Effect of replacing fish meal with soybean meal on growth, feed
utilization and carcass composition of cuneate drum
(
Nibea miichthioides
)
Yan Wang
a,⁎
, Ling-Jun Kong
a, Cui Li
a, Dominique P. Bureau
baLaboratory of Aquatic Ecology and Fish Nutrition, Shanghai Fisheries University, Shanghai, China bDepartment of Animal and Poultry Science, University of Guelph, Guelph, Ontario, Canada N1G 2W1
Received 12 July 2006; received in revised form 29 August 2006; accepted 29 August 2006
Abstract
The effect of replacing fish meal with soybean meal (SBM) in practical feeds for cuneate drum was evaluated in an 8-week net pen trial. The cuneate drum fingerlings (initial body weight 29.8 ± 1.3 g fish−1) were fed six isonitrogenous and isocaloric feeds
containing 39% digestible protein and 16 MJ kg−1digestible energy. The control feed was formulated to contain 40% herring meal,
whereas in the other five feeds SBM was included at 11.3, 22.5, 33.8, 45.0 and 56.3% to replace 20, 40, 60, 80 or 100% of the fish meal. There were no significant differences in feed intake between fish fed the control feed and feeds in which SBM replaced 20 to 80% of the fish meal, but fish fed the fish meal free feed had higher feed intake than the other treatments. Weight gain linearly declined with the decrease of fish meal level. Final body weight (FBW) of fish fed the feeds in which SBM replaced 20% of the fish meal did not significantly differ from fish fed the control feed. Replacing 40 to 100% of the fish meal resulted in lower FBW and nitrogen retention efficiency (NRE), and higher feed conversion ratio (FCR) than those of fish fed the control feed. Fish fed the feeds in which SBM replaced 60 to 100% of the fish meal had lower condition factor and hepatosomatic index than those of fish fed the control feed. No significant differences in carcass protein content was found among the treatments, but fish fed the feeds in which SBM replaced 60 to 100% of the fish meal had higher moisture and lower lipid content in carcass than those of fish fed the control feed. Results of the present study appear to indicate that cuneate drum has a limited ability to utilize SBM as a protein source in practical feeds.
© 2006 Elsevier B.V. All rights reserved.
Keywords:Cuneate drum;Nibea miichthioides; Soybean meal; Growth; Nitrogen retention efficiency
1. Introduction
Fish meal is an excellent but costly protein source for fish feed formulation, and is generally incorporated at
50% in commercial feeds for carnivorous fish species (Hertrampf and Piedad-Pascual, 2000). Reducing fish meal level is key to reducing feed cost for commercial fish farming and ensuring sustainability of this enterprise. It is essential to evaluate the suitability of alternate plant and animal protein meals as dietary protein sources for marine carnivorous fish species. Soybean meal (SBM) is a widely available, economical protein source with relatively high www.elsevier.com/locate/aqua-online
⁎ Corresponding author. Tel.: +86 21 65710764; fax: +86 21
65711600.
E-mail address:[email protected](Y. Wang).
digestible protein and energy contents and good amino acid profile (Hertrampf and Piedad-Pascual, 2000). The use of soybean proteins as a dietary protein has been examined for many commercial important fish species, such as rainbow trout (Cho et al., 1974; Dabrowski et al., 1989; Pongmaneerat and Watanabe, 1992; Oliva-Telesa et al., 1994; Kaushik et al., 1995; Refstie et al., 1997; Bureau et al., 1998), channel catfish (Wilson and Poe, 1985; Webster et al., 1992; Peres et al., 2003), tilapia (Shiau et al., 1989, 1990), red drum (Reigh and Ellis, 1992; McGoogan and Gatlin, 1997), Atlantic salmon (Refstie et al., 1998) and Asian seabass (Boonyaratpalin et al., 1998; Tantikitti et al., 2005).
Cuneate drum is a carnivorous sciaenid species native to near-shore waters of the China Sea, and has been widely cultured, with raw fish as feed, in net pens along the coast of the South and East China Sea. Feeding raw fish results in high feed costs and a high amount of nitrogenous waste. Feed formulae that have high nutritive value, are cost-effective, and produce less waste outputs are needed to improve economical and environmental sustainability of cuneate drum culture. The use of rendered animal protein ingredients, such as meat and bone meal (MBM), poultry by-product meal (PBM) and feather meal (FEM), as fish meal replacement in cuneate drum feeds has been determined (Wang et al., 2006b), but the ability of cuneate drum to utilize SBM remains not to be evaluated. Knowledge concerning fish meal replacement by SBM for sciaenid species is still limited (Reigh and Ellis, 1992; Davis et al., 1995; McGoogan and Gatlin, 1997). The present study aimed at evaluating the effect of replacing fish meal with SBM on growth, feed utilization and carcass composition of cuneate drum reared in net pens.
