) or NotI (Promega; sense). The linearized cDNA cally stressed animals and in controls, silver grains were was in vitro-transcribed with the Riboprobe in vitro-tran-counted over single neurons. scription system (Promega), using T7-polymerase to gener-ate the antisense and SP6-polymerase to genergener-ate the sense
35
probe in the presence of [ S]UTP (250mCi; ICN Pharma-ceuticals, Costa Mesa, CA). The probes were purified with
2. Materials and methods S400 HR MicroSpin columns (Pharmacia). Labeling
ef-ficiency was analyzed in a scintillation counter and integri-2.1. Animal experiments ty was checked on a 6% polyacrylamide gel.
Male tree shrews (Tupaia belangeri ) from the breeding 2.3. Immunocytochemistry colony at the German Primate Center were used [8]. All
2.4. In situ hybridization labeled neuron) was subtracted from specific labeling. Sections of a control and a stressed animal mounted on the To identify the neurons expressing a2A-adrenoceptor same slide were analyzed pair wise (for details see Section mRNA, antibody-stained paraffin sections were pretreated 3). For statistical evaluation, data were subjected to before the in situ hybridization. Briefly, slides were ANOVA and Newman–Keuls post hoc test.
hydrated in graded alcohols, rinsed in 0.9% NaCl and phosphate-buffered saline (PBS) for 5 min each and
post-fixed in 4% PFA (10 min). They were then treated with 3. Results
proteinase K (20 mg / ml; Boehringer, Mannheim,
Ger-many) at 378C, 60 min, washed in PBS, acetylated in 0.1 3.1. a2 A-cDNA and cRNA probe M triethanolamine, and processed for in situ hybridization
(see below). Three clones of the tree shrew a2A-adrenoceptor gene For quantification of receptor expression in stressed were obtained which yielded identical DNA-sequences animals and controls, frozen brains were cut on a cryostat. representing 89% of the expected receptor coding sequence Sections (10 mm) were thaw-mounted on gelatin-coated (Fig. 1). Alignment of the tree shrew cDNA sequence with slides. Sections from one subordinate and one control those from the human (clone C10) and the rat gene (clone animal were mounted pair wise on the same slide. The RG20) showed 92% DNA sequence identity with the cryostat sections were then dried at room temperature for human and 88% identity with the rat sequence. Antisense 20 min, fixed in 4% PFA, rinsed in PBS, dehydrated run-off transcription of the linearized cDNA-clone in the
35
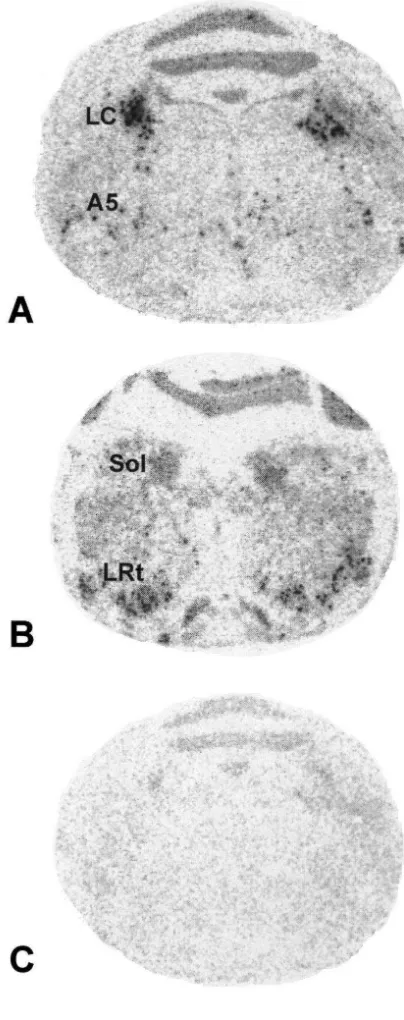
through graded alcohols, air-dried and stored at 2808C. presence of [ S]UTP generated a 1057-nucleotide cRNA Before the in situ hybridization, cryostat sections were probe complementary to nucleotides 238–1219 of the re-hydrated, post-fixed a second time with 4% PFA, and cloned cDNA fragment and 75 nucleotides of the vector then acetylated. For in situ hybridization, all sections were sequence (Fig. 1). In situ hybridization of brain sections processed as described with two modifications: (1) the with this probe resulted in a specific hybridization pattern hybridization was carried out at 608C for 18 h, and (2) visualized by film autoradiography (Fig. 2). At the level of when washing the sections, the RNase treatment was the LC, the most pronounced hybridization was detected in followed by a high stringency wash (0.23 SSC, 60 min, the LC itself and scattered labeling was observed in the 658C) [21]. area of the noradrenergic cell group A5. Moderate labeling To obtain an overview of the hybridization pattern, was observed in the solitary tract nucleus. The lateral labeled sections were exposed on BioMax film (Kodak, reticular nucleus was strongly labeled. Hybridization with Rochester, NY, USA) for 7 days at 48C. To visualize the sense probe yielded only a faint and diffuse au-hybridization on the cellular level, slides were coated with toradiographic image with no distinct labeling of brain NTB 2 nuclear emulsion (Kodak, Rochester, NY, USA) stem structures (Fig. 2).
