Volume 47, Number 1, March 2014
Research Report
The role of Hsp0, CD-8 and IFN-γ in immunopathobiogenesis of
periapical granuloma in dental caries
risya Cilmiaty1and Mandojo rukmo2
1 Department of Dental and Oral, Faculty of Medicine, University of Sebelas Maret, Surakarta-Indonesia 2 Department of Conservative Dentistry, Faculty of Dental Medicine, Universitas Airlangga, Surabaya-Indonesia
abstraCt
background: The incidence of dental caries with periapical granulomas in Indonesia is quite high. However, the mechanism of the formation of periapical granulomas in dental caries caused by bacterial infection in immunopathobiogenesis cannot be explained completely. Thus, this explanation is necessary in order to be used as a basis for diagnostic, preventive and therapeutic measures.
Purpose: This research was aimed to determine the role of Hsp60, CD-8 and IFN-γ in immunopatobiogenesis of periapical granuloma
in dental caries. Methods: This research was an analytic observational study with cross sectional approach. Samples of this research were 36 teeth of patients with dental caries, consisting of 18 caries teeth with periapical granulomas and 18 caries teeth without periapical granulomas. The variables observed in this research were Hsp60, CD-8 and IFN-γ. Measurements were conducted by using immunohistochemical methods on periapical tissue. results: The mean of Hsp60, CD-8 and IFN-γ in granuloma group was significantly
higher than those in non granuloma group (p<0.05). The positive role of IFN-γ on the incidence of granulomas appeared to be more prominent. Conclusion: The study suggested that in immunopathobiogenesis of periapical granuloma in dental caries, Hsp60, CD-8 and IFN-γ played important roles, but the role of IFN-γ was found to be more prominent.
Key words: Dental caries, immunopathobiogenesis, periapical granuloma, Hsp60, CD-8, IFN-γ
abstraK
latar belakang: Angka kejadian gigi karies dengan granuloma periapikal di Indonesia cukup tinggi, Namun mekanisme terbentuknya granuloma periapikal pada gigi karies yang disebabkan oleh infeksi bakteri secara imunopatobiogenesis belum dapat dijelaskan secara tuntas. Adanya penjelasan ini diperlukan agar dapat digunakan sebagai dasar pengembangan diagnosis, langkah preventif dan terapinya. tujuan: Penelitian ini bertujuan untuk mengetahui peran Hsp60, CD-8 dan IFN-γ dalam immunopatobiogenesis dari granuloma periapikal karies gigi. Metode: Penelitian ini merupakan penelitian observasional analitik dengan pendekatan cross sectional. Sampel dari penelitian ini adalah 36 gigi pasien dengan karies, yang terdiri dari 18 karies gigi dengan granuloma periapikal dan 18 karies gigi tanpa granuloma periapikal. Variabel yang diamati adalah Hsp60, CD-8 dan IFN-γ. Pengukuran dilakukan dengan menggunakan metode imunohistokimia pada jaringan periapikal. results: Rerata Hsp60, CD-8 dan IFN-γ pada kelompok granuloma secara signifikan lebih tinggi dibanding kelompok non granuloma (p <0,05). Peran positif dari IFN-γ terhadap kejadian granuloma tampaknya lebih menonjol. simpulan: Studi ini menunjukkan bahwa dalam imunopatobiogenesis dari granuloma periapikal karies gigi, Hsp60, CD-8 dan IFN-γ memainkan peran penting, tetapi peran IFN-γ ditemukan lebih menonjol.
Kata kunci: Gigi karies, imunopatobiogenesis, granuloma periapikal, Hsp60, CD-8, IFN-γ
introduCtion
Dental caries with chronic periapical can be considered as a multifactorial infectious disease caused by irritants in necrotic pulp moving into periapical tissues. Cases involving chronic periapical disease was necrotic pulp can be treated with root canal treatment. The continuous exposure of those irritants to the periapical tissues will stimulate host defense in the form of periapical granulomas. The healing process of periapical granulomas is actually associated with the body’s immune response. Thus, it is possible for recurrence despite the application of root canal treatment, or it may even develop into a radicular cyst, which is more difficult to treat. Therefore, if the process to being granuloma formation can be prevented, many difficulties in healing process of periapical granulomas can be overcome.