2. Material and methods
2.1. Feed formulation and pellet preparation
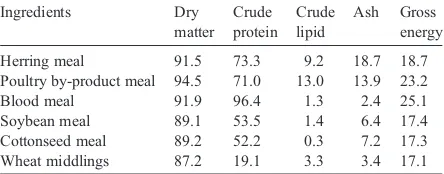
Defatted (solvent extracted) soybean meal was purchased from the East Sea Crop and Oil Corporation (Shanghai, China). Rendered animal by-product ingre-dients, including PBM and blood meal (BM), was supplied by National Rendered Association (Hong Kong, SAR). Other feed ingredients were obtained from Xinnong Feed Company (Shanghai, China). The proximate composition and gross energy content of the ingredients are shown inTable 1.
A practical feed (39% digestible protein and 16 MJ kg−1 digestible energy) containing 400 g kg−1 herring meal which had previously been demonstrated to support good growth performance (Wang et al., 2006a) was used as the control. In the other five feeds, 20, 40, 60, 80 or
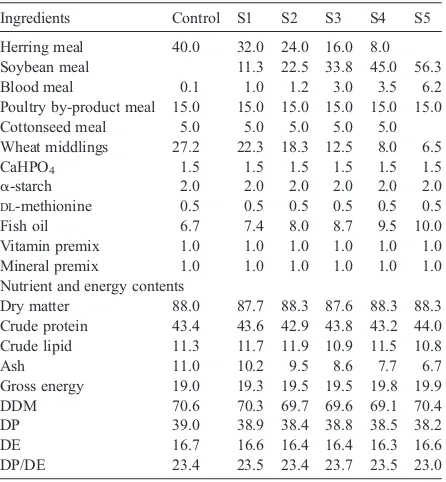
100% of the fish meal was replaced by SBM. These five additional feeds were formulated to be isonitrogenous (39% DP) and isocaloric (16 MJ kg−1DE). All the feeds were supplemented with 0.5%DL-methionine since this amino acid was predicted to be the first limiting amino acid. The formulation, proximate composition and gross energy of the test feeds are shown inTable 2, and amino acid profile of the herring meal, SBM and test feeds in Table 3.
The dry ingredients were ground with a hammer grinder, and mixed with a 30-l Hobart kitchen mixer. Slow sinking pellets (diameter 4 mm and length 8 mm) were made using a laboratory-scale, single screw extruder (extruding temperature 100 °C to 120 °C). The pellets were dried at room temperature.
2.2. Fish, husbandry and feeding
An 8-week feeding trial was carried out in net pens in Shenao Bay, Shantou (Guangdong, China). Cuneate drum (Nibea miichthioides) fingerlings were obtained from a local marine fish hatchery. The fish were reared in net pens (3 m × 3 m × 2 m) and were gradually weaned from raw fish onto the control feed. Two weeks prior to the start of the feeding trial, 800 fish were selected and acclimated to 20 experimental pens (1 m × 1 m × 1.5 m) at 40 fish per pen, and fed the control feed twice daily. At the start of the trial, the acclimated fish were deprived of feed for 24 h, pooled, and 18 groups of 30 fish each were group weighed, and randomly sorted into 18 experimental pens. Each feed treatment had 3 replicates. Eight sub-samples of 3 fish each were collected from the remaining acclimated fish for the determination of initial carcass composition. The sampled fish were frozen at−20 °C until analysis.
During the trial, the fish were hand fed at 08:00 and 16:00 h daily following the method described inWang et al. (2006a)except days of strong waves or high temperature. Dead fish were recorded and weighed for calculating feed conversion ratio (FCR). Water temperature, measured Table 1
Proximate composition (%) and gross energy content (MJ kg−1) of the
ingredients
Herring meal 91.5 73.3 9.2 18.7 18.7
Poultry by-product meal 94.5 71.0 13.0 13.9 23.2
Blood meal 91.9 96.4 1.3 2.4 25.1
Soybean meal 89.1 53.5 1.4 6.4 17.4
Cottonseed meal 89.2 52.2 0.3 7.2 17.3
Wheat middlings 87.2 19.1 3.3 3.4 17.1
daily, was 25 °C to 32 °C, and salinity, measured weekly, was 31 to 32‰during the trial. At the end of the trial, the
fish were collected from each pen and group weighed. Three fish were sampled from each pen for the determination of final carcass composition.