and exposed for 25 days at 48C. Silver grains were
developed in D 19 (Kodak), slides were rinsed in water 3.2. a2 A-Adrenoceptor RNA expressing neurons and fixed with Unifix (Kodak). Sections were faintly
counter stained with 0.05% toluidine blue in 0.1% di- Neurons expressing a2A-adrenoceptor mRNA were first sodium tetraborate. For quantification of silver grains over visualized on cryostat sections that had been hybridized
35
single neurons in cryostat sections from stressed tree with the S-labeled cRNA probe and lightly counter shrews and controls, emulsion-coated sections were in- stained with toluidine blue (Fig. 3). Cells were classified as spected under a microscope with a CCD camera connected being specifically labeled when the number of silver grains to an image analysis system using the grain counting over the cell was more than 5 times the number of grains
35
program MCID/ M1 4.2 (Imaging Research, Ontario, in the surrounding area. Hybridization with the S-labeled Canada). For this purpose, slides had been labeled with a sense probe yielded no cells in the respective brain areas code unknown to the investigator. The specificity of the that fulfilled this criterion.
Fig. 1. Nucleic acid sequence of the tree shrewa2A-adrenoceptor cDNA representing 89% of the expected receptor coding sequence, and alignments with the partiala2A-adrenoceptor cDNAs of human (clone C10; nucleotides 88–1306 [15]) and rat (clone RG20; 88–1306 [16]). Dashes represent identical nucleotides, asterisks mark missing nucleotides. Underlined letters denote the forward and reverse oligonucleotide primers used for PCR-amplification of the tree shrew receptor cDNA.
sibly glial cells sometimes showed slight accumulations of silver grains.
3.3. Immunocytochemcal identification of antisense labeled cells
To identify the neurons that expressed mRNA for the
a2A-adrenoceptor, paraffin sections were immuno-cytochemically stained with antibodies, either against tyrosine hydroxylase (TH), the rate-limiting enzyme of the noradrenaline biosynthesis pathway, against phenyl-ethanolamine-N-methyltransferase (PNMT), the enzyme generally used to visualize adrenergic neurons, or against glutamate or GABA. After the immunocytochemical pro-cedure, sections were processed for in situ hybridization.
The noradrenergic neurons in the LC were strongly stained by the TH antibody and showed pronounced accumulations of silver grains corresponding to a2A -ad-renoceptor RNA in cell nuclei and the cytoplasm (Fig. 4). In the solitary tract nucleus, only few TH-labeled cells showed minor accumulations of silver grains (not shown). PNMT immunopositive neurons in the solitary tract nu-cleus were not labeled by thea2A-adrenoceptor probe (Fig. 4). Instead, the hybridization signal was seen over toluidine blue stained nuclei of cells that were not labeled by the PNMT antibody. In the area of the adrenergic cell group C1, PNMT-immunopositive neurons showed only slight accumulations of silver grains (Fig. 4). In the vicinity of PNMT-positive neurons, immunonegative struc-tures displayed high numbers of silver grains. On sections stained with the antibody against glutamate, these struc-tures were identified as large immunopositive neurons that showed pronounced accumulations of silver grains with numbers of grains varying from cell to cell (Fig. 4). Cells stained with the GABA antibody were never labeled by the receptor antisense probe, neither in the LC, nor in solitary tract nucleus and lateral reticular nucleus. No specific labeling of antibody-stained cells was detected with the
35
S-labeled sense probe in the analyzed areas.
3.4. Quantification ofa2 A-adrenoceptor expression
a2A-Adrenoceptor expression in brain stem nuclei of chronically stressed tree shrews and controls was
quan-35
Fig. 2. Autoradiograms showing hybridization with the S-labeled
tified by determining the numbers of silver grains over antisense riboprobe generated from thea2A-adrenoceptor cDNA (A,B),
35 single neurons on cryostat sections coated with
photo-and with the S-labeled sense probe (C). The neuroanatomical levels of
the sections are P 2.0 (A,C) and P 7.5 (B) according to the tree shrew graphic emulsion. For quantification, the microscopic atlas [36]. Abbreviations: A5, noradrenergic cell group A5. LC, locus picture was projected onto a computer screen, labeled cells coeruleus; LRt, lateral reticular nucleus; Sol, solitary tract nucleus. Bar
in the observation area were manually delineated, and represents 4 mm.
silver grains were counted by computer-based image analysis yielding pixels representing relative numbers of concentrated over the cell nuclei as was the case in the LC silver grains per cell. Only those cells were analyzed that and the solitary tract nucleus. In contrast, in the lateral showed at least 5 times more silver grains than surround-reticular nucleus, large neurons with high numbers of ing areas.
