Modulated synthesis and characterization of Ni-UiO66
Dini Iflakhah and Ratna Ediati
Department of Chemistry ITS, Kampus ITS Keputih Sukolilo, Surabaya 60111, Indonesia
Abstract
Modulated synthesis of Ni-UiO66 is reported via direct conventional solvothermal method. Zirconium tetrachloride (ZrCl4), 1,4-benzen dicarbocarboxylic acid (H2BDC), and
nickel (II) nitrate (Ni(NO3)2.6H2O) were dissolved in mixture of N-N -dimethyl formamide
(DMF) and variant concentrations of acetic acid (CH3COOH). Acetic acid was used as
modulator in which variants concentrations were 5eq, 10eq, and 20eq. All mixtures were heated at 140 °C for 6h. The structures of Ni-UiO66 obtained from modulated synthesis were confirmed to be isostructural to UiO-66 by powder X-Ray diffraction (PXRD). The data present a unique crystalline phase, which is in good agreement with the UiO-66 pattern by Cavka. The characterization PXRD pattern of Ni-UiO66 has main peak with a high intensity at 2 of 7.3°, as well as other characteristic peaks with lower intensity at 2 of 8.6, 10.3, 20.6 and 30.5°. The crystallinity of Ni-UiO66 can be enhanced by adding modulator to the reaction mixture. Ni-UiO66 with variant modulator is 20eq has the highest crystalline. The IR spectra of UiO-66 and Ni-UiO66 are almost the same, further confirming the isostructural to UiO-66. SEM images of UiO-66 and Ni-UiO66 show the modulator leads to the morphology of intergrown particles decreasing.
Keywords UiO-66, bimetallic MOF, modulated synthesis
1. Introduction
Metal Organic Framework (MOF) is coordination polymer consist of metal ion or polynuclear unit and organic compound as linker (Zhou et al., 2013). Thus, the combination of such unbelievable levels of porosity, surface area, pore size and wide chemical inorganic organic composition has recently brought these materials to the attention of many researchers both in academia and industry, with over 1000 publications on metal organic frameworks (Czaja et al., 2009). MOF was applied as hidrogen storage (Kuppler et al., 2009), gas separation applications (Lee, 2012) and heterogeneous catalysis (Burrows et al., 2013, Vermoortele et al., 2013). The most representative MOFs is UiO-66 with thermal stability up to 500oC. UiO-66 or Zr-MOF is one of member group MOF consist of zirconium (Zr) and 1,4
benzene dicarboxylic acid (BDC) as organic linker. The 3D structure of UiO-66 was built by an inner Zr6O4(OH)4core in which the triangular faces of the Zr6-octahedron are alternatively
capped by 3-O and 3-OH groups (Cavka et al., 2008).
pivalik acid, trichloroacetic acid and acid trifluoroasetik. Ren (2014) have reported also modulation UiO-66 using formic acid to produce crystals of crystalline and reduced agglomeration by increasing the concentration of formic acid is added.
Bimetallic Ni-MOF5 was reported by Li and co-workers in 2012. The results demonstrated that the Ni-MOF5 not only exhibit larger specific surface areas and larger pores than the MOF-5, but also significantly enhance water resistance of the framework. Herein, we report the modulated synthesis of bimetallic Ni-UiO66 with solvothermal method.
2. Materials and methods
2.1
Materials
Zirconium tetrachloride (ZrCl4Sigma-Aldrich 99,0%), 1,4-benzen dicarbocarboxylic acid
(BDC, Sigma-Aldrich 99.0%), N-N -dimethyl formamide (DMF, Merck 99.8%), Chloroform (CHCl3, Merck 99.9%), Acetic acid (CH3COOH, Sigma-Aldrirh, 99.85%), Nickel (II) nitrate
(Ni(NO3)2.6H2O, Sigma-Aldrich, 96%). All chemicals were pure analysis and used without
purification.
2.2 Procedures
Synthesis of Ni-UiO66. ZrCl4 (0.3381 g, 1.5 mmol), BDC (0.25 g, 1.5 mmol),
Ni(NO3)26H2O (0.0654 g, 0.225 mmol) were dissolved in 45 ml DMF at room temperature.
10% HCl (0.052 ml, 1.5 mmol) was added. The mixture was stirred at room temperature for 1h. The mixture was transferred into a 50 ml vial, which was sealed and maintained at 140oC
for 6h. The vial was then removed from the oven and allowed to cool to room temperature. The white solid was filtrated, washed with DMF and chloroform and dried at vacuum condition.
Ni-UiO66 Mod5The mixture of Ni-UiO66 as described above was prepared. 5eq of acetic acid (0.5 ml, 7.5 mmol) was added. The mixture was stirred at room temperature for 1h. The mixture was transferred into a 50 ml vial, which was sealed and maintained at 140oC
for 6h. The vial was then removed from the oven and allowed to cool to room temperature. The white solid was filtrated, washed with DMF and chloroform and dried at vacuum condition.