Unfortunately, the mechanism of immunopathobiogenesis of periapical granulomas caused by chronic periapical in dental caries still cannot be explained. Many researches only focus on the causes of dental pulp necrosis, such as tumor necrosis factor α (TNF α) and interleukin-6 (IL-6) in periapical lesions.1 The mechanism of periapical granuloma
is necessary to be studied since the number of cases of dental pulp necrosis is more commonly found in patients with dental caries.
Histological changes in the periapical tissues caused by bacterial invasion, moreover, will be indicated by the presence of granulation tissue containing with macrophages, lymphocytes, plasma cells, neutrophils, and fibrovascular elements in varying numbers. At the same time, there will also be damage to the periapical tissues and bone resorption.3 Periapical granuloma is actually composed
of granulation tissue surrounded by a cell wall of fibrous connective tissue.
In histopathological examination of the chronic lesions, it can be known the existence of lymphocytes, plasma cells, neutrophils, histiocytes, eusinophils, and epithelial cell rests of Mallesse.4 Lymphocytes are the predominant cell type
(50%), which number is closely related to the total number of the following cells, namely CDs -4 T cells and CD-8 T cells. Therefore, in chronic lesions the number of CD-8 T cells is increased. All of their structures are surrounded by a capsule of fibrous connective tissues consisting of CD-8 T lymphocytes.3 Therefore, it can be said that pathological
periapical granuloma is frequently caused by dental caries.1
Dental caries is considered as a chronic pathological process caused by microorganisms found in dental caries tissue as a potential immunogen leading to changes in the pulp tissue related to immune response.5, 6 In other words, bacteria can
be considered as an important factor in the development and growth of dental caries.
The presence of anaerobic bacteria considered as pathogenic can trigger macrophages to form periapical granulomas. In the process of periapical granuloma formation, various components have important roles, namely Hsp60 as chaperone playing a role in the fraction of
proteins involved in APC. It is because Hsp60 synthesized in the exosome is used to assist the synthesis and maturation of proteins to become functional ones. Consequently, the processing of epitope can be run, and Hsp60 will be recognized by CTL/CD-8, which then will secrete IFN-γ. 7, 8
Furthermore, IFN-γ released by both Th-1 and CTL/CD-8 will induce macrophage activities. Thus, those macrophages will migrate around those containing intracellular bacteria, and then create granuloma.9 It is
because the presence of pathogenic bacteria will trigger some histiocytes to develop into macrophages and APC leading to the formation of granulomas, while some others will develop into phagocytic causing no granulomas.The study was aimed to determine the role of Hsp60, CD-8 and IFN-γ in immunopaobiogenesis of periapical granuloma in dental caries.
MaterialsandMethods
This research was an analytic observational research with cross sectional approach. The samples of this research were selected from the population based on inclusion criteria (purposive sampling). The samples were divided into two groups, consist of group with granulomas and group with no-granulomas. The subjects were 36 patients who were indicated for tooth extraction and to accept informed consent about the purposes and objectives of this research.
Those patients classified into group with granulomas must have a diagnosis of dental pulp necrosis with periapical granulomas. Diagnose was determined through clinical and radiographic examinations. Clinically the depth of cavity reached the pulp and radiographic figure showed that there was radiolucent area with distinct border with 0.5 mm-2 of diameter. In the other hand, those patients who were classified into non-granulomas group must have a diagnosis of dental pulp necrosis without periapical granulomas indicated clinically by the depth of pulp cavity and the radiographic figure showed no periapical radiolucency.
