T
he challenge for today’s scientists is to maintain a broad per-spective of interactive signaling pathways, while dissecting their parts sufficiently to render them understandable. Our goal is to stimulate the reader to consider the phosphoinositide (PI) pathway as a functional component of a complex growth response and to move beyond the reductionist’s perspective of confining PI signaling to the generation of a transient, Ins(1,4,5)P3-induced Ca21oscillation. (For additional coverage of plant PIs and the enzymes involved in their metabolism see Refs 1,2.)
Inositol phospholipids as regulators of growth
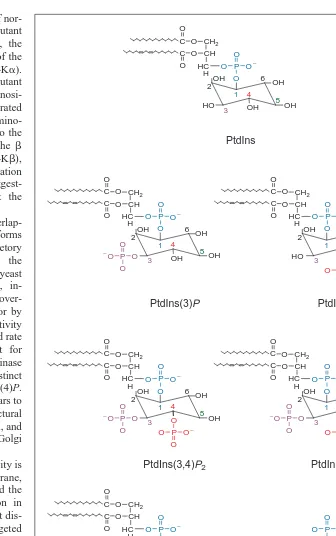
Most plant responses to external stimuli involve a change in growth and therefore membrane biogenesis. Membrane trafficking and signaling are inexorably linked in regulating cellular metab-olism and controlling growth. To coordinate these processes, evo-lution appears to have capitalized on the stereospecificity of the PIs. During membrane trafficking, individual inositol phospho-lipids on the vesicle surface specify functional information like cogs on a wheel. As vesicles traffic from the endoplasmic reticu-lum (ER) to the plasma membrane, and from the ER to the vacuole and retrograde pathways, inositol phospholipids attract specific proteins necessary for budding, docking and fusion3,4. Similarly, the presence of a multitude of polyphosphorylated inositol lipids and soluble inositol phosphates imparts specificity to signaling pathways by recruiting and activating signaling proteins into functionally distinct complexes. In the following sections, the major inositol lipids (Fig. 1) and their potential roles in membrane biogenesis, signaling and plant growth are briefly described.
Biosynthesis and function of PtdIns(3) P
The first evidence for the exacting requirements for stereospecific isomers of phosphatidylinositol (PtdIns) phosphate in membrane trafficking was revealed when the gene for yeast PtdIns-3-kinase, VPS34, was shown to be essential for trafficking of hydrolytic enzymes to the vacuole5
. The plant PtdIns-3-kinase is related to the yeast Vps34p, which uses only PtdIns as a substrate. In plants, PtdIns-3-kinase activity is associated with nodule formation during symbiotic nitrogen fixation1,6. The temporal increase in PtdIns-3-kinase activity in root nodules, where the peribacteroid membrane is being formed, and the selective inhibition of vacuolar trafficking by wortmannin in tobacco cells1,7
, are consistent with the yeast data, in that PtdIns(3)P is vital for vesicle trafficking. However, lit-tle is known about the plant enzyme and additional characteriz-ation of these systems is warranted. This is especially true because
wortmannin has been shown not only to inhibit PtdIns-3-kinase, but also to bind and inhibit PtdIns-4-kinases8.
Even though its activity is low in vitro, PtdIns-3-kinase is clearly essential for growth because the antisense expression of the gene for PtdIns-3-kinase results in transgenic plants that grow poorly, if at all9. This apparent incongruity might be explained in part by recent work showing that the soybean PtdIns-3-kinase co-localizes with nuclear transcription sites, suggesting that PtdIns(3)P is important for regulating transcription10
.
Prevalent in animal cells, but not in plants or yeast are two additional families of PI-3-kinases, the class I and class II PI-3-kinases1. Unlike the Vps34p-type PtdIns-3-kinase, the class I and II PI-3-kinases can phosphorylate PtdIns, PtdIns(4)P, PtdIns(5)P and PtdIns(4,5)P2 to produce PtdIns(3)P, PtdIns(3,4)P2, PtdIns(3,5)P2 and PtdIns(3,4,5)P3, respectively. The physiologi-cal relevance of the class II PI-3-kinases is not understood8. The class I PI-3-kinase and its stereospecific lipid products are involved in signaling pathways that are activated via Ras and phosphotyrosine-coupled plasma-membrane receptors. The fact that the class I PI-3-kinase and PtdIns(3,4,5)P3appear to be absent in plants is consistent with the lack of plasma-membrane-associ-ated tyrosine-receptor kinases11. In summary, the molecular and biochemical data support the thesis that essential biochemical functions of nuclear and ER-localized PtdIns-3-phosphate have been evolutionarily conserved between plants and animals, whereas the evolution of plasma-membrane signaling is specific to the organism, controlling responses to stimuli and ensuring survival under changing environmental conditions.
Biosynthesis and function of PtdIns(4) P
PtdIns(4)P is important for membrane biogenesis and vesicle trafficking from the ER to the Golgi and plasma membrane4. For example, PtdIns-4-kinase activity is necessary for vesicle for-mation and stimulated secretion of catecholamines in chromaffin cells, and for regulated exocytosis of the small synaptic vesicles from nerve terminals12. Exploiting yeast genetics to demonstrate the necessity of PtdIns(4)P in membrane trafficking and to elucidate the physiological roles of the distinct PtdIns-4-kinase isoforms has corroborated these findings.