Ni-UiO66 Mod10 or Ni-UiO66 Mod20The procedure was similar as described above. However, instead of 5eq of acetic acid, 10eq (0.86 ml, 15 mmol) or 20eq (1.71 ml, 30 mmol) was used.
2.3
Characterization
X-ray diffractometer (PXRD, D8 Advance-Bruker aXS) using Cu Ka radiation (k = 1.54056 Å) in the range of 2 = 0 50 was used to confirm the isostructural Ni-UiO66 obtained from modulated synthesis with UiO-66 by Cavka (2009). FTIR (Spectrum 100-FT-IR Spectrometer, Perkin-Elmer) was used to check the stability of the functional groups on the organic ligands. The spectrum was scanned from 600 to 4000 cm-1.SEM (Zeiss EVO MA 10). Analysis was used
3. Results and discussion
3.1 Modulated synthesis of Ni-UiO66
Conventional solvothermal method was used for modulated synthesis of Ni-UiO66 with the variant concentration of modulator were 5, 10 and 20 eq. After all reactants were dissolved in 45 ml DMF, the mixture was sealed and heated at 140oC for 6h. The mixture
seemed two layer and its colour were bluish while heating process and then turned back to green after cooling. It was shown at Figure 1. The greenish and bluish color occurred due to the effect of adding ion Ni2+. The solid was collected by centrifuge or filtration. The amounts
of samples was shown in Figure 2. The graph at Figure 2 shows that the greater the modulator is added, the heavier the mass produced. Competition reaction between ligand BDC with modulator, acetic acid occurs during the process of solvothermal. As described in the previous research that the carboxylate groups on the ligand acts as a bridge between clusters because BDC has carboxylic acid groups on both ends, but acetic acid has only one carboxylic acid group at the end. Thus, the polymerization process stalled when the metal reacts with acetic acid. The ends of the polymerization process is what causes the increased crystallinity (Tsuruoka, 2009).
(a) b)
Figure 1.The mixture of Ni-UiO66 at: (a) room temperature and (b) 140oC.
Figure 2.Mass products of Ni-UiO66 obtained from modulated synthesis.
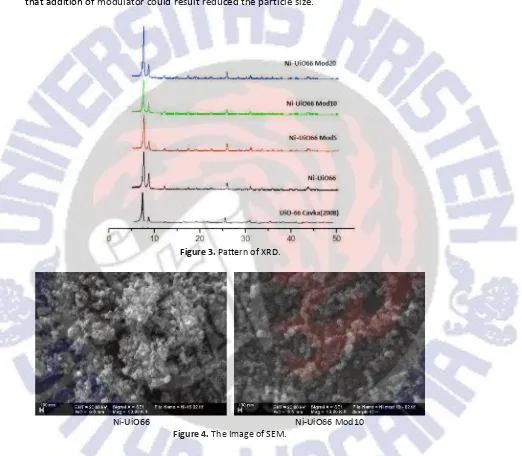
results argued by Tsuruoka (2009). The competition reaction between ligand BDC and acetic acid ligands to the metal lead to a decrease the speed of crystal formation, thereby reducing the formation of intergrown crystals, the crystals become more crystalline. Based on the Scherrer equation, particle size of Ni-UiO66 and Ni-UiO66 Mod 20 are 38 nm and 35 nm. In another research, particle size of UiO-66 is 63 nm (Abid, 2012), respectively, which suggests that addition of modulator could result reduced the particle size.
Figure 3.Pattern of XRD.
Ni-UiO66 Ni-UiO66 Mod10
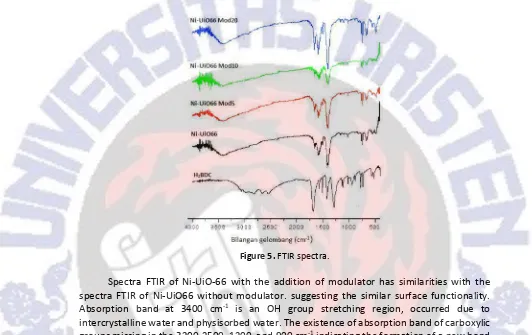
Figure 4.The Image of SEM.
The particle size of Ni-UiO66 obtained from modulated synthesis and morphology crystal can be shown at SEM Image at Figure 4. It is seen that particle size of Ni-UiO66 was similar with cubic shape at about 70 nm but the particle size of Ni-UiO66 obtained from modulated synthesis was reduced, which confirm the results from XRD analysis. Addition of acetic acid as modulator in synthesis solution will increase in BDC and metal solubility. Secondly, the metal ion will not aggregate to form larger crystals.
Figure 5 shows spectra of H2BDC and Ni-UiO66 with and without modulator.
bands in the 3200-2500 cm-1which is the stretching area of OH from carboxylate groups,
absorption bands in the 1730-1700 cm-1 which is the stretching area of C = O and the
absorption band in the 1320- 1210 cm-1which is the stretching area of CO. In addition, at
1600-1465 cm-1region, absorption appears bands stretching and the region 800-711 cm-1
appears bending characteristic absorption band of benzene rings.
Figure 5.FTIR spectra.
