Colour and Anthocyanin Stability
of Red
Raspberry
Jam
Cristina Garc
•
a-Viguera,
1
*
Pilar Zafrilla,
1
Francisco Arte
s,
1
Fernando Romero,
2
Pedro Abella
n
2
and Francisco A Toma
s-Barbera
n
1
1
Dpto Ciencia y Tecnolog•a de Alimentos, CEBAS-CSIC, PO Box 4195, 30080, Murcia, Spain
2
Dpto de Calidad y Desarrollo, Hero Espan
8
a, SA Alcantarilla, Spain
(Received 3 November 1997 ; revised version received 23 February 1998 ; accepted 6 April 1998)
Abstract :Anthocyanin and colour stability of red raspberry jams made from two di†erent varieties (“ZevaÏ and “HeritageÏ) were analysed during 6 months, stored at three temperatures (20, 30 and 37¡C). Also the inÑuence of freezing the fruit, previously to jam manufacture, was evaluated. Di†erent anthocyanin composi-tion was detected for both cultivars and while “ZevaÏ fruit had a higher total anthocyanin content, Heritage variety produced jams with a higher redness hue. The development of browning was directly related to storage temperature but not to thawing or the variety of fruit used.(1998 Society of Chemical Industry. J Sci Food Agric78, 565È573 (1998)
Key words : raspberry ; jams ; anthocyanins ; cultivar ; colour ; storage ; stability
INTRODUCTION
Colour stability of red fruit or red fruit products is inÑuenced by many factors which have been previously reported (Markakis 1982). Temperature and time of
processing (Decareu et al 1956 ; Markakis 1982 ; Pilano
et al 1985) and storage (Meschter 1953) were found to exert a great inÑuence on anthocyanin stability. Loss of anthocyanins and/or formation of brown compounds in strawberry and red raspberry products during storage have been attributed to many factors such as pH and acidity, phenolic compounds, sugars and sugar degrada-tion products, oxygen, ascorbic acid, fruit maturity and
thawing time (Markakiset al1957 ; Wrolstadet al1970 ;
Abers and Wrolstad 1979 ; Spayd and Morris 1981 ;
Rommelet al1990 ; Withyet al1993).
The quality of the colour may inÑuence consumerÏs acceptance. It is therefore essential that jam is prepared and stored at a temperature which will maximise colour stability. Nevertheless, as far as we are aware, no studies have been done on this aspect.
The purpose of the present work was to determine the inÑuence of the cultivar on red raspberry jam
antho-* To whom correspondence should be addressed. Contract/grant sponsor : MEC-CSIC
Contract/grant sponsor : Hero Espan8a SA
cyanin composition and stability, and to deÐne the changes in colour and pigment composition which may occur during processing and storage at di†erent tem-peratures for 6 months. The inÑuence of freezing the fruit previously to jam manufacture was also examined as pigment degradation may take place during thawing.
MATERIAL AND METHODS
Material
Samples of two red raspberry (Rubus idaeus) cultivars,
“ZevaÏ (Socovos, Albacete, Spain) and “HeritageÏ
(Nerpio, Albacete, Spain), at processing maturity, were harvested on the August 1995. Half of the total fruit harvested was processed in Hero SA, on the same day,
while the rest was immediately frozen (at [20¡C) in
about 3 kg lots and jam was manufactured after 24 h. Prior to jam preparation, samples were removed from the bags to allow semi-thawing. Jam was made under the speciÐc conditions of the Industry in order to obtain a Ðnal product that contains 450 g fruit kg~1, 2 g pectin kg~1, 0É4 g citric acid kg~1and a Ðnal sucrose concen-tration up to 63 ¡Brix. Fruit and sugar were mixed with 565
pectin and citric acid, processed for 15 min at 78¡C under vacuum (500 mmHg), heated at 92¡C and allowed to cool down to 88¡C before Ðlling glass jars (45 g). Glass jars were closed and cooled gradually with water and kept in the dark, at three di†erent tem-peratures (20, 30 and 37¡C), for 6 months.