In an elegant mutant screen, yeast mutants were identified that were compromised in various steps of intracellular lipid trans-port13. One mutant that was unable to transport phosphatidylserine (PS) from the ER to the Golgi was isolated and found to accumu-late PS in the ER, with diminished phosphatidylethanolamine
Inositol signaling and plant growth
Jill M. Stevenson, Imara Y. Perera, Ingo Heilmann, Staffan Persson and Wendy F. Boss
(PE) formation in the Golgi in spite of nor-mal PS-decarboxylase activity. The mutant defect was complemented by STT4, the yeast ortholog (functional homolog) of the
aisoform of PtdIns-4-kinase (PtdIns4Ka). The rescue of the yeast PS transport mutant indicates the importance of phosphoinosi-tides, specifically PtdIns(4)P generated from PtdIns4Ka, in intracellular amino-phospholipid transport from the ER to the Golgi. PIK1, the yeast ortholog of the b
isoform of PtdIns-4-kinase (PtdIns4Kb), was not identified in the complementation screen of .10 000 transformants, suggest-ing that it would not complement the aminophospholipid transport mutant.
Additional data supporting non-overlap-ping roles for the PtdIns-4-kinase isoforms has recently been reported in yeast secretory mutants14. Although overexpressing the gene for PtdIns4Kadid not rescue the yeast SEC14 secretory mutant phenotype, in-creasing PtdIns(4)P levels, either by over-expressing the gene for PtdIns4Kb, or by eliminating PtdIns(4)P phosphatase activity did14. This implies that the location and rate of PtdIns(4)P turnover is important for secretion and that the two PtdIns-4-kinase isoforms,aandb, are functionally distinct and generate different pools of PtdIns(4)P. In animals and yeast, PtdIns4Kbappears to be more involved in sustaining the structural integrity of the cytoskeleton and Golgi, and plays a vital role in secretion from the Golgi apparatus15,16
.
In plant cells, PtdIns-4-kinase activity is associated with the plasma membrane, nucleus, endomembranes, cytosol and the cytoskeleton1
. This wide distribution in subcellular compartments implies that dis-tinct pools of PtdIns(4)P are targeted differentially to or synthesized in these subcellular compartments and might per-form distinct physiological roles as they clearly do in yeast.
In spite of a wide range of subcellular locations reported, only two PtdIns-4-kinase genes have been cloned from any organ-ism8
. The gene for PtdIns4Kais the larger of the two and it encodes a 200–230 kDa polypeptide. This isoform contains a pleck-strin homology (PH) domain, a poorly con-served 100 amino acid motif that binds polyphosphorylated inositol lipids and thereby targets the protein in which it resides to the membrane17. The gene for PtdIns4Kb is smaller and encodes a polypeptide of
~110–126 kDa, and does not have a PH domain. Both genes have been cloned from Arabidopsis, one encoding a polypeptide of 205 kDa (AtPtdIns4Ka)18and the other encoding a polypeptide of 126 kDa (AtPtdIns4Kb)19
. However, as in other systems, a small molecular weight (65 kDa) PtdIns-4-kinase has been found20. It remains to be determined if the small molecular weight plant en-zyme is an as yet unknown PtdIns-4-kinase isoform, or if it is an
alternative splice variant or proteolytic fragment of one of the known isoforms. Antibodies generated to the C-terminal third of AtPtdIns4Ka, which includes the catalytic, PH and lipid-kinase unique domains, crossreact with all three isoforms of the proteins in carrot suspension culture cells and Arabidopsis seedlings, indicating significant sequence similarity (J.M. Stevenson and W.F. Boss, unpublished).
Fig. 1. The phosphoinositide stereoisomers known to exist in plants. Abbreviations: Ins(1,4,5)P3, inositol (1,4,5) trisphosphate; PtdIns, phosphatidylinositol; PtdIns(3)P,
phos-phatidylinositol-3-monophosphate; PtdIns(3,4)P2, phosphatidylinositol (3,4) bisphosphate;
PtdIns(3,5)P2, phosphatidylinositol (3,5) bisphosphate; PtdIns(4)P,
phosphatidylinositol-4-monophosphate; PtdIns(4,5)P2, phosphatidylinositol (4,5) bisphosphate.
In spinach, the putative PtdIns4Kb isoform and a 65 kDa isoform co-purify with the plasma membrane20, whereas the PtdIns4Kaisoform is most prevalent in the microsomal F-actin fraction18. The differential localization of the PtdIns-4-kinase isoforms implies that the lipid kinases will dictate the location of the different PtdIns(4)P pools and that distinct isoforms will have non-overlapping functions, as in yeast.
An added means of regulating PtdIns(4)P distribution within cells was revealed in studies of the PH domain of PtdIns4Ka. The PH domain of Arabidopsis PtdIns 4-kinase, AtPtdIns4Ka, binds to PtdIns(4)P in preference to other polyphosphoinositides and phospholipids18. Binding to the product of the reaction might enable the enzyme to retain the PtdIns(4)P in a specific intracellu-lar pool and dictate its fate by awaiting competitive binding by a PtdInsP kinase, PtdIns transfer protein, actin-binding proteins or other vesicle-trafficking machinery. Clearly, regulation of the PtdIns(4)P pools within the cell is important, and determining the function of the PtdIns-4-kinase isoforms in plants will bring new insights as to their individual contributions to membrane trafficking and signaling.
Biosynthesis and function of PtdIns(4,5) P2
As the precursor of the second messengers Ins(1,4,5)P3and diacyl-glycerol, PtdIns(4,5)P2plays a pivotal role in signaling. In addi-tion, PtdIns(4,5)P2is important for the regulation of cytoskeletal dynamics, vesicle trafficking and ion transport. PtdIns(4,5)P
2 interacts with many actin-binding proteins, including profilin, gel-solin, a-actinin and cofilin21,22, and overexpression of a PIP-5-kinase type Iaisoform in COS-7 cells results in a massive increase in actin polymerization23. PtdIns(4,5)P
2also plays a role in vesicle formation and in endocytosis24
. PtdIns(4,5)P
2modulates proteins involved in vesicle trafficking, such as the small G protein ADP-ribosylation factor 1 (Ref. 25) and phospholipase D (Ref. 26).