Spectra FTIR of Ni-UiO-66 with the addition of modulator has similarities with the spectra FTIR of Ni-UiO66 without modulator. suggesting the similar surface functionality. Absorption band at 3400 cm-1 is an OH group stretching region, occurred due to
intercrystalline water and physisorbed water. The existence of absorption band of carboxylic groups missing in the 3200-2500, 1200, and 900 cm-1indicating the formation of a new bond
between the carboxylic groups with cluster Zr6O6 where ligand BDC is a bridge between
clusters Zr6O6. Absorption bands at 680 and 470 cm-1indicates the formation of clusters of Zr
and absorption bands 447-470 cm-1region is an area stretching 3-OH. (Valenzanoet al.,
2011). Significant differences between solids Ni-UiO66 with and without the modulator shown in the absorption band in the 1700 and 1400 cm-1which is the absorption of the CO
group of carboxylic acid groups. At Ni-UiO66 Mod5 intensity absorption band in the 1700 and 1400 cm-1was higher than the Ni-UiO66. The larger the modulator is added, the higher the
intensity of absorption.
Thermal stability analysis of Ni-UiO66 Mod20 was conducted using a TGA. Weight loss profiles and weight loss rate are presented in Figure 6. It is clear that three-stage weight loss occurred on Ni-UiO66 Mod20. The first weight loss appearing before 50-100oC, 9,32% is due
to the evaporation of surface adsorbed water on Ni-UiO66 Mod20. The second weight loss at 120-200oC, 18,76% is attributed to the decomposition of DMF, which was not exchanged
by chloroform. The third stage of weight loss at 470-580oC, 28,06% is ascribed to the
decomposition of Ni-UiO66 Mod20 to ZrO2. Compared with Ni-UiO66 exhibited less weight
Figure 6.TGA profile.
4. Conclusion and remarks
Modulated synthesis of Ni-UiO66 is reported via direct conventional solvothermal method. The adding of acetic acid as modulator produce some effects on structure and properties of Ni-UiO66. XRD, FTIR, and SEM analyses indicated that the crystalline structure, functionality and morphology remained unchanged. XRD pattern and SEM images of Ni-UiO66 obtained from modulated synthesis shows the cristalinity increase and the particle size smaller. TGA profile show Ni-UiO66 obtained from modulated synthesis has slight higher stability compare to Ni-UiO66 without modulator.
Acknowledgment
The authors would like to thank Laboratory Materials and Energy of Chemistry in faculty of mathematics and science (MIPA), laboratory energy ITS and laboratory material characterization of materials and metallurgical engineering department of industrial technology faculty (FTI) for their cooperation in this research.
References
Abid, H.R., Pham, G.H., Ang, H.M., Tade, M.O., & Wanga, S. (2012). Adsorption of CH4and CO2on
Zr-metal organic frameworks.Journal of Colloid and Interface Science,366, 120 124.
Burrows, A. (2013), Post-synthetic modification of MOFs. Metal Organic Frameworks as Heterogeneous Catalyst,RSC Publishing, Catalysis Series No. 12, 31 75.
Cavka, J.H., Jakobsen, S., Olsbye, U., Guillou, N., Lamberti, C., Bordiga, S., & Lillerud, K.P. (2008). A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. Journal American Chemistry Society,130(42), 13850 13851.
Czaja, A.U., Trukhan, N., & Müller, U. (2009). Industrial applications of metal organic frameworks. Chem. Soc. Rev.,38, 1284 1293
Kuppler, R.J., Timmons, D.J., Fang, Q.R., Li, J.R., Makal, T.A., Young, M.D., Yuan, D., Zhao, D., Zhuang, W., & Zhou, H.C. (2009). Potential applications of metal-organic frameworks. Coordination Chemistry Reviews,253, 3042 3066
Li, J., Cheng, S., Zhao, Q., Long, P., & Dong, J. (2009). Synthesis and hydrogen-storage behavior of metal organic framework MOF-5.International Journal of Hydrogen Energy,34, 1377 1382. Ren, J., Langmi, H.W., North, B.C., Mathe, M., & Bessarabov, D. (2014). Modulated synthesis of
Tsuruoka, T., Furukawa, S., Takashima, Y., Yoshida, K., Isoda, S., & Kitagawa, S. (2009). Nanoporous nanorods fabricated by coordination modulation and oriented attachment growth.Angewandte Chemistry International,29, 4739 4743.
Valenzano, L., Civalleri, B., Chavan, S., Bordiga, S., Nilsen, M.H., Jakobsen, S., Lillerud, K.P., & Lamberti, C. (2011). Disclosing the complex structure of UiO-66 metal organic framework: A synergic combination of experiment and theory.Chem. Mater,23, 1700 1718.
Vermoortele, F., Bueken, B., Bars, G.L., Van de Voorde, B., Vandichel, M., Houthoofd, K., Vimont, A., Daturi, M., Van Speybroeck, V., Kirschhock, C., & De Vos, D.E., (2013). Synthesis modulation as a tool to increase the catalytic activity of metal-organic framework: The unique case of UiO-66 (Zr). Journal American Chemistry Society, 135, 11465 11468.