www.elsevier.com / locate / bres
Research report
Effects of brain oxygenation on metabolic, hemodynamic, ionic and
electrical responses to spreading depression in the rat
*
Judith Sonn, Avraham Mayevsky
Faculty of Life Sciences, Bar-Ilan University, Ramat Gan 52900, Israel
Accepted 8 August 2000
Abstract
1
The effect of cortical spreading depression (CSD) on oxygen demand (extracellular K ), oxygen supply (cerebral blood flow – CBF) and oxygen balance (mitochondrial NADH) was studied by a special multiprobe assembly (MPA), during hypoxia and partial ischemia. The MPA was constructed and applied to monitor the CSD wave from its front line until complete recovery, continuously and simultaneously. CSD under hypoxia or partial ischemia led to an initial increase in NADH levels and a further decrease in CBF during the first phase of the CSD wave, indicating a decrease of tissue capability to compensate for an increase in oxygen demand. Furthermore, the special design of the MPA enabled identifying the close interrelation between oxygen demand, supply and balance during CSD propagation. In conclusion, brain oxygenation was shown to have a clear effect on tissue responses to CSD. 2000 Elsevier Science B.V. All rights reserved.
Theme: Other systems of the CNS
Topic: Brain metabolism and blood flow
1
Keywords: Cortical spreading depression; Mitochondrial NADH; Extracellular K ; Brain oxygenation; Hypoxia; Ischemia
Cortical spreading depression (CSD) first described by during various levels of brain oxygenation: normoxia, Leao [11], is a wave of reversible EEG suppression that hypoxia and partial ischemia.
propagates at a rate of 2–5 mm / min across the cortical Increasing oxygen demand during these states, by surface, accompanied by a negative deflection of the direct inducing CSD waves, will cause different changes in the current (DC) potential and a severe disruption in ion monitored parameters during CSD propagation. Back et al. homeostasis [6,7,16,17]. Recovery from CSD is dependent [1] showed no changes in cerebral blood flow (CBF) and a upon activation of the ion pumps causing an increase in decrease in tissue pO during spontaneous CSD waves in2
metabolic activity [8,10,14,16] and a rise in oxygen ischemic brain tissue, whereas induction of CSD waves demand [12,15]. In normal brain tissue, this chain of (by KCl solution) in normal tissue showed a significant events will be compensated by an increase in cerebral increase in CBF and in tissue pO . Since the CSD wave2
blood supply [6,10,15,19]. This coupling will be disturbed propagation is a multifactorial event, our strategy was to in tissue suffering from lack in oxygen supply. Then, the construct a multiprobe assembly (MPA) containing probes disruption in ion homeostasis and the increase in intracel- to evaluate most of the parameters involved during CSD
21
lular Ca ions, acidosis, release of noxious substances wave propagation, from its front line at the same time. such as glutamate and related excitatory amino acids This will verify the parallel changes in the various (EAAs), will enhance brain damage [2,4,5,17]. parameters after CSD initiation, and will help us to The aim of this study was to identify the sequence of identify which parameter / s start the chain responses and responses developed under the CSD phenomenon, initiated the reaction of the later ones. The MPA measured:
1 1
extracellular K concentration [K ] ,e mitochondrial
1
NADH / NAD ratio, cerebral blood flow and volume
*Corresponding author. Tel.:1972-3-5318218; fax:1972-3-6354459.
(CBF and CBV), steady DC potential and bipolar
electro-E-mail addresses: [email protected] (J. Sonn),
[email protected] (A. Mayevsky). corticography (ECoG).