Anthocyanin extraction
Five grams of fruit (fresh or frozen) was extracted with 15 ml acetone, in order to produce pectin clotting, for 5 min at room temperature. The extract was Ðltered, concentrated under vacuum (35¡C), and the residue redissolved in 5 ml acidiÐed water (30 ml formic acid
litre~1), this aqueous solution was adsorbed onto a C
18
Sep-Pak cartridge (Waters Associates, Milford, MA, USA). The cartridge was washed with 30 ml formic acid
litre~1, and the pigments were eluted with 30 ml formic
acid litre~1 of methanol. The methanolic extract was
concentrated and redissolved in a mixture of aqueous
50 ml formic acid litre~1 containing 150 ml methanol
litre~1 (1 ml) and Ðltered through a 0É45lm Type
Millex HV 13 Millipore Ðlter (Millipore Corp, Bedford, MA, USA) before HPLC analysis. Each jam (5 g) was extracted by stirring with 75 ml of a methanol/acetic acid/water (25 : 1 : 24) mixture, for 20 min at room
tem-perature, as previously reported (Garc•a-Viguera et al
1997). All extractions were done in triplicate.
Flavonol extraction
Fifty grams of fresh fruit was mixed with 200 ml of methanol and homogenised with an Ultra-Turrax T25 (Jankel & Kunkel, IKA-Labortechnik, Germany). The homogenate was Ðltered, 50 ml of the Ðltrate was mixed with 20 ml of distilled water and concentrated under vacuum (35¡C) up to 20 ml. The aqueous solution was
adsorbed onto a C Sep-Pak cartridge, following the
18
same method as with the anthocyanins but with non-acidiÐed solvents. All extractions were done in triplicate.
HPLC analysis of anthocyanins
For fruit anthocyanin identiÐcation the same apparatus
as in Garc•a-Viguera et al (1997) was used. For jam
analysis a Merck-Hitachi L-6200 intelligent pump
(Darmstadt, Germany) chromatograph was used,
equipped with a Merck-Hitachi UV-VIS detector L-4200 and auto-injector Merck-Hitachi AS-2000 A. Chromatograms were recorded and processed on a D-2500 Chromato-Integrator (Merck-Hitachi). Each
sample (20ll) was analysed on a Lichrochart 100
RP-18 reversed-phase column (125]4 mm, particle
size 5lm) using a mobile phase of 50 ml formic acid
litre~1 (solvent A) and methanol (solvent B). Elution
was performed at a Ñow rate of 1 ml min~1using a
gra-dient starting with 150 ml methanol litre~1, increasing
to 300 ml methanol litre~1at 15 min, isocratic elution
for 5 min and increasing to 950 ml methanol litre~1at
25 min. Detection was achieved at 520 nm. All analyses were done in triplicate and results expressed as mean
value. Reproducibility of the HPLC analyses was ca
^6%.
HPLC analysis of Ñavonols
The same equipment as mentioned before, for
antho-cyanin fruit analyses, was used. Each sample (20ll) was
analysed on a Lichrosorb RP-18 (Merck, Darmstadt,
Germany) reversed-phase column (250]4 mm, particle
size 5lm), using the same mobile phase as mentioned
above. Elution was performed at a Ñow rate of
1 ml min~1using a gradient starting with 200 ml
meth-anol litre~1, increasing to 500 ml methanol litre~1 at
20 min, to 600 ml methanol litre~1 at 30 min and to
950 ml methanol litre~1 at 35 min. Detection was
achieved at 360 nm. All analyses were done in triplicate and results expressed as the mean value.
Repro-ducibility of the HPLC analyses wasca^6%.
Flavonoids identiÐcation and quantiÐcation
The di†erent compounds were characterised by chro-matographic comparison with authentic standards (cyanidin 3-rutinoside from Apin Chemicals (UK) and the rest of anthocyanins provided by Dr Bridle (IFR, Reading Laboratory, UK)). Kaempferol and quercetin 3-glucosides previously isolated in our laboratory, their spectra recorded with the diode array detector, and by their mobility on HPLC. All anthocyanins were quanti-Ðed as cyanidin 3-rutinoside due to the lack of a
suffi-cient amount of other standards, and Ñavonol
derivatives as quercetin glucoside and kaempferol 3-glucoside. The total anthocyanins were calculated by addition of the amounts of the anthocyanins detected in each chromatogram.