PtdIns(4,5)P2 is involved in both anterograde and retrograde vesicle trafficking. PtdIns(4,5)P
2 and PtdIns(4)P have been shown to stimulate vesicle budding in reconstitution assays with coat proteins24
. Endocytosis of G-protein-coupled receptors by a complex formation of b-arrestin, AP-2 and clathrin is dependent on PtdIns(4,5)P
2turnover 27,28
. The requirement for PtdIns(4,5)P 2 turnover for retrograde trafficking was underscored recently by studies with synaptojanin knockout mice. Mice that lacked the inositol polyphosphate 59phosphatase, synaptojanin, had in-creased PtdIns(4,5)P
2levels, and their inability to dephosphoryl-ate PtdIns(4,5)P
2for synaptic vesicle recycling proved to be fatal 29.
Similarly, PtdIns(4,5)P2accumulation can lead to defects in traf-ficking from the lysosome to the extracellular space. Individuals suffering from Lowe’s syndrome lack the lysosome-associated inositol polyphosphate 59phosphatase, OCRL-1 (Lowe’s oculo-cerebrorenal syndrome), which leads to a two- to threefold increase in PtdIns(4,5)P2 levels in kidney cells and eventually results in renal failure30
. PtdIns(4,5)P
2 can regulate ion transport by modulating the activity of integral membrane proteins, such as the P-type ATPases1, and the ATP-sensitive K1
channel31. PtdIns(4,5)P 2can also mediate the subcellular location and activity of proteins recruited to the membrane via their PH domains during signaling, such as phospholipase C d1 and bARK (β-adrenergic receptor kinase) associated with G-protein-coupled receptors17. In this capacity, PtdIns(4,5)P2might act as a nucleation site for protein scaffolding. However, plant isoforms of PLCdidentified so far do not contain a PH domain.
As with PtdIns(4)P, the myriad of functions of PtdIns(4,5)P 2 can be dictated by a family of PIP kinases that differ in their local-ization, regulation and substrate specificity. Two major types of
mammalian PtdIns phosphate kinases (each containing three iso-forms) have been characterized. The type I enzymes phosphoryl-ate PtdIns(4)P preferentially, whereas the type II enzymes have a higher affinity for PtdIns(5)P [in either case the end product will be PtdIns(4,5)P2]. In addition, both enzymes use PtdIns(3)P as a substrate in vitro21. This is probably also the biosynthetic route for PtdIns(3,4)P2and PtdIns(3,5)P2in plants
1 .
Yeast contain two PtdInsP kinase orthologs. MSS4, which encodes the major PtdIns(4)P(5)-kinase, is an essential gene that was first identified as a multicopy suppressor of the PtdIns-4-kinase STT4 (Ref. 8). Mss4p is localized in the plasma membrane and conditional mutants exhibit disruption of cell polarity and the actin cytoskeleton32,33
. The second yeast PtdIns phosphate kinase ortholog, FAB1 is a PtdIns(3)P(5)-kinase involved in regulating vesicle trafficking and vacuolar morphology and function. Fab1p has a functional alliance with Vps34p and is responsible for phos-phorylating PtdIns(3)P to generate PtdIns(3,5)P2 which accumu-lates in yeast in response to hyperosmotic shock5. The PtdIns(3,5)P
2 isomer has not been functionally characterized in plants. In some plants, there is an increase in the PtdIns(3,5)P2isomer in response to osmotic stress34
, whereas in others, the PtdIns(4,5)P2isomer in-creases35. Whether these are truly species differences or differences in methods or the physiological status of the cells remains to be seen. PtdInsP kinase activity is associated with different subcellular fractions, including the ER, plasma membrane, cytoskeleton and the nucleus1,8. The distribution of the kinases changes in response to agonist stimulation in vivo, suggesting that location of the enzyme is the key to its regulation. Phosphorylation of the kinases has been proposed as a reversible means of affecting both their activity and localization21. The different types of PtdInsP kinases and their regulated translocation might generate different cellular pools of PtdIns(4,5)P2. Recent studies offer intriguing evidence for the presence of such dynamic and functionally distinct pools of PtdIns(4,5)P2in plants
36,37. One method of visualizing PtdIns(4,5)P 2 within unstimulated cells is to express the cDNA encoding a fusion protein of green fluorescent protein (GFP) with the human PLCd–PH domain, which binds with higher affinity to PtdIns(4,5)P
2 than any other polyphosphorylated inositol lipid. This fusion pro-tein was first used in plant cells to identify PtdIns(4,5)P2 within membranes of pollen tubes and to block pollen-tube growth37. Whether the observed effects on pollen-tube growth resulted from the PH domain binding to PtdIns(4,5)P2or Ins(1,4,5)P3remains to be determined. The PLCd–PH domain binds to Ins(1,4,5)P3with an affinity that is eight times higher than its affinity for PtdIns(4,5)P2 (Ref. 17). Nonetheless, the overexpression of the human PLCd–PH domain is a valuable tool for blocking both PtdIns(4,5)P
2 and Ins(1,4,5)P3metabolism.
Although the levels of PtdIns(4,5)P
2in plants are low, there is good correlative evidence of changes in Ins(1,4,5)P
3 levels in response to various stresses2and for the up-regulation of the activity of the enzymes involved in the synthesis and hydrolysis of PtdIns (4,5)P2(Refs 38,39). To date, one Arabidopsis PtdInsP kinase cDNA has been characterized and the corresponding recombinant polypep-tide was shown to have PtdInsP kinase activity38,40. In addition, sev-eral more putative genes have been annotated from the Arabidopsis genome-sequencing initiative. If these multiple members of the PtdIns phosphate kinase family prove to have as much functional complexity as their animal counterparts they will probably define unique microdomains for regulating subcellular metabolism.