In the group with granulomas, periapical tissues around tooth extraction were taken by using a pair of tweezers. Meanwhile, in the group with non-granulomas, periapical tissues taken were tissues attached to the extracted tooth. Those periapical tissues were partially fixed in 10% buffered formalin, then histopathologically examined by using Hematoxylin Eusin (HE) staining, and observed by using a microscope with a magnification of 400 times.
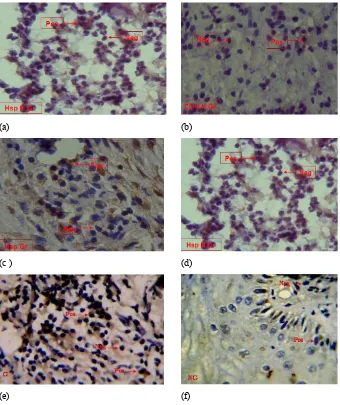
figure 1. The incision of apex tissue: (a) granuloma using CD-8; (b) non-granuloma using CD-8; (c) granuloma using Hsp60; (d) non-granuloma using Hsp60; (e) granuloma using IFN-γ; (f) non-granuloma using IFN-γ. Brownish color for marking positive reaction to Hsp60 in macrophages, and brown color for marking positive reaction to CD-8 and IFN-γ in lymphocytes. Magnification 400 x.
5
Ȗ
(a) (b)
(c ) (d)
(e) (f)
Ȗ
Ȗ of the examination showed the number of cells producing
Hsp60, CD-8 and IFN-γ that would give a positive reaction to monoclonal antibodies, divided by the total (number of cells) and multiplied by 100%.21
Finally, multivariation statistical analysis was used to measure the differences of the average number of cells producing Hsp60, CD-8 and IFN-γ among the groups. Besides that, discriminant analysis was also conducted to obtain the mechanism of periapical granulomas and the patterns of variable contribution between in the group with granulomas and in the group with non-granulomas, so the dominant variable used to distinguish them could be determined.
results
The results of immunohistochemical examination using monoclonal antibody, anti-Hsp60, CD-8 lymphocytes and IFN-γ induced into granuloma and non-granuloma tissues can be seen in Figure 1. The calculation results of cells producing Hsp60, lymphocytes CD-8, and IFN-γ in the group with granulomas and in the group with non-granulomas were obtained by using immunehistochemical method. The mean and standard deviation of those three variables on each group can be seen in Table 1.
table 1. The mean and standard deviation of immunity components, namely Hsp60, CD-8 lymphocytes and IFN-γ
table 2. Fisher’s linear discriminant function with variable IFN-γ granulomas (Table 1). The results of multivariate analysis using Wilks ‘Lambda test shows p<.001. This means that there was a significant difference between the mean of Hsp60, CD-8 lymphocytes and IFN-γ in the group with granulomas and that in the group with non-granulomas.
Moreover, based on the results of a statistical test with stepwise method, it is known that IFN-γ was the only one dominant variable in distinguishing the group with granulomas and the group with non-granuloma. The minimum of F value of IFN-γ was about 0.1, while the F values of Hsp60 and CD-8 lymphocytes were 0.998 and 0.087. Since the F values of Hsp60 and CD-8 lymphocytes were higher than the maximum limit of 0.05 for inclusion in the analysis, Hsp60 and CD-8 lymphocytes did not become discriminative with the grouping of granulomas/ non-granulomas. However, the discriminant analysis resulted in Fisher’s linear discriminant function as shown in Table 2.
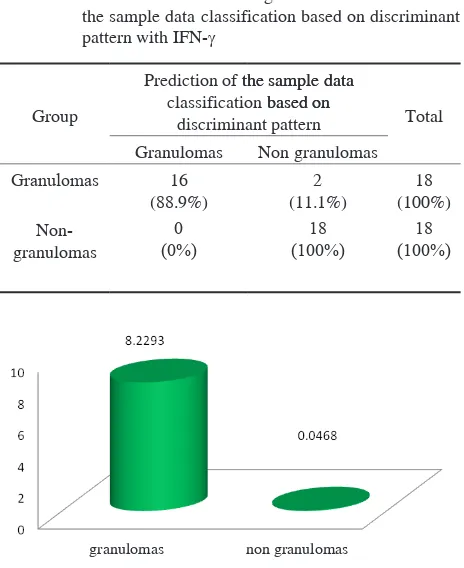
Cross tabulation result from discriminant pattern can bee seen on the Table 3. Based on Fisher’s linear discriminant function above, it was found that there were two groups of samples with granulomas incorrectly classified as the group with non-granulomas, while for the sample of non granulomas there was not any incorrect classification. The means of difference among those two groups was 94.4%, namely 88.9% for the group with granulomas and 100% for the group with non-granulomas. The results of cross-tabulations of the discriminant pattern can be seen in Table 3.