Titratable acidity, soluble solids and pH determination
Two grams of jam was stirred with 75 ml distilled water during 15 min and acidity was determinated by
auto-burette model 736 (Barcelona, Spain) and expressed as grams of citric acid per 100 ml of extract, in accordance with AOAC (1984).
Soluble solids concentration (¡Brix) and pH were measured in an Atago 1T (Japan) refractometer at 20¡C and a Crison micropH 2000 (Barcelona, Spain) pHmeter with glass electrode, respectively.
Colour measurements
A tristimulus colour spectrophotometer Minolta
CM-508i (Osaka, Japan) was used to obtain the absorp-tion spectra from which L*a*b* values were calculated using illuminant D65 and a 10¡ observer according to the CIELAB 76 convention (McLaren 1980). Hue angle
(H) was calculated from H\arctan b*/a*. Data were
recorded and processed on a Minolta ChromaControl S, PC based colorimetric data system. All measures were done in triplicate ; the mean values are reported in the data.
RESULTS
Changes in titratable acidity (TA), soluble solids (ÄBrix)
and pH
TA was slightly higher for those jams elaborated with Zeva cultivar (freshB0É86 and frozenB0É81) than with
“HeritageÏ (fresh B0É72 and frozen B0É70). Neverthe-less, no signiÐcant variations were observed in this parameter during storage at the three di†erent tem-peratures (20, 30 and 37¡C). The total soluble solids
showed a constant value (B65¡Brix) along the
experi-ment for all the analysed jams. And no changes in pH through storage were noticed (B3É33).
Anthocyanin composition of raspberry fruits and changes during processing
The anthocyanins present in fresh raspberry fruits of both cultivars (“ZevaÏ and “HeritageÏ) were analysed by HPLC. Qualitative and quantitative di†erences were found when analysing the two cultivars. While four
cya-nidin based anthocyanins (3-sophoroside,
3-glucosylrutinoside, 3-glucoside and 3-rutinoside) were found in “ZevaÏ, fruit of the cultivar Heritage contained only the 3-sophoroside and 3-glucoside. These results were consistent with previously reported data on the composition of Heritage cv (Francis 1972), although nothing has been published, as far as we are aware, on the anthocyanin composition in “ZevaÏ. Quantitative data on the total anthocyanin content showed that “ZevaÏ presented a higher amount (double than “Heri-tageÏ) of these pigments (Table 1). Cyanidin 3-glucoside was the main pigment in cultivar Zeva, while in cultivar Heritage this was cyanidin 3-sophoroside.
TABLE 1
Anthocyanin losses during red raspberry jam manufacturing (means^SD,n\3)a
Fresh fruit Frozen fruit Fresh fruit Frozen fruit (cv Zeva) (cv Zeva) (cv Heritage) (cv Heritage) Cy-3-soph
Fruit 339É91 (^40É59) 293É03 (^34É99) 596É61 (^4É50) 515É75 (^20É74) Jam 211É30 (^7É37) 182É58 (^18É30) 518É78 (^61É76) 399É61 (^2É23) Cy-3-glc rut
Fruit 99É92 (^11É37) 86É14 (^9É80) ND ND
Jam 68É73 (^1É80) 57É56 (^7É59) ND ND
Cy-3-glc
Fruit 941É77 (^103É14) 811É87 (^88É91) 464É16 (^34É77) 375É68 (^1É93) Jam 542É59 (^10É76) 504É54 (^63É39) 364É17 (^43É56) 275É00 (^24É24) Cy-3-rut
Fruit 444É42 (^54É98) 383É12 (^47É39) ND ND
Jam 260É13 (13É64) 235É85 (^36É85) ND ND
T otal
Fruit 1826É02 1574É16 1060É77 891É43
Jam 1082É75 980É53 882É95 674É61
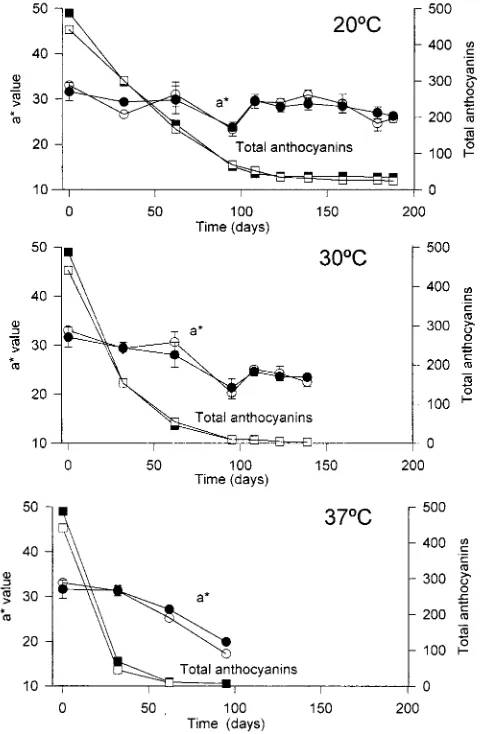
Fig 1. Total anthocyanin (=)and a* value(…) degradation of red raspberry jams, made with Zeva variety, stored at 20, 30 and 37¡C. Black symbols represent jams prepared with
fresh fruit, white symbols represent frozen fruit.