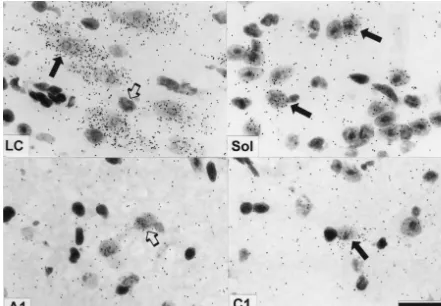
Fig. 3. Expression ofa2A-adrenoceptor RNA in neurons of the locus coeruleus (LC), the solitary tract nucleus (Sol), the area of the noradrenergic cell group A1, and of the adrenergic cell group C1. Sections were lightly counterstained with toluidine blue to visualize cell nuclei. Filled arrows indicate labeled cells, open arrows denote unlabeled cells. Note that in the LC, many silver grains are located over neurons, whereas in the solitary tract nucleus, only a few neurons are labeled with low numbers of silver grains. In the areas of the noradrenergic cell groups A1 and the adrenergic cell group C1, there are only minor accumulations of silver grains over cell nuclei. Bar represents 30mm.
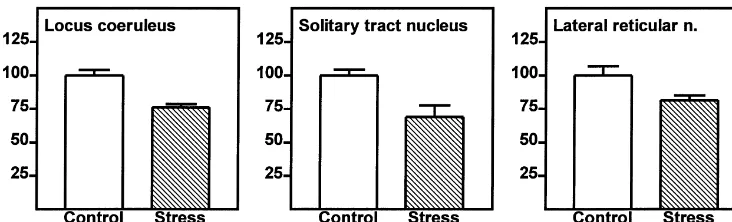
nucleus where noradrenergic cells are loosely clustered (animal no. 2 versus 3, P,0.05), but overall, this was not (Fig. 4). At this level, seven to 10 labeled cells per significant (one-way ANOVA, P50.066). Also two-way hemisphere were analyzed (14–20 cells per section). There ANOVA showed a reliable effect of the treatment (F5
were no reliable differences in numbers of labeled neurons 138.2, P50.0013), and a difference between individuals between the hemispheres. Since stress had the same effect (F517.6, P50.045). Therefore, in the LC of subordinates, on silver grain numbers in the nuclei as in the cytoplasm of the number of silver grains was significantly lower than in neurons, silver grains over these cellular compartments controls (224.0%; Fig. 5).
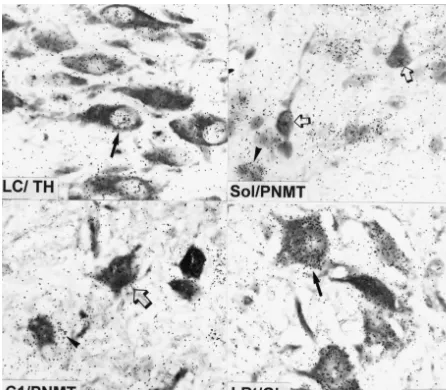
Fig. 4. TH-immunopositive neurons in the locus coeruleus (LC), PNMT-immunopositive neurons in the solitary tract nucleus (Sol), PNMT-immuno-positive neurons in area C1, and glutamate (Glu) immunoPNMT-immuno-positive neurons in the lateral reticular nucleus (LRt). Paraffin sections were first stained with the respective antibody and then processed for in situ-hybridization to visualize a2A-adrenoceptor expression (silver grains). LC: note that the TH-immunopositive neurons show high numbers of silver grains representing RNA encoding the receptor (filled arrow). Sol: section was counterstained with toluidine blue to visualize cell nuclei. Note that PNMT-immunopositive cells show only background levels of silver grains (open arrow), whereas toluidine
35
blue stained nuclei of immunonegative cells are labeled by the S-labeled cRNA probe (arrow head). C1: note that PNMT-immunopositive cells show only slight accumulations of silver grains (open arrow). Instead, the surrounding of the PNMT cell reveals patches of silver grains (arrow head). LRt: note that the large glutamate-immunopositive neurons in the lateral reticular nucleus display high numbers of silver grains (filled arrow). Bar represents 30mm.