Furthermore, the positive role of IFN-γ on the incidence of granulomas appeared more clearly as seen in Figure 2, where the mean and standard deviations of both variables
distinguishing the group with granulomas and the group with non-granulomas granuloma were calculated based on Fisher’s function.
disCussion
Based on Table 1, it is known that the average number of cells producing Hsp60 in the group suffering from granulomas (5.68) was significantly different that in the group suffering from non-granulomas (0.95). Similarly, a research conducted by Suzuki et al.10 also showed that there was an increase in the local expression of Hsp60 in periapical inflammatory lesions. This increasing of Hsp60 expressions in patients with periapical granulomas is caused by immune response to the inflammatory later leading to the expression of MHC class I.7,9 The producing of the expression of MHC class I then will trigger a series of processes to produce several cytokines, including IFN-γ, TNF-α and TNF-b. On the other hand, the presence of MHC class I expression on the cell surface will also be recognized by CD-8 lymphocytes, thereby increasing the expression of CD-8. However, in the cases of periapical granulomas, the increasing of CD-8 expression does not occur since the release of IFN-γ activates monocytes (macrophages) which will surround intracellular bacteria. It happened because there is stimulation of IFN-γ , especially MAF, which then will lead to the formation of granuloma.8,11
table 3. Cross tabulation of the original data classification and the sample data classification based on discriminant pattern with IFN-γ
Group
Prediction of the sample datathe sample data classification based on based on
discriminant pattern Total
The average number of cells producing CD-8 in the group with granulomas (3.71) was significantly different that in the group with non-granulomas (0.19). The difference is caused by high immunity possessed by those patients with granulomas. As a result, bacteria entering to the body will be phagocytized by monocytes, so they will get phagolysosome and be neutralized by enzyme system (lysozyme). Bacteria that are resistant to lysozyme will be destroyed by monocytes with reactive oxygen species (ROS) system, especially super oxidant radicals, so the bacteria will get oxidative stress. To protect proteins contained in the body of bacteria from damage, the bacteria then will release stress proteins (Hsp).
When the attacks of intracellular bacteria occur continuously, the host cell will release excessive ROS. As a result, anti-ROS produced by the host will become unbalanced. In other words, ROS is greater than anti-ROS (scavenger enzyme), so the host cells will get oxidative stress. The host cells that distress then will lead to the damage of proteins in the host cells. Thus, to prevent the damage, the host cell releases stress protein, Hsp60. Hsp60 is synthesized in the exosome and serves as chaperones, which will help folding and can be used to assist the synthesis and maturation of proteins to become functional ones. Consequently, the processing of epitope can be run, and Hsp60 will be recognized by CTL / CD-8, which then will secrete IFN-γ.7, 8
CD-8 lymphocyte can be considered as a specific phenotype of mononuclear cells used to differentiate asymptomatic lesions and symptomatic lesions in patients with periapical lesions. According to Colic et al.,12 the
percentage of CD-8 cells in symptomatic lesions is higher than that in asymptomatic lesions. In other words, the research proves the existence of CD-8 lymphocytes in asymptomatic lesions although it does not distinguish further the percentage of CD-8 cells in granuloma tissue and that in non-granuloma tissue in patients with asymptomatic periapical lesions. also means that intracellular signals will be increased, so cytokines playing a role in delivering extracellular signal, namely IL-12, will be improved. The cytokines also play a role in inducing T lymphocytes to secrete IFN-γ. The secretion of IFN-γ then will stimulate the activation of macrophages playing a role to prevent the diffusion of bacteria, thus, the cells containing intracellular bacteria will be surrounded by macrophages, and then granuloma will be formed.8,11 Similarly, a research conducted by
Breloer et al.,13 also showed that the in vitro release of
IFN-γ can dramatically be increased as the increasing of Hsp60 moving to T cells and macrophages, but either the production of IL-2 or the proliferation of T cells will not be increased. It means that the induction of IFN-γ is dependent
entirely on the ability of macrophages to produce IL-12. In patients suffering from dental caries with periapical granuloma, immunity components, namely Hsp60, CD-8 lymphocytes and IFN-γ, have an important contribution as indicated with the increasing of cells producing CD-8, IFN-γ and Hsp60.