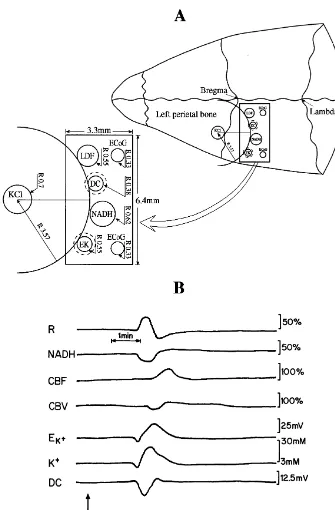
The probes were embedded in a Delrin rectangular allowed the CSD wave to be monitored from its front line cannula (6.433.3 mm) (Fig. 1A). It was found that the (by all the detected parameters) until complete recovery. probes should be arranged on a circumference where the The MPA contained: surface mini-electrodes for measuring
1
KCl cannula (CSD initiation area) is located in the center [K ] and DC steady potential, fiber optic probes that weree
of the circle. This enabled the probes to be arranged used to monitor intramitochondrial NADH redox state according to the shape of the CSD wave determined and (NADH fluorescence) and reflected light, as well as
Fig. 1. (A) Schematic presentation of the special multiprobe assembly (MPA) developed in order to study the responses to cortical spreading depression. The right side shows the MPA located on the cerebral cortex. The localization of the various sensors is shown on the left side of the figure. LDF – light guide connected to the laser Doppler flowmeter; NADH – light guide connected to the fluorometer / reflectometer for NADH fluorescence and 366 nm
1
reflectance; EK – selective K electrode; DC – direct current; KCl – push–pull cannula; ECoG – electrocorticography electrodes. The R values represent the radii of the particular probes. (B) Typical responses of the normoxic rat brain to cortical spreading depression (CSD). R, NADH – 366 nm reflectance
1
and corrected NADH fluorescence. CBF, CBV – cerebral blood flow and volume measured by the Laser Doppler flowmeter. EK1, K – potential of the
1 1
1
relative CBF and CBV by laser Doppler flowmetry and noticed in the K trace as an initial decrease in potassium. spontaneous bipolar ECoG via two platinum electrodes. A clear oxidation in the NADH redox state (decrease All details regarding the various probe construction and signal) was accompanied by a large increase in CBF. It is calibration were published previously [13,15,16]. important to note that the edge of the various probes was
The Institutional Animal Care and Use Committee of the positioned on the same front line.
Bar Ilan University approved all experimental protocols. Statistical analysis of the responses to CSD showed that
1
Male Wistar rats weighing 200–250 g were anesthetized under normoxic conditions, [K ] was elevated by 10–18e
with a 0.3 ml / 100 g intraperitoneal (i.p.) injection of mM (P,0.001), DC potential decreased by 15 mV (P,
Equithesin (each ml contains 42.51 mg chloral hydrate, 0.0001). NADH levels decreased (oxidation cycle) by 13% 9.72 mg pentobarbital, 21.25 mg magnesium sulfate, (P,0.0005) and CBF increased in a range of 71–106%
1
44.43%, w / v, propylene glycol, 11.5% alcohol and dis- (P,0.01, P,0.0005). The levels of the elevated [K ] aree
tilled water). The cranium was exposed and a thin rectan- much lower compared to microelectrodes recording [6], gular Plexiglas pattern was located. The pattern contained due to the surface monitoring in our experiments. two scratches: the MPA probe (6.433.3 mm) and the KCl During CSD propagation the ECoG signal became very push–pull cannula (2 mm diameter) in the exact shape as it low in amplitude (close to isoelectric), and returned to is schematically illustrated in Fig. 1A. This pattern was normal after recovery. Under hypoxia and partial ischemia used to mark the exact shape and position of the MPA CSD wave duration increased (slower recovery) as com-probe and the KCl cannula on the bone. Two holes were pared to normoxic ECoG trace.
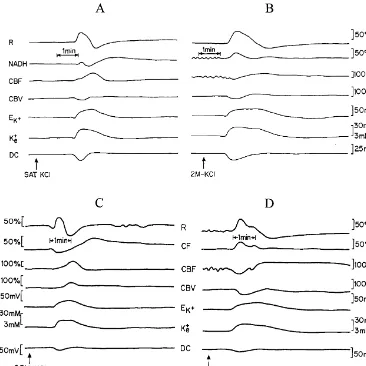
drilled: one in the frontal bone for the KCl cannula and the Induced hypoxia itself caused a significant increase in other in the ipsilateral parietal bone for the MPA implanta- NADH (14–15%, P,0.0005) and in CBF for about the tion (Fig. 1A). No harm was done to the sagittal sinus same level (P,0.05). Since partial ischemia was obtained during MPA implantation. The dura mater was gently 24 h before CSD was initiated, initial levels of CBF and removed. The push–pull cannula enabled flushing of the NADH before bilateral carotid occlusion are unknown. brain with diluted KCl solution in order to induce CSD Four CSD waves shown in Fig. 2 present the effects of waves (0.5 M-saturated KCl). Two other holes were drilled hypoxia and partial ischemia on CSD propagation. The for screws located in the contralateral hemisphere that comparison between hypoxia (Fig. 2B) and normoxia (Fig. helped to hold the system securely to the cranium. All 2A) was made in the same animal and it is clear that components were fixed to the brain using dental acrylic lowering the supply of O by breathing low FiO led to a2 2