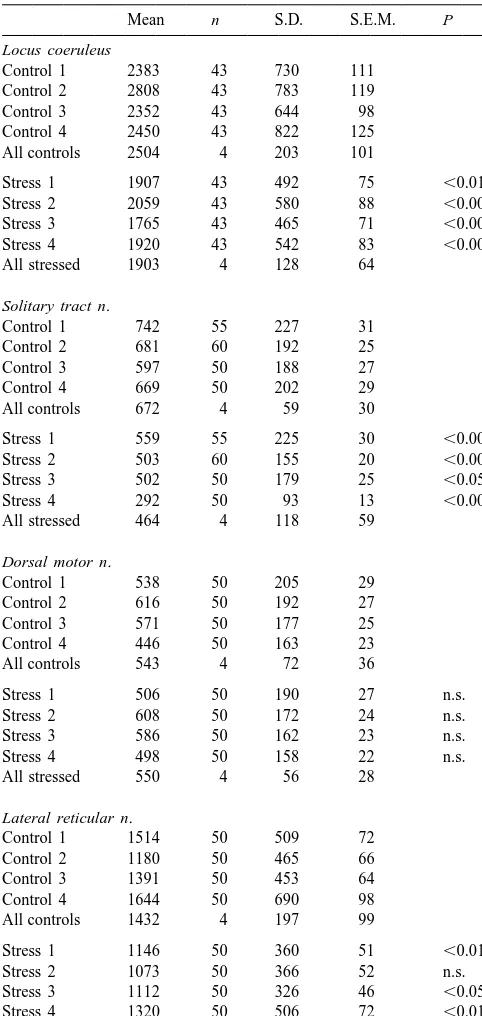
Table 1 3.5. Peripheral reactions during chronic subordination Effect of chronic stress on numbers of silver grains (expressed as stress
a
pixels / cell) in brain stem nuclei (n5number of cells)
Mean n S.D. S.E.M. P In accordance with our previous reports, the chronic
Locus coeruleus psychosocial stress exposure significantly reduced body
Control 1 2383 43 730 111 weight of subordinate males to 91.763.9% of the initial Control 2 2808 43 783 119 value (P,0.05). Body weight of control animals remained
Control 3 2352 43 644 98
constant during the whole experimental period. Cortisol in
Control 4 2450 43 822 125
urine of subordinates was significantly increased (mean of All controls 2504 4 203 101
all days during the stress period: 0.2060.01 ng /mmol
Stress 1 1907 43 492 75 ,0.01
creatinine versus 0.0860.01 ng /mmol creatinine during the
Stress 2 2059 43 580 88 ,0.001
no-stress period; P,0.01), whereas urinary cortisol in
Stress 3 1765 43 465 71 ,0.001
Stress 4 1920 43 542 83 ,0.001 control animals remained low throughout the experimental All stressed 1903 4 128 64 period (0.0960.03 ng /mmol creatinine).
Solitary tract n.
Control 1 742 55 227 31
4. Discussion
Control 2 681 60 192 25
Control 3 597 50 188 27
Control 4 669 50 202 29 The present study shows that chronic subordination
All controls 672 4 59 30
stress reduces expression of the a2A-adrenoceptor mRNA Stress 1 559 55 225 30 ,0.001 in noradrenergic neurons of the locus coeruleus, in solitary Stress 2 503 60 155 20 ,0.001 tract neurons that might be noradrenergic and in the
Stress 3 502 50 179 25 ,0.05
glutamatergic neurons of the lateral reticular nucleus.
Stress 4 292 50 93 13 ,0.001
All stressed 464 4 118 59
4.1. Sequence of the tree shrewa2 A-adrenoceptor cDNA Dorsal motor n.
Control 1 538 50 205 29 The cloned 1.22-kb a -adrenoceptor cDNA-fragment
2A
Control 2 616 50 192 27
of the tree shrew contained 89% of the expected receptor
Control 3 571 50 177 25
coding sequence. Sequence alignment of this fragment
Control 4 446 50 163 23
All controls 543 4 72 36 with clones from human and rat showed a higher extent of identity with the human receptor cDNA (92%) than with
Stress 1 506 50 190 27 n.s.
the rat (88%) [15,16]. At position 515, the tree shrew
Stress 2 608 50 172 24 n.s.
Stress 3 586 50 162 23 n.s. a2A-adrenoceptor sequence displays a guanine nucleotide Stress 4 498 50 158 22 n.s. which corresponds to the same nucleotide at position 602
All stressed 550 4 56 28
in the human receptor gene, whereas the rat cDNA contains a cytosine at the corresponding site. In the human Lateral reticular n.
and tree shrew a -adrenoceptor proteins this encodes a
Control 1 1514 50 509 72 2A
Control 2 1180 50 465 66 cysteine at amino acid position 201; the corresponding Control 3 1391 50 453 64 amino acid in the rat receptor (referred to as the a
-2D
Control 4 1644 50 690 98
adrenoceptor subtype) is serine. The pharmacological
All controls 1432 4 197 99
consequences of this amino acid substitution have been Stress 1 1146 50 360 51 ,0.01 discussed elsewhere [20].
Stress 2 1073 50 366 52 n.s.