Immunopathobiogenesis of periapical granulomas can show a relationship between those three components to the pattern of cells producing high CD-8, and IFN-γ, and Hsp60. It means that those three immunity components have positive contribution in the immunopathobiogenesis of periapical granuloma. For instance, CD-8 lymphocyte expression will directly trigger the apoptosis of CD-8 lymphocytes, and then serve as a proapoptotic to inhibit CD-4 (Th2 cells). These barriers will affect the balance resulting in the increasing of Th1.14 On the other hand,
Hsp60 will also stimulate Th1 through IL-12, so cytokines (IFN-γ) will be secreted. Cells producing IFN-γ will trigger the proliferation of CD-8, so CD-8 lymphocytes will be increased and IFN-γ produced will also be high.
Hsp60 is a mediator of immune stimulation with different mechanisms, and can be affected by LPS. Hsp60 and LPS, according to Osterloh et al.,15 can increases
cytokine (IFN-γ) produced by Th1 cells. The presence of Hsp60 will induce the production of IFN-α, so that Hsp60 functions as a classic signal that induces tissue damage to the innate immune system. If there is a bacterial infection, then Hsp60 binds to LPS and facilitates the detection of microbes by recognizing Pathogen-Associated Molecular Pattern (PAMP) early and increasing TLR signaling. The result of a research conducted by Suzuki et al.,10 showed
that the epithelial residue of Malassez had a negative reaction to Hsp60 indicating no protective function of Hsp60 against inflammation. This finding explains another function of Hsp60 in immunepathobiogenesis of periapical granulomas since Hsp60 is previously more often associated with epithelial proliferation and migration process into periapical lesions.
Similarly, the results of an in vitro research conducted by Breloer et al.13 showed that Hsp60 led to a pro-inflammatory
response of cells in the adaptive immune system. It is also known that Hsp60 triggers specific IFN-γ secretion with a high number of T cells in Peritoneal Exudates Cells (PEC) as the cells displaying the antigen (APC). In short, these results indicate that Hsp60 as endogenous molecules can cause inflammatory response. These also indicate that the activation of cells of the innate and adaptive immune system by Hsp60 is strongly influenced by the type of APC.
The formation of granuloma can be considered as a chronic inflammatory process due to the failure of the acute inflammatory process, causing activation of persistent antigens and accumulation of macrophages continuously. It is because IFN-γ released by T cells causes the transformation of macrophages into epitheloid cells and datia cells.11 Similarly, although the ability of macrophages
causing no inflammatory reaction excessive, there is still humoral immunologic reaction. In this immunological reaction, macrophages will secrete IL-12, which will induce lymphocytes (Th1), so IFN-γ will be produced. Then the expression of IFN-γ will trigger proliferation of CD-8 lymphocytes. Consequently, it will secrete IFN-γ and activate macrophages. Therefore, in patients suffering from dental caries with periapical granulomas, there will be no destruction of macrophages containing intracellular bacteria, but the activity of macrophages to surround those macrophages containing intracellular bacteria will be increased.8
The study suggested that in the immunopathobiogenesis of periapical granuloma in dental caries, Hsp60, CD-8 and IFN-γ played important rules, but the role of IFN-γ was found to be more prominent.
referenCes
1. Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol 2008; 79(8 Suppl): 1585-91.