Ins(1,4,5)P3: a means of coordinating growth
Although the sequence of events leading up to stimulus-mediated Ins(1,4,5)P3 pro-duction has not been delineated in plants, any stimulus that increases cytosolic Ca21 in a PtdIns(4,5)P2microdomain should, in theory, activate plant PLCs to produce a transient increase in Ins(1,4,5)P3(Ref. 2). It is not surprising that a transient increase in Ins(1,4,5)P3occurs in plants in response to many stimuli. Although increases in Ins(1,4,5)P3levels might be a result of ini-tiating reversible responses, such as guard-cell closure, as sessile organisms, plants must be able to distinguish between transient perturbations and a persistent stress that would require a growth response. This leads to the question, is there evidence that long-term increases in Ins(1,4,5)P3 levels are associated with plant-cell growth? The answer is ‘yes’. In both tip-growing cells37,41 and elongating cells of gravistimulated maize pulvini42, the data indicate a role for PtdIns(4,5)P2 turnover in regulating cell elongation.
In maize pulvini, between 2 and 4 h of gravistimulation are required to induce a bending response. That is, it takes at least 2 h in a horizontal position for the cells on the lower side of the pulvinus to be com-mitted to elongate, even if the plant is returned to a vertical position. When maize plants are placed horizontally, there is a rapid (10 s), transient fivefold increase in
Ins(1,4,5)P3levels in the tissue on the lower side of the pulvinus. Over the next 2 h, these levels increase alternately in both the upper and lower tissue. Between 2 and 8 h, Ins(1,4,5)P3levels increase on the lower side by up to six times more than in the vertical control and then decrease as bending of the pulvinus becomes visible (Fig. 2). One interpretation of these data is that the early fluctuations in Ins(1,4,5)P3(prior to 2 h) are a part of the process that coordinates the cells within a tissue as they make a commitment to elongate. After the presentation time (2 h), but prior to visible growth (8 h), the sustained increase in Ins(1,4,5)P3 levels might predict the growth response. If this is true, then the increase in Ins(1,4,5)P3should precede measurable increases in auxin (Fig. 2; Refs 43–45). Significantly, the sustained increase in PI metabolism might reflect a need for the inositol lipid turnover associated with increased membrane biogenesis and cytoskeletal restructuring, as well as an increase in Ins(1,4,5)P3-mediated Ca
21
release to initiate and sustain cell elongation. If Ins(1,4,5)P3-mediated Ca
21
oscillations are involved in the growth response, plants might require a sustained increase in Ins(1,4,5)P3 to generate these oscillations. The duration of Ins(1,4,5)P3-mediated Ca
21
oscillations will depend, in part, on the properties of the receptor and the rate of Ins(1,4,5)P3 metab-olism; both of which appear to differ in plants and animals. Animals have evolved to contain at least three isoforms of Ins(1,4,5)P3receptors that have been cloned and characterized
46.
Although the animal receptors share ~70% amino acid identity, they differ in their affinity for Ins(1,4,5)P3 and show varying sus-ceptibility for feedback inhibition by Ca21
(Ref. 47). The major Ins(1,4,5)P3-sensitive Ca
21
store in plants appears to be the vacu-ole48
. Electrophysiological studies indicate that the vacuolar Ca21 channel is not Ca21
regulated, which implies that this channel
alone (unlike the ER-localized channels in animal cells) would be unable to sustain Ca21
oscillations48
. Functionally, therefore, the plant Ins(1,4,5)P3 receptor might be most similar to the subtype 3 receptor in animals, suggesting that a sustained increase in Ins(1,4,5)P3would be a requisite to maintain Ins(1,4,5)P3-mediated Ca21
oscillations.
Homology-based approaches to clone the elusive plant Ins(1,4,5)P3receptor have been largely unsuccessful, suggesting that the plant and animal receptors might not share much sequence similarity. The only putative plant Ins(1,4,5)P3receptor that has been purified and characterized biochemically to date appears to be much smaller in size compared with any of the animal counterparts and its activity is enhanced by inositol phosphate metabolites49.
In plant cells, the proteins involved in terminating the Ins(1,4,5)P3signal by hydrolyzing Ins(1,4,5)P3are also different from those in animal cells. In plants, both in vivo50
and in vitro51 data indicate that Ins(1,4,5)P3 is hydrolyzed by both inositol polyphosphate 19phosphatase (InsP19ptase) and InsP59ptase to form Ins(4,5)P2and Ins(1,4)P2, respectively; whereas, in animal cells InsP59ptase is the first enzyme in the hydrolytic pathway. The plant InsP19ptase (IMP) has been cloned and characterized52. Furthermore, at least ten InsP ptase cDNAs have been identified in Arabidopsis on the basis of sequence similarity (G.E. Gillaspy, pers. commun.). Because inositol is an essential metabolite synthe-sized de novo by dephosphorylation of Ins1P by an InsP19ptase, it is crucial that all of the InsP ptase isoforms are identified and characterized and that the different metabolic roles are delineated. Theoretically, altering the production of selective isoforms will affect the duration of the Ins(1,4,5)P3 signal and could thereby affect the rate of cell growth without eliminating de novo synthesis of inositol.