Sections of stressed animals and controls had been mounted on the same
The combination of immunocytochemistry and in situ slide and were analyzed pair wise. Significant differences (P) between the
con-Fig. 5. Silver grains in single neurons of chronically stressed male tree shrews expressed as percentages of controls (mean6S.E.M.). Differences between groups are significant (see Table 1).
ditions had to be modified to suppress non-specific label- was not detectable in PNMT-containing cells of the Sol. ing. This was achieved by treating the antibody-stained There might be two explanations for this: (i) the expres-paraffin sections with proteinase K prior to the hybridiza- sion level of the a2A-adrenoceptor gene in these cells is tion (see Section 2). As a control experiment, we also low, or (ii) the turnover of the receptor-specific mRNA is
35
hybridized antibody-stained sections with the S-labeled higher than in the LC neurons. Since in the other adren-sense probe. In these sections, no cells in the LC, the ergic cell group, C1 in the ventrolateral medulla, PNMT-solitary tract nucleus, and the lateral reticular nucleus were immunopositive neurons were also only lightly labeled by radio-labeled. However, throughout the rostral medulla the antisense probe, one has to conclude that adrenergic oblongata, there were some very large neurons that showed neurons in the Sol either contain noa2A-adrenoceptor gene accumulation of silver grains (presumably motor neurons; transcript, or only at a very low level. Similar results were diameter of cell body approximately 35mm). obtained for the TH-immunopositive neurons in Sol and the A1 cell group, respectively. In the Sol, TH-containing 4.3. a2 A-Adrenoceptor expression in nor /adrenergic and neurons displayed scant but specific antisense
hybridiza-glutamatergic neurons tion, but A1 neurons displayed no specific antisense
labeling. Therefore, the expression level of a2A -adreno-A specific in situ hybridization pattern was obtained ceptors in medullary noradrenergic neurons at the level of with the antisense riboprobe generated from the tree shrew the obex might by lower or the RNA turnover might be
a2A-adrenoceptor cDNA. Like in other species, this re- faster than in the LC.
ceptor subtype is strongly expressed in the main norad- A pronounced antisense hybridization was detected in renergic cell group in the brain, the LC [25,33,39]. Within the large neurons of the lateral reticular nucleus (LRt) that LC neurons, silver grains were found over nucleus and are known to be glutamatergic [14,24]. These findings cytoplasm. The nuclear labeling probably represents hy- show that in the brain stem, the a2A-adrenoceptor is not bridization with the primary gene transcript (heteronuclear only expressed in noradrenergic neurons, which agrees RNA) because the coding region of the a2A-adrenoceptor with previous electron microscopic studies on the ventrola-gene is not interrupted by introns [15]. Cytoplasmic teral medulla where a2A-adrenoceptor immunoreactivity labeling presumably represents hybridization with mRNA. was not limited to catecholaminergic neurons [23]. Hybrid-In accordance with previous reports indicating that the ization of the receptor-specific probe was never observed
a2A-adrenoceptor is expressed in noradrenergic neurons, in cells that were stained by the GABA antibody in LC, the antisense-labeled cells in the LC of the tree shrew were Sol and LRt, indicating that at least in these nuclei, immunoreactive for tyrosine hydroxylase [2,17,32]. a2A-adrenoceptor expressing cells are not GABAergic.
In contrast to the LC, the solitary tract nucleus (Sol)
showed only few cells that were moderately labeled by the 4.4. Quantification ofa2 A-adrenoceptor RNA in stressed receptor probe. These labeled cells were loosely arranged animals
in the medial part of the nucleus and around the
Our earlier observations indicated that a2-adrenergic elucidated. Besides the regulation of gene transcription, radioligand binding is reduced in the brain stem of tree altered degradation of receptor mRNA might also be shrews subjected to subordination stress [6]. In the present involved in stress-mediated down-regulation of a2A -ad-study, quantification of hybridization signals over neurons renoceptor expression.