During jam processing the total anthocyanin content per gram of fruit (fresh weight) was reduced on a
17È24% when using “HeritageÏ, while the percentage of
loss increased to 37È40% when jams were prepared
with “ZevaÏ red raspberries (Table 1). Thawing resulted
in jam with a smaller anthocyanin content (9È24%
lower). This is not unexpected, since during freezing and thawing, the cell structures are disrupted and the plasti-dic oxidative enzymes (polyphenol oxidases, PPO) and the vacuolar substrates (phenolics) interact and during thawing there is a loss of phenolic pigments due to this enzymatic process.
Anthocyanin stability during jam storage
Storage temperature was the main responsible factor for anthocyanin loss. Thus, the degradation rate increased
when increasing temperature in all studied jams. The higher loss in pigment composition, at 30 and 37¡C, was observed during the Ðrst month, while jams stored at 20¡C showed a progressive decrease of anthocyanins during the Ðrst 3 months (Figs 1 and 2). Some minor di†erences in jams stored at 20¡C can also be pointed out in this aspect, as jams made with “ZevaÏ (Fig 1) showed an anthocyanin degradation rate slower than those made with “HeritageÏ (Fig 2). Nevertheless, at the end of the experiment the total anthocyanin content
was reduced down to 4È7% of the initial anthocyanins,
in all analysed jams.
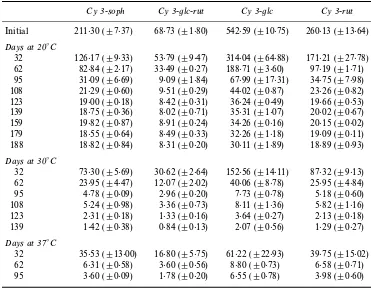
It is also remarkable that cyanidin 3-glucoside, the major pigment of “ZevaÏ, was the most unstable antho-cyanin during jam processing and storage (Tables 2 and
3). This is in accordance with Rommelet al(1990) that
have reported that this pigment was more reactive and therefore more likely to polymerise than other antho-cyanins, with an associated increase in browning. Nevertheless, the stability of this pigment was similar to that of cyanidin 3-sophoroside in “HeritageÏ (Tables 4 and 5).