cement. The animals were placed in a cage made of few typical changes in the responses to CSD. As seen, the Plexiglas and allowed to recover from surgery for at least electrical and ionic responses were similar although the 60–90 min before the experiment started. duration of the wave was much longer under hypoxia (Fig. A single cycle of CSD was induced before and after 2B). The main difference between normoxia (Fig. 2A) and hypoxia (13 rats). A control group underwent the same hypoxia (Fig. 2B) was in the responses of the mito-experimental procedures under normoxic conditions (11 chondrial and hemodynamic signals namely, instead of a rats). Three CSD waves (one per hour) were induced after decrease in the NADH levels and a large increase in CBF, partial ischemia (nine rats) and in sham rats (eight rats). the NADH showed an increase while CBF decreased CSD waves were induced by epidural application of initially (Fig. 2B). Furthermore, slower recovery from the diluted KCl solution in lightly anesthetized rats. depolarization event was noticed. The same type of effects Hypoxia was achieved by inhalation of a gas mixture was recorded under partial ischemia (Fig. 2D) as compato containing 12% O2188% N . Arterial blood samples,2 normoxia (sham rat – Fig. 2C). The duration of the under hypoxia, showed a significant decrease (P,0.005) in ischemic wave was longer and the metabolic and hemo-pO2 (from 107.7364.9 to 57.1868.62 mmHg), pCO2 dynamic responses were inverted under partial ischemia, (from 34.2362.1 to 26.3862.9 mmHg), O2 saturation NADH increased and CBF decreased. It must be empha-(from 96.0160.47% to 81.0665.37%) and content (from sized that the responses to CSD under partial ischemia as 19.3362.1 to 15.9661.64%, v / v). compared to hypoxia were more severe. The reduced cycle Partial ischemia was induced by permanent bilateral in NADH was not followed by an oxidized one and the carotid artery occlusion, and 24 h afterwards, the rats were decrease in CBF was more pronounced.
reanesthetized and underwent preparation for the experi- As expected, in the normal brain CSD wave propagation
1
Fig. 2. Effects of hypoxia (B) and partial ischemia (D) on the various responses to CSD compared to the control normoxic state (A) and to the normoxic sham brain (C), respectively. All abbreviations are as in Fig. 1B. CSD waves were induced by 0.5 M KCl solution, 2 M KCl solution, or by saturated (SAT.) KCl solution (arrows).
increase turnover rate are typical to the state 4 state 3 mitochondrial and hemodynamic signals. Instead of a large transition measured in isolated mitochondria [3]. increase in CBF and a decrease in the NADH levels, the As is known, hypoxia stimulates the production of a NADH showed an increase (reduction cycle) while CBF wide variety of vasodilator metabolites, such as adenosine, decreased (at least initially). Back et al. [1] showed that the
21 1 1
NO, Ca , H and K levels, prostaglandins and others, response of CBF did not change during CSD under causing an increase in CBF [18]. Due to our results, it ischemia. The main common denominators between hypo-seems that the augmentation in CBF did not compensate xia and ischemia are oxygen insufficiency and a release of the lack in oxygen during hypoxia, resulting in an increase vasoactive substances. This may lead to a massive decrease in mitochondrial NADH redox state. Increasing O2 de- in vascular resistance. A further release of dilator sub-mand, by inducing CSD waves under hypoxia and partial stances during CSD can be ineffective and therefore no ischemia (low O delivery) showed that the electrical and2 changes in CBF may be found. Furthermore, inducing ionic responses were almost similar. These responses can CSD under hypoxia or ischemia may cause a release of be related to the ‘all or none’ type reaction of the nervous endothelium-derived contracting factors from vascular system reaching a threshold of CSD wave. In addition, the endothelial cells, inducing vasoconstriction, as mentioned duration of the waves was much longer under these by Pearce [18] under hypoxia. Moreover, the flow varia-perturbations (Fig. 2B and D), showing a delay in the tions during CSD under normal conditions, can partially be
1
recovery phase as compared to the normoxic CSD waves explained by a direct action of [K ] on cerebral vessels. Ite
(Fig. 2A and C). Similar responses in CSD wave duration has been shown that an increase of up to 10 mM caused under hypoxia and ischemia were reported previously dilation of pial vessels while further increase lead to
1
[1,16]. The extension in wave duration under these per- constriction [9]. Therefore, a further increase in [K ]e
vasocon-hippocampal cell damage after ischemia, Acta Neurol. Scand. 78
striction, causing a decrease in CBF. However, the specific
(1988) 529–536.
mechanism involved remains to be elucidated.