in the LC and in the solitary tract nucleus showed a
significant stress-dependent reduction ofa2A-adrenoceptor 4.6. Implications of stress-reduceda2 A-adrenoceptor RNA expression. It therefore appears that the reduced expression
3
[ H]RX821002 binding observed earlier in brain stem
nuclei of subordinate tree shrews is due to reduced Although the present results show that that not only receptor gene expression [6]. In this former study, the noradrenergic but also glutamatergic neurons in the brain extent of down-regulation of a2-adrenergic binding sites stem express thea2A-adrenoceptor, they support the view was only 10% in the LC, whereas in the present analysis, that this receptor subtype is an autoreceptor in noradrener-the receptor RNA levels were reduced by 24% in noradrener-the gic neurons, at least in the LC [2,17,32]. The stress-stressed animals. One possible reason for this discrepancy induced down-regulation of autoreceptor expression proba-is that the a2-adrenoceptor radioligand labeled all three bly contributes to an imbalance in the noradrenergic
a2-adrenoceptor subtypes (A, B, C) leading to an undere- system which, during periods of chronic stress, is persis-stimation of the stress effect ona2A-adrenoceptor expres- tently or repetitively activated [1]. This chronic activation sion. may be due to the low autoreceptor expression that results In the Sol, a2A-adrenoreceptor expression was quan- in reduced capacity for negative feed-back and increased tified in neurons located in the medial part of the nucleus firing rates of noradrenergic LC neurons [1], thus enhanc-and around the subnucleus gelatinosus. The distribution ing release of noradrenaline at projection sites of the LC pattern of these cells indicated that they might represent [26]. Since the LC is an important integrator of the stress noradrenergic and / or adrenergic neurons. However, since response that participates in the regulation of sleep, the double-labeling experiments showed co-expression arousal, learning and memory, and controls endocrine and with TH in only very few cells, and not at all with PNMT, autonomic nervous functions through a widespread system no definitive conclusion may be made concerning the of efferent fibers, the autoreceptor down-regulation has identity of thea2A-adrenoceptor expressing cells in the Sol consequences for many brain functions [37]. In the where receptor expression is reduced during stress. In areas subordinate male tree shrews, the resulting noradrenergic A1 and C1, the number of antisense-labeled cells was too hyperactivity may contribute to the affective state of low for a statistically relevant quantification of silver subordination stress resulting in behavioral changes and in grains. In addition, the hybridization signal of the strongly an impairment of cognitive performance [9,27].
antisense-labeled LRt neurons tended to mask the hybridi- The co-expression of a2A-adrenoceptors and glutamate zation signals from the A1 and C1 cells. in LRt neurons agrees with pharmacological results in-dicating that a2-adrenoceptor agonists such as clonidine 4.5. Mechanisms ofa2 A-adrenoceptor down-regulation may at least in part exert their hypotensive effects via LRt neurons [10]. Iontophoretically applied noradrenaline and Previous investigations on a2-adrenoceptor regulation systemically injected clonidine depressed LRt neuronal have focused on mechanisms of rapid (minutes) and long- firing [3]. Also, a2-adrenoceptors in the LRt modulate term (hours) agonist-induced receptor desensitization in spinal antinociception in rats [13,19]. The stress-induced cell culture [5]. Little is known about the effects of chronic changes ina2A-adrenoceptor expression in the LRt might stress and the accompanying persistent or repetitive agonist therefore affect cardiovascular as well as nociceptive exposure on these receptors in brains of mammals. Several functions in tree shrews, although the physiological conse-lines of evidence emphasize the importance of agonists in quences of the receptor changes have not yet been ana-transcriptional and post-ana-transcriptional control, but hetero- lyzed in detail. In addition, since the LRt projects to the logous regulation could also explain the present findings cerebellar cortex, an impairment of cerebellar functions [12]. Recently it was shown that a2A-adrenoceptor RNA should also be considered [38,41].
transcription in rat astroglial cultures is down-regulated by increased levels of intracellular cyclic AMP and by protein
kinase C (PKC) activation [31]. The promoter region of 5. Conclusions
the a2A-adrenoceptor gene of the rat can bind several
[13] A.J. Janss, G.F. Gebhart, Spinal monoaminergic receptors mediate brain noradrenergic system, a characteristic feature of
the antinociception produced by glutamate in the medullary lateral chronic stress. Furthermore, the a2A-adrenoceptor is reticular nucleus, J. Neurosci. 7 (1987) 2862–2873.
strongly expressed in glutamatergic neurons of the lateral [14] T. Kaneko, K. Itoh, R. Shigemoto, N. Mizuno, Glutaminase-like reticular nucleus, and chronic stress reduces its expression immunoreactivity in the lower brainstem and cerebellum of the adult in these neurons. Therefore, in addition to its role as rat, Neuroscience 32 (1989) 79–98.
[15] B.K. Kobilka, H. Matsui, T.S. Kobilka, T.L. Yang Feng, U. Francke, autoreceptor, thea2A-adrenoceptor appears to form a link
M.G. Caron, R.J. Lefkowitz, J.W. Regan, Cloning, sequencing, and between the noradrenergic and the glutamatergic system.
expression of the gene coding for the human plateleta2-adrenergic receptor, Science 238 (1987) 650–656.
[16] S.M. Lanier, S. Downing, E. Duzic, C.J. Homcy, Isolation of rat genomic clones encoding subtypes of the a -adrenergic receptor.
Acknowledgements 2
Identification of a unique receptor subtype, J. Biol. Chem. 266 (1991) 10470–10478.