Fig 2. Total anthocyanin(=) anda* value(…) degradation of red raspberry jams, made with Heritage variety, stored at 20, 30 and 37¡C. Black symbols represent jams prepared with
TABLE 2
Anthocyanins stability of red raspberry jam elaborated with fresh fruit (cv Zeva) during storage at 20, 30 and 37¡C (mean^SD,n\3)a
Cy 3-soph Cy 3-glc-rut Cy 3-glc Cy 3-rut Initial 211É30 (^7É37) 68É73 (^1É80) 542É59 (^10É75) 260É13 (^13É64) Days at 20¡C
32 126É17 (^9É33) 53É79 (^9É47) 314É04 (^64É88) 171É21 (^27É78) 62 82É84 (^2É17) 33É49 (^0É27) 188É71 (^3É60) 97É19 (^1É71) 95 31É09 (^6É69) 9É09 (^1É84) 67É99 (^17É31) 34É75 (^7É98) 108 21É29 (^0É60) 9É51 (^0É29) 44É02 (^0É87) 23É26 (^0É82) 123 19É00 (^0É18) 8É42 (^0É31) 36É24 (^0É49) 19É66 (^0É53) 139 18É75 (^0É36) 8É02 (^0É71) 35É31 (^1É07) 20É02 (^0É67) 159 19É82 (^0É87) 8É91 (^0É24) 34É26 (^0É16) 20É15 (^0É02) 179 18É55 (^0É64) 8É49 (^0É33) 32É26 (^1É18) 19É09 (^0É11) 188 18É82 (^0É84) 8É31 (^0É20) 30É11 (^1É89) 18É89 (^0É93) Days at 30¡C
32 73É30 (^5É69) 30É62 (^2É64) 152É56 (^14É11) 87É32 (^9É13) 62 23É95 (^4É47) 12É07 (^2É02) 40É06 (^8É78) 25É95 (^4É84) 95 4É78 (^0É09) 2É96 (^0É20) 7É73 (^0É78) 5É18 (^0É60) 108 5É24 (^0É98) 3É36 (^0É73) 8É11 (^1É36) 5É82 (^1É16) 123 2É31 (^0É18) 1É33 (^0É16) 3É64 (^0É27) 2É13 (^0É18) 139 1É42 (^0É38) 0É84 (^0É13) 2É07 (^0É56) 1É29 (^0É27) Days at 37¡C
32 35É53 (^13É00) 16É80 (^5É75) 61É22 (^22É93) 39É75 (^15É02) 62 6É31 (^0É58) 3É60 (^0É56) 8É80 (^0É73) 6É58 (^0É71) 95 3É60 (^0É09) 1É78 (^0É20) 6É55 (^0É78) 3É98 (^0É60) aValues are microgram of anthocyanin per gram of fruit in jam (fresh weight). HPLC repro-ducibility wasca^6%. Cy 3-soph, Cyanidin 3-sophoroside ; Cy 3-glc-rut, Cyanidin 3-glucosyl rutinoside ; Cy 3-glc, Cyanidin 3-glucoside ; Cy 3-rut, Cyanidin 3-rutinoside ; ND, non-detected.
Jam prepared with frozen fruit presented a similar degradation slope than that made with fresh fruit (Figs 1 and 2).
Colour quality alteration
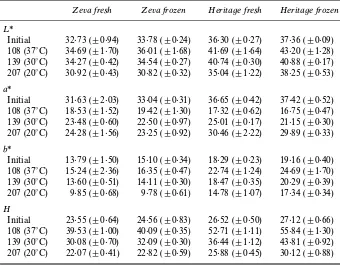
It is important to mention that while all the previous changes were noticed in anthocyanin concentration, only minor changes were found when analysing the colour of these products stored at 20¡C measured with a reÑectance spectrophotometer. Thereby, no signiÐcant
di†erences where noticed when analysing the L* value,
for the two cultivars and for jams made with fresh or frozen fruit, or even during the storage at the di†erent temperatures (Table 6), indicating no changes in
light-ness. A higher decrease in a* value was detected when
jam was stored at higher temperatures. This e†ect being more remarkable in “HeritageÏ than in “ZevaÏ, due to the fact that “HeritageÏ presented an initial a* value higher than “ZevaÏ, while the decrease was higher in that Ðrst cultivar reaching the same value at the end of the experiment. On the other hand, only minor di†erences
were observed inb* values. However, changes ina* and
b* values showed that theHvalue variations were more
important for those jams made with frozen fruit and/or Heritage variety and stored at higher temperatures, indicating an increase in browning. Thus, when jams were stored at 20¡C no signiÐcant di†erences were observed in comparison with those products made with Zeva cultivar or fresh fruit.