Fig. 2. Transient and long-term changes in inositol (1,4,5) trisphosphate [Ins(1,4,5)P3]
levels in the gravistimulated maize pulvinus. Starting at 10 s, transient oscillatory changes in Ins(1,4,5)P3occur in both the upper and lower sides of the pulvinus over the first 2 h of
gravi-stimulation (red bar). Over the first 2–8 h of gravigravi-stimulation, there is a gradual long-term increase in Ins(1,4,5)P3levels on the lower side (unbroken red line) compared with the
upper side (broken red line) and preceding gravitropic bending. Bending (green) is first detectable around 8 h and reaches a maximum at 48 h. The blue arrows indicate the timing of the increases in auxin and invertase levels on the lower side of the pulvinus (W. Zhao, J.C. Long and G.K. Muday, unpublished).
Trends in Plant Science Persisting gravistimulus
pmol Ins(1,4,5)
P3
/ g FW
0 1000 2000 3000 4000 5000
Time (h)
10 30
20 50
40
Angle of bending (deg
rees)
Ins(1,4,5)P3
oscillations
Ins(1,4,5)P3 on
lower side
Ins(1,4,5)P3 on upper side
Gravitropic bending
Presentation time
0 2 4 6 8 10 15 36 48
Increase in invertase on the lower side Increase in auxin
In addition, phosphorylation of Ins(1,4,5)P3affects the duration of the Ins(1,4,5)P3signal. In yeast, InsP6, which results from the phosphorylation of Ins(1,4,5)P3, is essential for transport of RNA out of the nucleus53
. Although phytate metabolism has been studied for years with regard to seed development and embryo for-mation54
, there is little information on the roles of the complex InsP isomers in signaling49and regulating plant-cell growth. (For comparison see http://dir.niehs.nih.gov/dirlst/shears.htm where de-velopments from the animal literature are continuously updated.)
If a sustained increase in Ins(1,4,5)P3is necessary for a growth response, then inhibiting PtdIns(4,5)P2 turnover should inhibit growth. Molecular studies that overexpress the human PLCd–PH domain to prevent PtdIns(4,5)P2hydrolysis or to bind Ins(1,4,5)P3 suggest that PtdIns(4,5)P2metabolism is essential for pollen-tube growth37. Furthermore, treatment with the PLC inhibitor, U73122, reduced gravitropic bending by 60% and abolished the long-term increase in Ins(1,4,5)P3 levels on the lower side of oat pulvini (I.Y. Perera et al., unpublished). Although U73122 appears to be effective at inhibiting recombinant PLC activity in vitro and abscisic acid-induced Ca21
increases in guard cells in vivo55 , the mechanism of action and the degree of specificity of the compound are not known. Presumably, U73122 inhibits all isoforms of PI–PLC, and it will take a molecular genetic approach to separate PLC-mediated signaling from growth-related pro-cesses such as membrane biogenesis. Caution should be exercised when interpreting data from other putative PLC inhibitors2
. One example is neomycin, a positively charged aminoglycoside and protein-synthesis inhibitor, which also inhibits PLC-mediated hydrolysis of PtdIns(4,5)P2when added at a 1:1 ratio to liposomes in vitro, but is ineffective in vivo2,56
. Most of the neomycin added will bind to the cell wall, which is why kanamycin, a less positively
charged analog, is commonly used as a protein-synthesis inhibitor. Furthermore, any neomycin that does enter the cell will not bind PtdIns(4,5)P2 with high enough affinity to displace membrane proteins that are already bound.
Although sustained increases in Ins(1,4,5)P3and Ca
21
can predict growth, continuous elevation of Ca21
could also, over time, decrease growth and even lead to apoptosis57
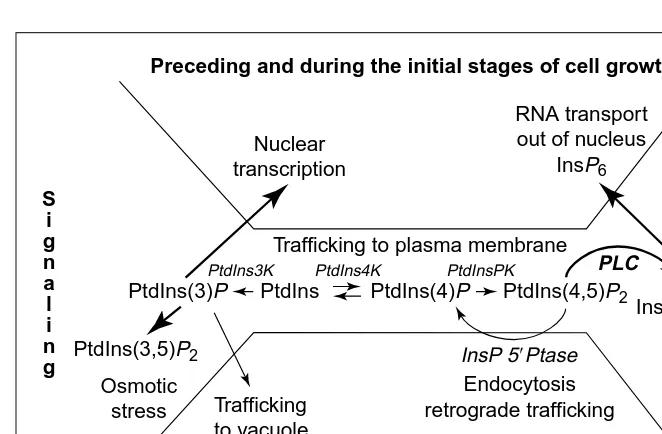
. It is therefore not surprising that Ins(1,4,5)P3 decreases in the maize pulvinus when bending is detectable and when cells are beginning to elongate (8 to 10 h after gravistimulation). One mechanism to decrease Ins(1,4,5)P3levels and yet sustain PtdIns(4,5)P2 turnover would be to switch from a predominantly PLC-mediated PtdIns-(4,5)P2 pathway to phosphatase-mediated PtdIns(4,5)P2 metabolism as cell enlarge-ment ensues. There are not enough data from either pollen tube or the maize pulvini to determine which pathway predominates during cell elongation. Clearly, an increase in retrograde trafficking would be an efficient means of meeting the increased demands for tonoplast biogenesis and for secretion of new plasma membrane and polysaccharides as the cell enlarges. Although further biochemical and molecu-lar studies are essential before we can determine if PtdIns(4,5)P2-mediated path-ways are crucial for regulating retrograde trafficking or cell enlargement in plants, inarguably, inositol phospholipids provide a source of regulatory metabolites that could coordinate both intracellular and intercellular growth responses. The potential shifts in PI metabolism associated with initial signaling and cell growth are shown in Figure 3.