This work was supported by the German Science [17] A. Lee, D.L. Rosin, E.J. Van Bockstaele, Alpha -adrenergic
re-2A
Foundation (SFB 406), by the German Academic Ex- ceptors in the rat nucleus locus coeruleus: subcellular localization in change Service (DAAD, HSP II AUFE), and in part by catecholaminergic dendrites, astrocytes, and presynaptic axon
termi-nals, Brain Res. 795 (1998) 157–169. EU-BMH4-98-3373. We thank S. Rudolph, M. Vorwald
[18] R. Link, D. Daunt, G. Barsh, A. Chruscinski, B.K. Kobilka, Cloning and A. Heutz for technical assistance, and Dr. G. Dowe
of two mouse genes encoding a2-adrenergic receptor subtypes and ¨
(MPI Biophysical Chemistry, Gottingen) and J. Ruuskanen identification of a single amino acid in the mousea -C10 homolog
2
(University of Turku) for help with the DNA sequencing. responsible for an interspecies variation in antagonist binding, Mol. Pharmacol. 42 (1992) 16–27.
[19] H. Mansikka, A. Pertovaara, The role ofa2-adrenoceptors of the medullary lateral reticular nucleus in spinal antinociception in rats,
References Brain Res. Bull. 37 (1995) 633–638.
¨ ´
[20] A. Marjamaki, H. Frang, M. Pihlavisto, A.M. Hoffren, T. Salminen, [1] E.D. Abercrombie, B.L. Jacobs, Single-unit response of noradren- M.S. Johnson, J. Kallio, J.A. Javitch, M. Scheinin, Chloro-ergic neurons in the locus coeruleus of freely moving cats. II. ethylclonidine and 2-aminoethyl methanethiosulfonate recognize two different conformations of the human a -adrenergic receptor, Adaption to chronically presented stressful stimuli, J. Neurosci. 7 2A
J. Biol. Chem. 274 (1999) 21867–21872. (1987) 2844–2848.
¨
[2] C. Aoki, C.G. Go, C. Venkatesan, H. Kurose, Perikaryal and synaptic [21] U. Meyer, M. Kruhoffer, G. Flugge, E. Fuchs, Cloning of glucocor-localization ofa2A-adrenergic receptor-like immunoreactivity, Brain ticoid receptor and mineralocorticoid receptor cDNA and gene Res. 650 (1994) 181–204. expression in the central nervous system of the tree shrew (Tupaia [3] P.M. Cahusac, R.G. Hill,a2-Adrenergic receptors on neurones in the belangeri ), Mol. Brain Res. 55 (1998) 243–253.
¨
region of the lateral reticular nucleus of the rat, Neurosci. Lett. 42 [22] A. Mittendorf, L. Denoroy, G. Flugge, Anatomy of the adrenergic (1983) 279–284. system in the medulla oblongata of the tree shrew: PNMT immuno-[4] L.F. Callado, J.A. Stamford, a2A- but not a2B / C-adrenoceptors reactive structures within the nucleus tractus solitarii, J. Comp.
modulate noradrenaline release in rat locus coeruleus: voltammetric Neurol. 274 (1988) 178–189.
data, Eur. J. Pharmacol. 366 (1999) 35–39. [23] T.A. Milner, D.L. Rosin, A. Lee, S.A. Aicher, Alpha2A-adrenergic [5] M.G. Eason, S.B. Liggett, Subtype-selective desensitization ofa2- receptors are primarily presynaptic heteroreceptors in the C1 area of adrenergic receptors. Different mechanisms control short and long the rat rostral ventrolateral medulla, Brain Res 821 (1999) 200–211. term agonist-promoted desensitization of a-2C10, a-2C4, and a- [24] A. Najlerahim, P.J. Harrison, A.J. Barton, J. Heffernan, R.C. 2C2, J. Biol. Chem. 267 (1992) 25473–25479. Pearson, Distribution of messenger RNAs encoding the enzymes
¨
[6] G. Flugge, Alterations in the central nervous a2-adrenoceptor glutaminase, aspartate aminotransferase and glutamic acid decarbox-system under chronic psychosocial stress, Neuroscience 75 (1996) ylase in rat brain, Mol. Brain Res. 7 (1990) 317–333.
¨
187–196. [25] A.P. Nicholas, V. Pieribone, T. Hokfelt, Distributions of mRNAs for ¨
[7] G. Flugge, A. Jurdzinski, S. Brandt, E. Fuchs, Alpha -adrenergic2 a2- adrenergic receptor subtypes in rat brain: an in situ hybridization binding sites in the medulla oblongata of tree shrews demonstrated study, J. Comp. Neurol. 328 (1993) 575–594.
by in vitro autoradiography: species related differences in com- [26] L.K. Nisenbaum, M.J. Zigmond, A.F. Sved, E.D. Abercrombie, parison to the rat, J. Comp. Neurol. 297 (1990) 253–266. Prior exposure to chronic stress results in enhanced synthesis and [8] E. Fuchs, Tree shrews, in: T. Poole (Ed.), The UFAW Handbook on release of hippocampal norepinephrine in response to a novel
the Care and Management of Laboratory Animals, Vol. 1, Blackwell stressor, J. Neurosci. 11 (1991) 1478–1484.