Flavonol composition of raspberry fruits and changes during processing
TABLE 3
Anthocyanins stability of red raspberry jam elaborated with frozen fruit (cv Zeva) during storage at 20, 30 and 37¡Ca
Cy 3-soph Cy 3-glc-rut Cy 3-glc Cy 3-rut Initial 182É58 (^18É30) 57É56 (^7É59) 504É54 (^63É39) 235É85 (^36É85) Days at 20¡C
32 129É81 (^12É71) 47É46 (^3É47) 328É77 (^35É66) 163É56 (^14É44) 62 77É53 (^7É35) 30É80 (^2É36) 173É16 (^17É66) 89É12 (^7É75) 95 32É71 (^0É80) 8É82 (^0É04) 74É81 (^3É18) 36É49 (^1É12) 108 27É35 (^2É69) 8É09 (^0É69) 58É57 (^8É11) 22É62 (^4É71) 123 16É64 (^0É71) 7É47 (^0É11) 33É06 (^1É53) 19É09 (^0É76) 139 16É47 (^1É42) 7É31 (^0É51) 30É33 (^2É22) 17É35 (^1É82) 159 13É73 (^1É69) 6É31 (^0É80) 23É93 (^2É87) 13É58 (^1É69) 179 15É64 (^0É64) 6É13 (^0É42) 24É22 (^1É47) 12É53 (^0É84) 188 13É47 (^3É98) 3É62 (^0É02) 24É46 (^1É02) 12É15 (^0É60) Days at 30¡C
32 72É17 (^9É78) 28É29 (^3É22) 157É16 (^18É02) 83É35 (^7É60) 62 28É75 (^4É38) 13É89 (^1É98) 48É75 (^7É27) 30É60 (^4É67) 95 5É40 (^0É51) 2É98 (^0É49) 8É27 (^1É44) 5É29 (^0É13) 108 4É49 (^0É49) 2É87 (^0É20) 7É31 (^0É49) 4É89 (^0É62) 123 2É69 (^1É33) 1É38 (^0É69) 4É22 (^2É04) 2É47 (^1É29) 139 1É67 (^0É36) 0É82 (^0É16) 2É42 (^0É38) 1É47 (^0É22) Days at 37¡C
32 23É18 (^1É47) 11É02 (^0É76) 40É93 (^2É91) 25É04 (^2É58) 62 4É82 (^1É00) 2É64 (^0É64) 7É31 (^0É84) 5É75 (^1É47)
aValues are expressed as in Table 2.
TABLE 4
Anthocyanins stability of red raspberry jam elaborated with fresh fruit (cv Heritage) during storage at 20, 30 and 37¡Ca
Cy 3-soph Cy 3-glc Initial 518É78 (^61É76) 364É17 (^43É56) Days at 20¡C
31 308É70 (^56É51) 182É38 (^33É51) 53 125É48 (^15É22) 70É64 (^8É80) 88 77É77 (^15É20) 42É48 (^8É20) 106 74É84 (^4É29) 33É40 (^3É58) 120 65É28 (^8É89) 32É02 (^11É71) 139 60É04 (^5É56) 27É75 (^2É51) 155 39É82 (^7É04) 11É75 (^1É60) 175 36É82 (^6É09) 10É84 (^0É33) 191 28É22 (^3É42) 10É29 (^0É60) Days at 30¡C
31 189É07 (^47É80) 96É63 (^24É73) 53 43É62 (^4É29) 18É49 (^2É29) 88 17É11 (^1É36) 6É15 (^0É62) 106 16É58 (^0É09) 5É82 (^0É11) 120 15É27 (^2É62) 4É73 (^0É69) 139 11É84 (^0É20) 4É16 (^0É16) Days at 37¡C
31 105É94 (^12É69) 49É80 (^4É53) 53 6É20 (^1É04) 1É51 (^0É47)
aValues are expressed as in Table 2.
the content of this Ñavonoid per gram of fruit was
reduced only on a ^6% for both varieties, while when
kaempferol 3-glycoside is considered the content is
reduced on a^22% (Table 7).
DISCUSSION
The results obtained with jams stored at di†erent tem-peratures are in accordance with previous reports on
other food products (Spayd and Morris 1981 ; Withyet
al1993). Thus, the stability of anthocyanins and the rate of their degradation is markedly inÑuenced by tem-perature, and formation of chalcone form is favoured by increasing temperature, during storage and processing, at the expense of the other species (Markakis 1982 ; Jackman and Smith 1996). It has also been demon-strated that the concentration of polymeric pigments increase with temperature and storage time, and this has an important inÑuence in the colouration of juices and red wines (Somers 1971 ; Adams and Ongley 1973 ;
Bakker and Timberlake 1986 ; Withyet al1993). In this
aspect it is remarkable that the rate of colour loss is much slower than the rate of anthocyanin degradation (Figs 1 and 2).