The broader implications of a change in lipid metabolism are intriguing and provide a wealth of material for future studies. We have only touched on the complexities of inositol metabolism in plants. Virtually nothing is known about the functional role of the scyllo-inositol stereoisomer of PIs (Ref. 58). Until recently, it was always assumed that the inositol lipids identified in plant systems were PtdIns, PtdIns(3)P, PtdIns(4)P and PtdIns(4,5)P2. We now know that there are PtdIns(3,4)P2, PtdIns(3,5)P2and possibly some as yet uncharacterized stereoisomers that are involved in signaling and membrane trafficking. These isomers will need to be consid-ered when interpreting past and future data. Inositol conjugates that can affect hormone-stimulated reactions and inositol metabo-lites that serve as osmometabo-lites within the cytosol of osmotically stressed cells must also be considered in the overall pathway54.
What does the future hold?
As we begin to combine biochemical and molecular genetic ap-proaches to understand physiological responses on a macro scale it will be important to characterize the changes in inositol signal-ing as plants mature and respond to changes in nutrient flux and environmental conditions36
. Crucial to interpreting these data will be an understanding of the subcellular localization of the proteins and lipids. To this end, the recent development of useful tools such as the fluorescently labeled inositol phospholipids59 and GFP-fusion proteins of lipid-binding domains will help to identify microdomains where signaling events are occurring. In addition, Fig. 3. The dynamic interplay between the stereospecific phosphoinositide (PI) isomers and
cell growth. In addition to generating inositol (1,4,5) trisphosphate [Ins(1,4,5)P3]-induced
Ca21
oscillations, PIs have been reported to affect membrane biogenesis, cytoskeletal struc-ture, ion transport, and RNA synthesis and transport. Temporal shifts in PI metabolism can occur with growth and development. For example, phospholipase-C-mediated phos-phatidylinositol (4,5) bisphosphate [PtdIns(4,5)P2] turnover provides Ins(1,4,5)P3for
initiat-ing signalinitiat-ing and providinitiat-ing a substrate for InsP6biosynthesis. PtdIns(4,5)P2turnover by
Ins 59phosphatase might favor cell expansion by increasing retrograde trafficking important for increased demands of an expanding tonoplast membrane. Because the PIs influence so many cellular processes, the steady-state profile of the lipid isomers and their metabolites should reflect the physiological status of the cell.
Trends in Plant Science
PtdInsPK
InsP 59Ptase
Ca2+
RNA transport out of nucleus
InsP6
Nuclear transcription
Endocytosis retrograde trafficking Trafficking
to vacuole
PtdIns(3,5)P2
Osmotic stress
PLC
Trafficking to plasma membrane
PtdIns3K PtdIns4K
Cell expansion
Preceding and during the initial stages of cell growth
S i g n a l i n g S
i g n a l i n g
PtdIns PtdIns(4)P PtdIns(4,5)P2
Ins(1,4,5)P3
further insight will be gained from genomic analyses, and the study of the physiology and biochemistry of transgenic plants with altered PI metabolism. We anticipate that these studies will reveal essential information about the role of inositol lipids as key regulators of growth.
In summary, living organisms have exploited membrane lipids, using them as regulatory metabolites and second messengers. As sessile organisms, plants need a system to determine whether a stimulus is transient or sustained before making a commitment to grow. Once committed, the plant must coordinate a growth response both within cells and throughout the tissue. The multiple stereoisomers of the phosphoinositides, provide both water and lipid-soluble metabolites that can diffuse within and between cells, creating microdomains that can coordinate and transmit information. Our challenge is to understand the key events that orchestrate the symphony of interacting pathways that result in stimulus-induced growth.
Acknowledgements
We apologize to the authors who could not be cited because of lim-ited reference space. We would like to acknowledge the support of the National Science Foundation (MCB-9604285), the National Aeronautics and Space Administration (NAGW-4984) and the North Carolina Agriculture Research Service to W.F.B., and a DAAD fellowship HSPIII financed by the German Federal Ministry of Education, Science, Research and Technology to I.H. We also acknowledge Bjørn Drøbak, Steve C. Huber, Gloria K. Muday and Glenda Gillaspy for sharing their unpublished results.
References
1 Drøbak, B.K. et al. (1998) Phosphoinositide kinases and the synthesis of
polyphosphoinositides in higher plant cells. In International Review of
Cytology (Vol. 189) (Jeon, K.W., ed.), pp. 95–130, Academic Press
2 Munnik, T. et al. (1998) Phospholipid signaling in plants. Biochim. Biophys.
Acta 1389, 222–272
3 Corvera, S. et al. (1999) Phosphoinositides in membrane traffic. Curr. Opin.
Cell Biol. 11, 460–465
4 Roth, M.G. (1999) Lipid regulators of membrane traffic through the Golgi
complex. Trends Cell Biol. 9, 174–179
5 Wurmser, E.A. et al. (1999) Phosphoinositide 3-kinases and their FYVE
domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J. Biol. Chem. 274, 9129–9132
6 Hong, Z. and Verma, D.P.S. (1994) A phosphatidylinositol 3-kinase is induced
during soybean nodule organogenesis and is associated with membrane proliferation. Proc. Natl. Acad. Sci. U. S. A. 91, 9617–9621
7 Matsuoka, K. et al. (1995) Different sensitivity to wortmannin of two vacuolar
sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130, 1307–1318
8 Fruman, D.A. et al. (1998) Phosphoinositide kinases. Annu. Rev. Biochem. 67,
481–507
9 Welters, P. et al. (1994) AtVPS34, a phosphatidylinositol 3-kinase of
Arabidopsis thaliana, an essential protein with homology to a
calcium-dependent lipid binding domain. Proc. Natl. Acad. Sci. U. S. A. 91, 11398–11402
10 Bunney, T.D. et al. Association of phosphatidylinositol 3-kinase with nuclear
transcription sites in higher plants. Plant Cell (in press)
11 Lease, K. et al. (1998) Challenges in understanding RLK function. Curr.
Opin. Plant Biol. 5, 388–392
12 Wiedemann, C. et al. (1998) An essential role for a small synaptic
vesicle-associated phosphatidylinositol 4-kinase in neurotransmitter release.