Science, Oxford, 1999, pp. 235–245. [27] F. Ohl, E. Fuchs, Differential effects of chronic stress on memory [9] E. Fuchs, M. Kramer, B. Hermes, P. Netter, C. Hiemke, Psycho- processes in the tree shrew, Cogn. Brain Res. 7 (1999) 379–387.
social stress in tree shrews: clomipramine counteracts behavioral [28] O.P. Ottersen, J. Storm-Mathisen, Glutamate- and GABA-containing and endocrine changes, Pharmacol. Biochem. Behav. 54 (1996) neurons in the mouse and rat brain, as demonstrated with a new
219–228. immunocytochemical technique, J. Comp. Neurol. 229 (1984) 374–
[10] R.A. Gillis, P.J. Gatti, J.A. Quest, Mechanism of the antihyper- 392.
tensive effect ofa2-agonists, J. Cardiovasc. Pharmacol. 7 (Suppl. 8) [29] J.V. Priestley, G. Wotherspoon, D. Savery, S. Averill, M. Rattray, A (1985) S38–S44. combined in situ hybridization and immunofluorescence procedure [11] D.E. Handy, M.T. Zanella, A. Kanemaru, A. Tavares, C. Flordellis, allowing visualization of peptide mRNA and serotonin in single
H. Gavras, A negative regulatory element in the promoter region of sections, J. Neurosci. Methods 48 (1993) 99–110.
the rat a2A -adrenergic receptor gene overlaps an SP1 consensus [30] A. Raab, H. Storz, A long-term study on the impact of sociopsychic binding site, Biochem. J. 311 (1995) 541–547. stress in tree shrews (Tupaia belangeri ) on central and peripheral [12] D.A. Heck, D.B. Bylund, Differential regulation ofa2-adrenergic tyrosine hydroxylase activity, J. Comp. Physiol. 108 (1976) 115–
[31] M.A. Reutter, E.M. Richards, C. Sumners, Regulation of a2A- [37] R.J. Valentino, G.S. Aston-Jones, Physiological and anatomical adrenergic receptor mRNA in rat astroglial cultures: role of cyclic determinants of locus coeruleus discharge, in: F.E. Bloom, D.J. AMP and protein kinase C, J. Neurochem. 68 (1997) 47–57. Kupfer (Eds.), Psychopharmacology. The Fourth Generation of [32] D.L. Rosin, D. Zeng, R.L. Stornetta, F.R. Norton, T. Riley, M.D. Progress, Raven Press, New York, 1995, pp. 373–385.
Okusa, P.G. Guyenet, K.R. Lynch, Immunohistochemical localiza- [38] Z. Wang, R.H. Liu, S.J. Fung, V.K. Reddy, C.D. Barnes, Immuno-tion of a2A-adrenergic receptors in catecholaminergic and other histochemical localization of glutamate-containing neurons in the brain stem neurons in the rat, Neuroscience 56 (1993) 139–155. lateral reticular nucleus projecting to the cerebellar vermis in the [33] M. Scheinin, J.W. Lomasney, D.M. Hayden-Hixson, U.B. Schambra, kitten, Neurosci. Lett. 164 (1993) 117–120.
M.G. Caron, R.J. Lefkowitz, R.T. Fremeau, Distribution of a2- [39] R. Wang, L.B. MacMillan, R.T. Fremeau, M.A. Magnuson, J. adrenergic receptor subtype gene expression in rat brain, Mol. Brain Lindner, L.E. Limbird, Expression ofa2-adrenergic subtypes in the Res. 21 (1994) 133–149. mouse brain: evaluation of spatial and temporal information im-[34] J. Storm-Mathisen, A.K. Leknes, A.T. Bore, J.L. Vaaland, P. parted by 3 kb of 59regulatory sequence for thea2A-AR-receptor
Edminson, F.M. Haug, O.P. Ottersen, First visualization of gluta- gene in transgenic animals, Neuroscience 74 (1996) 199–218. mate and GABA in neurons by immunocytochemistry, Nature 301 [40] U.H. Winzer-Serhan, H.K. Raymon, R.S. Broide, Y. Chen, F.M. (1983) 517–520. Leslie, Expression of a2-adrenoceptors during rat brain develop-[35] E.M. Talley, D.L. Rosin, A. Lee, P.G. Guyenet, K. Lynch, Dis- ment. I. Alpha2A-messenger RNA expression, Neuroscience 76
tribution ofa2A-adrenergic receptor-like immunoreactivity in the rat (1997) 241–260.
![Fig. 1. Nucleic acid sequence of the tree shrew a-adrenoceptor cDNA representing 89% of the expected receptor coding sequence, and alignments withthe partial2A a-adrenoceptor cDNAs of human (clone C10; nucleotides 88–1306 [15]) and rat (clone RG20; 88–1306](https://thumb-ap.123doks.com/thumbv2/123dok/3140202.1382906/4.612.121.473.61.610/sequence-adrenoceptor-representing-receptor-sequence-alignments-adrenoceptor-nucleotides.webp)