TABLE 5
Anthocyanins stability of red raspberry jam elaborated with frozen fruit (cv Heritage) during storage at 20, 30 and 37¡Ca
Cy 3-soph Cy 3-glc
aValues are expressed as in Table 2.
TABLE 7
Flavonol losses during red raspberry jam manufacturing (mean^SD,n\3)a
aValues are micrograms of Ñavonoids per gram of fruit in jam (fresh weight). HPLC reproducibility was ca ^6%. Quer gly, Quercetin glycoside ; Kaemp gly, Kaempferol 3-glycoside.
fell within a narrow pH range from 3É22 to 3É46,
averag-ing 3É33, and thus, pH was not a signiÐcant factor in
colour expression in these jams. Therefore, other factors may have a signiÐcant role in the expression of colour in raspberry jams by co-pigmentation or some other physicochemical processes. In addition to anthocyanin, it has been reported that red raspberries also contain high concentrations of phenolic acids (Schwab and Herrmann 1985), Ñavonols (Henning 1981) and Ñavan-3-ols (Mossel and Herrmann 1974), which are known to
TABLE 6
L*a*b and Hue angle values obtained for red raspberry jams prepared with fresh or frozen fruit
Zeva fresh Zeva frozen Heritage fresh Heritage frozen
interact with anthocyanins to produce an increase in colour intensity and a bathocromic shift in the spectrum of the anthocyanin to give purple to blue colours (Francis 1975 ; Mazza and Brouillard 1990 ;
Garc•a-Viguera et al1994 ; Rivas-Gonzalo et al1995). All
pre-vious studies have been done in liquid solutions (juices, wines or model system). Nevertheless, it may be assumed that the same reactions take place in jams even if the bathocromic shift is not as evident as in liquid media and no precipitation is shown, due to the forma-tion of polymers.
Zeva cultivar presented a higher
monomeric-anthocyanin amount but “HeritageÏa* value was higher
during the experiment, conÐrming that the monomeric species are not the main factor in colour expression. This may be explained, partially, if the concentration of Ñavonols is considered, as colour is the result of the physical interaction of electrons between the antho-cyanin and co-pigments rings within the anthoantho-cyanin/ co-pigment complex, and Ñavonols have proved to be the most efficient co-pigments (Mazza and Miniati 1993). When the Ñavonol composition of both varieties was analysed quercetin and kaempferol 3-glycosides (glucoside plus glucoronide co-eluting) were found (Table 7). Nevertheless, “ZevaÏ presented a lower con-centration, of quercetin 3-glycoside (33É79lg g~1fresh
fruit), than “HeritageÏ (52É65lg g~1), while both
vari-eties contained similar amounts of kaempferol 3-glycoside (7É14lg g~1 and 7É17lg g~1, respectively).
Moreover, during processing only ^6% of quercetin
3-glycoside was lost (Table 7) ; therefore, a considerable amount of this Ñavonol is present in the jams. Quercetin derivatives are well known co-pigments (Mazza and Miniati 1993). Thus, the di†erences found in this co-pigment concentration could explain the higher red hue found for “HeritageÏ at the beginning of the assay, but not at the end of each experiment, when total
antho-cyanin concentration had decreased to less than ^10%
of the initial value. Therefore, the di†erences found in colour, during storage, are more likely to be due to pol-ymerisation phenomena, similar to what was mentioned above for wines or juices.
It can also be concluded that the colour of processed and stored at 20¡C products is not signiÐcantly a†ected by freezing for 24 h. Moreover, although total antho-cyanin concentration is lower when jam was made with frozen fruit, the rate of loss of these compounds during storage was not inÑuenced by this factor. Nevertheless, the development of browning was higher when jams were produced with frozen fruit and stored at higher temperatures.
ACKNOWLEDGEMENTS
CGV wish to thank the Spanish Ministerio de Educa-cion y Ciencia, via CSIC, for a contract and PZ to Hero
Espan8a SA for a grant. Thanks also to C Mart•nez Ataz
for technical assistance.
REFERENCES
Abers J F, Wrolstad R F 1979 Causative factors of colour deterioration in strawberry preserves during processing and storage.J Food Sci4475È78 (81).