J. Neurosci. 18, 5594–5602
13 Trotter, M.J. et al. (1998) A genetic screen for aminophospholipid transport
mutants identifies the phosphatidylinositol 4-kinase, Stt4p, as an essential component in phosphatidylserine metabolism. J. Biol. Chem. 273, 13189–13196
14 Hama, H. et al. (1999) Direct involvement of phosphatidylinositol
4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274, 34294–34300
15 Walch-Solimena, C. and Novick, P. (1999) The yeast
phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nat. Cell Biol. 1, 523–525
16 Godi, A. et al. (1999) ARF mediates recruitment of PtdIns-4-OH kinase-band stimulates synthesis of PtdIns(4,5)P2on the Golgi complex. Nat. Cell Biol. 1, 280–287
17 Lemmon, M.A. et al. (1996) PH domains: diverse sequences with a common
fold recruit signaling molecules to the cell surface. Cell 85, 621–624
18 Stevenson, J.M. et al. (1998) A phosphatidylinositol 4-kinase pleckstrin
homology domain that binds phosphatidylinositol 4-monophosphate. J. Biol.
Chem. 273, 22761–22767
19 Xue, H-W. et al. (1999) A plant 126-kDa phosphatidylinositol 4-kinase with a
novel repeat structure. J. Biol. Chem. 274, 5738–5745
20 Westergren, T. et al. (1999) Phosphatidylinositol 4-kinase associated with
spinach plasma membranes. Isolation and characterization of two distinct forms. Plant Physiol. 121, 507–516
21 Hinchliffe, K.A. et al. (1998) PIPkins, their substrates and their products: new
functions for old enzymes. Biochim. Biophys. Acta 1436, 87–104
22 Staiger, C.J. et al. (1997) Profilin and actin-depolymerizing factor: modulators
of actin organization in plants. Trends Plant Sci. 7, 275–281
23 Shibasaki, Y. et al. (1997) Massive actin polymerization induced by
phosphatidylinositol-4-phosphate 5-kinase in vivo. J. Biol. Chem. 272, 7578–7581
24 Toker, A. (1998) The synthesis and cellular roles of phosphatidylinositol 4,
5-bisphosphate. Curr. Opin. Cell Biol. 10, 254–261
25 Randazzo, P.A. (1997) Functional interaction of ADP-ribosylation factor 1
with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 272, 7688–7692
26 Wang, X. (1999) The role of phospholipase Din signaling cascades. Plant
Physiol. 120, 645–651
27 Gaidarov, I. and Keen, J.H. (1999) Phosphoinositide-AP-2 interactions required
for targeting to plasma membrane clathrin-coated pits. J. Cell Biol. 146, 755–764
28 Jost, M. et al. (1998) Phosphatidylinositol-4,5-bisphosphate is required for
endocytic coated vesicle formation. Curr. Biol. 8, 1399–1402
29 Cremona, O. et al. (1999) Essential role of phosphoinositide metabolism in
synaptic vesicle recycling. Cell 99, 179–188
30 Majerus, P.W. et al. (1999) The role of phosphatases in inositol signaling
reactions. J. Biol. Chem. 274, 10669–10672
31 Shyng, S-L. and Nichols, C.G. (1998) Membrane phospholipid control of
nucleotide sensitivity of KATPchannels. Science 282, 1138–1141
32 Desrivieres, S. et al. (1998) MSS4, a phosphatidylinositol-4-phosphate
5-kinase required for organization of the actin cytoskeleton in Saccharomyces
cerevisiae. J. Biol. Chem. 273, 15787–15793
33 Homma, K. et al. (1998) Phosphatidylinositol-4-phosphate 5-kinase localized
on the plasma membrane is essential for yeast cell morphogenesis. J. Biol.
Chem. 273, 15779–15786
34 Meijer, H.J.G. et al. (1999) Hyperosmotic stress induces rapid synthesis of
phosphatidyl-D-inositol 3,5-bisphosphate in plant cells. Planta 208, 294–298 35 Pical, C. et al. (1999) Salinity and hyperosmotic stress induce rapid increases
in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J. Biol. Chem. 274, 38232–38240
36 Heilmann, I. et al. (1999) Changes in phosphoinositide metabolism with days
in culture affect signal transduction pathways in Galdieria sulphuraria. Plant
Physiol. 119, 1331–1339
37 Kost, B. et al. (1999) Rac homologues and compartmentalized
phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell. Biol. 145, 317–330
38 Mikami, K. et al. (1998) A gene encoding phosphatidylinositol-4-phosphate
5-kinase is induced by water stress and abscisic acid in Arabidopsis thaliana.