[3] B. Chance, G.R. Williams, Respiratory enzymes in oxidative
phos-The decrease in O supply under a large increase in O2 2 phorylation, J. Biol. Chem. 217 (1955) 383–427.
demand during the CSD wave, resulted in a slower [4] G. Gido, T. Kristian, K.I. Katsura, B.K. Siesjo, The influence of
recovery from the depolarization event. This type of effects repeated spreading depression-induced calcium transients on neuro-nal viability in moderately hypoglycemic rats, Exp. Brain Res. 97
was more pronounced under partial ischemia, indicating a
(1994) 397–403.
further deficiency in O2 supply. Thus, our data indicate [5] G. Gido, T. Kristian, B.K. Siesjo, Induced spreading depressions in that inducing hypoxia or partial ischemia resulted in a energy-compromised neocortical tissue: calcium transients and change in the balance between O supply and demand.2 histopathological correlates, Neurobiol. Dis. 1 (1994) 31–41.
Using the special MPA in this study, we were able for [6] A.J. Hansen, B. Quistorff, A. Gjedde, Relationship between local
1
changes in cortical blood flow and extracellular K during spreading
the first time to prove the sequence of events during the
depression, Acta Physiol. Scand. 109 (1980) 1–6.
CSD wave propagation and to present the interrelation
[7] A.J. Hansen, T. Zeuthen, Extracellular ion concentration during
between CBF and NADH during the various phases of its spreading depression and ischemia in the rat brain cortex, Acta propagation. As found, the time to the decreased peak in Physiol. Scand. 113 (1981) 437–445.
CBF was initial to the increased peak in NADH during the [8] J. Krivanek, Some metabolic changes accompanying Leao’s spread-ing cortical depression, J. Neurochem. 6 (1961) 183–189.
first phase. The opposite interrelation can be seen in the
[9] W. Kuschinsky, M. Wahl, Local chemical and neurogenic regulation
later phase where parallel to the increase in CBF, a
of cerebral vascular resistance, Physiol. Rev. 58 (1978) 656–689.
decrease in NADH was noticed. The simultaneous and [10] M. Lauritzen, Cerebral blood flow in migraine and cortical spread-opposite changes between NADH and CBF were found to ing depression, Acta Neurol. Scand. 76 (1987) 1–40.
be more interrelated when O delivery was limited. These2 [11] A.A.P. Leao, Spreading depression of activity in cerebral cortex, J. Neurophysiol. 7 (1944) 359–390.
results may indicate an increase in the correlation between
[12] L.D. Lukyanova, J. Bures, Changes in pO2 due to spreading
CBF and NADH under limited O2 supply conditions, depression in the cortex and nucleus caudatus of the rat, Physiol. which is disclosed after increasing O demand by CSD.2 Bohemoslav. 16 (1967) 449–455.
[13] A. Mayevsky, Brain NADH redox state monitored in vivo by fiber optic surface fluorometry, Brain Res. Rev. 7 (1984) 49–68. [14] A. Mayevsky, B. Chance, Repetitive patterns of metabolic changes Acknowledgements
during cortical spreading depression of the awake rat, Brain Res. 65 (1974) 529–533.
The study was supported by a grant from the Research [15] A. Mayevsky, H.R. Weiss, Cerebral blood flow and oxygen con-Committee of Bar-Ilan University and the Health Sciences sumption in cortical spreading depression, J. Cereb. Blood Flow
Research Center, the Faculty of Life Sciences, Bar-Ilan Metab. 11 (1991) 829–836.
[16] A. Mayevsky, N. Zarchin, J. Sonn, Brain redox state and O balance
University. 2
in experimental spreading depression and ischemia, in: A. Lehmen-kuhler, K.-H. Grotemeyer, F. Tegtmeier (Eds.), Migraine – Basic Mechanisms and Treatment, Urban & Schwarzenberg,
Munchen-References Wier, Baltimore, MD, 1993, pp. 379–393.
[17] W.A.C. Mutch, A.J. Hansen, Extracellular pH changes during spreading depression and cerebral ischemia: mechanisms of brain [1] T. Back, K. Kohno, K.A. Hossmann, Cortical negative DC
deflec-pH regulation, J. Cereb. Blood Flow Metab. 4 (1984) 17–27. tions following middle cerebral artery occlusion and KCl-induced
[18] W.J. Pearce, Mechanism of hypoxic cerebral vasodilation, Phar-spreading depression: effect on blood flow, tissue oxygenation and
macol. Ther. 65 (1995) 75–91. electroencephalogram, J. Cereb. Blood Flow Metab. 14 (1994)
[19] R.D. Piper, G.A. Lambert, J.W. Duckworth, Cortical blood flow 12–19.
changes during spreading depression in cats, Am. J. Physiol. 261 [2] H. Benveniste, M.B. Jorgensen, N.H. Diemer, A.J. Hansen, Calcium