Adams J B, Ongley M J 1973 The degradation of antho-cyanins in canned strawberries. I. The e†ect of various pro-cessing parameters on the retention of pelargonidin 3-glucoside.J Food T echnol8139È145.
AOAC 1984Official Methods of Analysis (14th edn). Associ-ation of Official Analytical Chemists, Virginia, USA, pp 414È420.
Bakker J, Timberlake C F 1986 The mechanism of colour changes in port wines.Am J Enol V itic37288È292.
Brouillard R 1982 Chemical structure of anthocyanins. In : Anthocyanins as Food Colors, ed Markakis P. Academic Press, London, UK, pp 1È40.
Decareu R V, Livingston G E, Fellers C R 1956 Color changes in strawberry jellies.Food Res10125È128.
Francis F J 1972 Anthocyanins of Durham and Heritage rasp-berries.HortSci4398.
Francis F J 1975 Anthocyanins as food colours.Food T echnol 2952È54.
Garc•a-Viguera C, Bridle P, Bakker J 1994 The e†ect of pH on the formation of coloured compounds in model solution containing anthocyanins, catechin and acetaldehyde. V itis 3337È40.
Garc•a-Viguera C, Zafrilla P, Tomas-Barberan F A 1997 Determination of authenticity of fruit jams by HPLC analysis of anthocyanins.J Sci Food Agric73207È213. Henning W 1981 Flavonolglycoside der Erdbeered
(Fragaria]ananassaDuch.), Himbeeren (Rubus idaeus L.)
und Brombeeren (Rubus fruticosus L.). Z L ebensm Unters Forsch173180È187.
Jackman R L, Smith J L 1996 Anthocyanin and betalains. In : Natural Food Colorants, eds Hendry G A F & Houghton J D. Blackie Academic & Professional, Glasgow, UK, pp 249È309.
Markakis P 1982 Stability of anthocyanins in food. In :Antho -cyanins as Food Colors, ed Markakis P. Academic Press, New York, USA, pp 163È180.
Markakis P, Livingston G E, Fellers C R 1957 Quantitative aspects of strawberry pigment degradation. Food Res 22 117È130.
Mazza G, Brouillard R 1990 The mechanism of co-pigmentation of anthocyanins in aqueous solutions.Phyto -chemistry291097È1102.
Mazza G, Miniati E 1993 Anthocyanins in Fruit, V egetables and Grains. CRC Press, Boca Raton, FL, USA.
McLaren K 1980 Food colorimetry. In :Developments in Food ColoursÈI, ed Walford J. Applied Science Publishers Ltd, London, UK, pp 27È45.
Meschter E E 1953 Fruit colour loss : e†ects of carbohydrates and other factors on strawberry products. J Agric Food Chem1574È579.
Mossel H D, Herrmann K 1974 Die phenolischen Inhalts-sto†e des Obstes. IV. Die phenolishen inhaltsInhalts-sto†e der brombeeren und himbeeren und deren veranderugen wahrend Wachstum und reife der Frutche. Z L ebemns Unters Forsch154324È327.
colour and appearance of strawberry wine.J Food Sci 50 1121È1125.
Rivas-Gonzalo J C, Bravo-Haro S, Santos-Buelga C 1995 Detection of compounds formed through the reaction of malvidin 3-monoglucoside and catechin in the presence of acetaldehyde.J Agric Food Chem431444È1449.
Rommel A, Heatherbell D A, Wrolstad R E 1990 Red rasp-berry juice and wine : e†ect of processing and storage on anthocyanin pigment composition, color and appearance.J Food Sci551011È1017.
Rommel A, Wrolstad R E 1993 Composition of Ñavonols in red raspberry juice as inÑuenced by cultivar, processing and environmental factors.J Agric Food Chem411941È1950. Schwab W, Herrmann K 1985 Hydroxybenzoic and
hydroxy-cinnamic derivatives in soft fruits.Phytochemistry242761È 2764.
Spayd S E, Morris J R 1981 InÑuence of immature fruits on strawberry jam quality and storage stability.J Food Sci46 414È418.
Somers T C 1971 The polymeric nature of wine pigments. Phytochemistry102175È2186.
Withy L M, Nguyen T T, Wrolstad R E, Heatherbell D A 1993 Storage changes in anthocyanin content of red rasp-berry juice concentrate.J Food Sci58190È192.