Plant J. 15, 563–568
39 Hirayama, T. et al. (1995) A gene encoding a phosphatidylinositol-specific
phospholipase C is induced by dehydration and salt stress in Arabidopsis
D
o stomata act independently regardless of the conduct of those around them? Or, do neighbouring stomata dictate their behaviour? Fifteen years ago, most stomatal physiolo-gists would have assumed that stomata act independently, but recent discoveries now suggest that stomatal behaviour dictated by that of neighbouring stomata is also possible. Certainly, anyone studying stomatal responses at the level of the intact leaf would agree that stomata are complex and unpredictable, withstanding even the most determined efforts to understand their behaviour. Doubtless, this is partly because of the many environmental parameters that stomatarespond to, and to the level of interaction among these responses. However, it now appears that some of this unpredictability might result from complex interactions between stomata, leading to a form of emergent behaviour that is inherently difficult to predict. This phenomenon, which we term ‘patchy stomatal conductance’, has been the subject of several recent exhaustive reviews1–3. This review will therefore focus on three aspects of the issue: hydraulic interactions as a mechanism for patchiness, the impor-tance of scale in stomatal dynamics, and patchiness as an emergent collective property of stomata.
Patchy stomatal conductance:
emergent collective behaviour
of stomata
Keith A. Mott and Thomas N. Buckley
Until recently, most scientists have tacitly assumed that individual stomata respond indepen-dently and similarly to stimuli, showing minor random variation in aperture and behaviour. This implies that stomatal behaviour should not depend on the scale of observation. However, it is now clear that these assumptions are often incorrect. Leaves frequently exhibit dramatic spatial and temporal heterogeneity in stomatal behaviour. This phenomenon, in which small ‘patches’ of stomata respond differently from those in adjacent regions of the leaf, is called ‘patchy stomatal conductance’. It appears to represent a hitherto unknown type of emergent collective behaviour that manifests itself in populations of stomata in intact leaves.
40 Satterlee, J.S. and Sussman, M.R. (1997) An Arabidopsis phosphatidylinositol
4-phosphate 5-kinase homolog with seven novel repeats rich in aromatic and glycine residues (Accession no. AF01938) (PGR 97–150). Plant Physiol. 115, 864
41 Franklin-Tong, V.E. et al. (1996) Growth of pollen tubes of Papaver rhoeas
is regulated by a slow-moving calcium wave propagated by inositol 1,4,5-trisphosphate. Plant Cell 8, 1305–1321
42 Perera, I.Y. et al. (1999) Transient and sustained increases in inositol
1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc. Natl. Acad. Sci. U. S. A. 96, 5838–5843
43 Rosen, E. et al. (1999) Root gravitropism: a complex response to a simple
stimulus? Trends Plant Sci. 4, 407–412
44 Philippar, K. et al. (1999) Auxin-induced K1channel expression represents an
essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci.
U. S. A. 96, 12186–12191
45 Kaufman, P.B. et al. (1995) Hormones and the orientation of growth. In
Plant Hormones: Physiology, Biochemistry and Molecular Biology
(Davies, P.J., ed.), pp. 547–571, Kluwer
46 Patel, S. et al. (1999) Molecular properties of inositol 1,4,5-trisphosphate
receptors. Cell Calcium 25, 247–264
47 Taylor, C.W. (1998) Inositol trisphosphate receptors: Ca21-modulated
intracellular Ca21channels. Biochim. Biophys. Acta 1436, 19–33
48 Sanders, D. et al. (1999) Communicating with calcium. Plant Cell 11, 691–706 49 Dasgupta, S. et al. (1996) Interaction of myoinositoltrisphosphate-phytase
complex with the receptor for intracellular Ca21mobilization in plants. Biochemistry 35, 4994–5001
50 Brearley, C.A. et al. (1997) Metabolic evidence for PtdIns(4,5)P2-directed phospholipase C in permeabilized plant protoplasts. Biochem J. 324, 123–131
51 Drøbak, B.K. et al. (1991) Metabolism of inositol (1,4,5) trisphosphate by a soluble
enzyme fraction from pea (Pisum sativum) roots. Plant Physiol. 95, 412–419
52 Gillaspy, G.E. et al. (1995) Plant inositol monophosphatase is a
lithium-sensitive enzyme encoded by a multigene family. Plant Cell 7, 2175–2185
53 York, J.D. et al. (1999) A phospholipase C-dependent inositol polyphosphate
kinase pathway required for efficient messenger RNA export. Science 285, 96–100
54 Loewus, F. and Murthy, P.P. (2000) myo-Inositol metabolism in plants. Plant
Sci. 150, 1–19
55 Staxen, I. et al. (1999) Abscisic acid induces oscillations in guard-cell
cytosolic free calcium that involve phosphoinositide-specific phospholipase C.
Proc. Natl. Acad. Sci. U. S. A. 96, 1779–1784
56 Chapman, K. (1998) Phospholipase activity during plant growth and development
and in response to environmental stress. Trends Plant Sci. 3, 419–425
57 Berridge, M.J. et al. (1998) Calcium – a life and death signal. Nature 395, 645–648 58 Pliska-Matyshak, G. et al. (1997) Novel phosphoinositides in barley aleurone
cells, additional evidence for the presence of phosphatidyl-scyllo-inositol.
Plant Physiol. 113, 1385–1393
59 Tuominen, E.K. et al. (1999) Fluorescent phosphoinositide derivatives reveal
specific binding of gelsolin and other actin regulatory proteins to mixed lipid bilayers. Eur. J. Biochem. 263, 85–92
Jill M. Stevenson, Imara Y. Perera, Ingo Heilmann,
Staffan Persson and Wendy F. Boss*are at the Botany Dept, North Carolina State University, Raleigh, NC 27695, USA.
![Fig. 2. Transient and long-term changes in inositol (1,4,5) trisphosphate [Ins(1,4,5)P3] levels in the gravistimulated maize pulvinus](https://thumb-ap.123doks.com/thumbv2/123dok/1040007.930159/4.858.233.569.89.321/transient-changes-inositol-trisphosphate-levels-gravistimulated-maize-pulvinus.webp)