2. Badan Penelitian dan Pengembangan Kesehatan Departemen Kesehatan Republik Indonesia. Laporan survei kesehatan rumah tangga (SKRT). Jakarta: Studi Morbiditas dan Disabilitas dalam SURKESNAS; 2004.
3. Radics T. The role of inflammatory and immunological processes in the development of chronic apical periodontitis. Universitas of Debrecen Medical and Health Science Center Faculty of Dentistry. 2004.
4. García CC, Sempere FV, Diago MP, Bowen EM. The post-endodontic periapical lesion: histologic and etiopathogenic aspects. Med Oral Patol Oral Cir Bucal 2007; 12(8): E585-90.
5. Leprince JG, Zeitlin BD, Tolar M, Peters OA. Interactions between immune system and mesenchymal stem cells in dental pulp and periapical tissues. Int Endod J 2012; 45(8): 689-701.
6. Trowbridge HO. Immunological aspects of chronic inflammation and repair. J Endod 1990; 16(2): 54-61.
7. Clancy J. Basic Concepts in immunology: a Student’s survival guide. The McGraw-Hill Companies Inc; 1998.
8. Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 6th ed. USA: WB. Saunders Company; 2007. 9. Goldsby RA, Kindt TJ, Osborne BA. Immunology. 4th ed. New York:
WH Freeman and Company; 2000. p. 432-4.
10. Matsumoto MA, Ribeiro DA. Inducible nitric oxide expression correlates with the level of inflammation in periapical cysts. Eur J Dent 2007; 1(4): 212-5.
11. Bratawidjaja KG, Rengganis I. Imunologi dasar. Edisi ketujuh. Jakarta: Balai Penerbit Fakultas Kedokteran Indonesia; 2009.
12. Colic M, Lukic A, Vucˇevic´ D, Milosavljevic P, Majstorovi I,
Marjanovic M, Dimitrijevic J. Correlation between phenotypic characteristics of mononuclear cells isolated from human periapical lesions and their in vitro production of Th1 and Th2 cytokines. Archives of Oral Biology 2006; 51: 1120-30.
13. Vercoulen Y, van Teijlingen NH, de Kleer IM, Kamphuis S, Albani S, Prakken BJ. Heat shock protein 60 reactive T cells in juvenile idiopathic arthritis: what is new?. Arthritis Res Ther 2009; 11(3): 231.
14. Stolzing A, Sethe S, Scutt AM. Stressed stem cells: Temperature response in aged mesenchymal stem cells. Stem Cells Dev 2006; 15(4): 478-87.
15. Osterloh A, Kalinke U, Weiss S, Fleischer B, Breloer M. Synergistic and differential modulation of immune responses by Hsp60 and lipopolysaccharide. J Biol Chem 2007; 282(7): 4669-80. 16. Braakman I, Hebert DN. Protein folding in the endoplasmic
reticulum. Cold Spring Harb Perspect Biol 2013; 5(5): a013201. 17. Zimmermann R, Eyrisch S, Ahmad M, Helms V. Protein
translocation across the ER membrane. Biochim Biophys Acta 2011; 1808(3): 912-24.
18. Mori K, Kawahara T, Yoshida H, Yanagi H, Yura T. Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for unfolded protein-response pathway. Genes Cells 1996; I: 803-17.
19. Cox JS, Chapman RE, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell 1997; 8(9): 1805-14.
20. Sasaki H, Balto K, Kawashima N, Eastcott J, Hoshino K, Akira S, Stashenko P. Gamma interferon (IFN-gamma) and IFN-gamma-inducing cytokines interleukin-12 (IL-12) and IL-18 do not augment infection-stimulated bone resorption in vivo. Clin Diagn Lab Immunol 2004; 11(1): 106-10.