2.3. Chemical analysis
The fish and feeds sampled during the trial were autoclaved at 120 °C for 20 min, homogenized, and dried at 105 °C for 24 h prior to the chemical analysis. The
ingredients, feeds and fish were ground with a laboratory mill. Moisture, crude protein, crude lipid, ash, and gross energy content of the ingredients, feeds and fish were analyzed using the methods described in Wang et al. (2006a). Amino acids of the herring meal, SBM and feeds were analyzed using a SYCOM S-433D amino acid analyzer (SYKAM, German).
2.4. Data calculations and statistical analysis
Feed intake, specific growth rate (SGR), weight gain (WG), FCR and nitrogen retention efficiency (NRE), condition factor (CF), and hepatosomatic index (HSI) were calculated as follows:
Feed intakeð% d−1Þ ¼100I=½ðW
0þWtÞ=2t
SGRð% d−1Þ ¼ ½lnðW
t=NtÞ−lnðW0=N0Þ=t
WGðgÞ ¼ ðWt=Nt−W0=N0Þ
FCRðdry feed gain−1Þ ¼I=ðW
t−W0þWdÞ
NREð%Þ ¼100 ðWtCNt−W0CN0þWd CN0Þ=ðICNfÞ
CFðg cm−3Þ ¼100W s=L3s
HSIð%Þ ¼100Wl=Ws
whereI(g) is total feed fed on a dry weight basis,W0(g) is total initial body weight andWt(g) total final body weight,t(d) is duration of the feeding trial,Ntis number of fish at the end of the trial andN0at the start of the trial, Wd(g) is total weight of the dead fish,CNt(%) is nitrogen content in carcass at the end of the trial andCN0(%) at the start of the trial,CNf(%) is nitrogen content in the feeds,Ws(g fish−1) is body weight of the fish sampled at the end of the feeding trial andLs(cm) total length and Wl(g) liver weight.
Table 3
Essential amino acid profile (% dry weight) of the herring meal, soybean meal and test feeds
Ingredients or feeds Thr Val Cys Met Ile Leu Tyr Phe Lys His Arg
Herring meal 2.14 3.00 0.24 1.68 2.64 4.17 1.56 2.27 4.27 1.52 2.98
Soybean meal 1.57 2.31 0.26 0.44 2.22 3.49 1.34 2.29 2.78 1.40 3.03
Control 1.39 1.94 0.19 1.29 1.63 2.81 0.89 1.51 2.55 1.38 2.15
S1 1.47 2.07 0.14 1.30 1.74 3.00 1.00 1.69 2.51 1.41 2.29
S2 1.41 2.07 0.07 1.12 1.74 3.00 0.79 1.71 2.52 1.44 2.30
S3 1.42 2.17 0.17 1.07 1.78 3.20 1.03 1.91 2.44 1.43 2.48
S4 1.35 2.12 0.12 0.97 1.74 3.12 1.02 1.89 2.47 1.33 2.52
S5 1.39 2.28 0.22 0.88 1.77 3.34 1.07 1.99 2.45 1.46 2.50
Table 2
Formulation, proximate composition (%) and energy content (MJ kg−1)
of the test feeds
Ingredients Control S1 S2 S3 S4 S5
Herring meal 40.0 32.0 24.0 16.0 8.0
Soybean meal 11.3 22.5 33.8 45.0 56.3
Blood meal 0.1 1.0 1.2 3.0 3.5 6.2
Poultry by-product meal 15.0 15.0 15.0 15.0 15.0 15.0
Cottonseed meal 5.0 5.0 5.0 5.0 5.0
Wheat middlings 27.2 22.3 18.3 12.5 8.0 6.5
CaHPO4 1.5 1.5 1.5 1.5 1.5 1.5
α-starch 2.0 2.0 2.0 2.0 2.0 2.0
DL-methionine 0.5 0.5 0.5 0.5 0.5 0.5
Fish oil 6.7 7.4 8.0 8.7 9.5 10.0
Vitamin premix 1.0 1.0 1.0 1.0 1.0 1.0
Mineral premix 1.0 1.0 1.0 1.0 1.0 1.0
Nutrient and energy contents
Dry matter 88.0 87.7 88.3 87.6 88.3 88.3
Crude protein 43.4 43.6 42.9 43.8 43.2 44.0 Crude lipid 11.3 11.7 11.9 10.9 11.5 10.8
Ash 11.0 10.2 9.5 8.6 7.7 6.7
Gross energy 19.0 19.3 19.5 19.5 19.8 19.9
DDM 70.6 70.3 69.7 69.6 69.1 70.4
DP 39.0 38.9 38.4 38.8 38.5 38.2
DE 16.7 16.6 16.4 16.4 16.3 16.6
DP/DE 23.4 23.5 23.4 23.7 23.5 23.0
Vitamin premix and mineral premix were described inWang et al. (2006a).
One-way analysis of variance was performed to examine differences in survival, feed intake, SGR, final body weight (FBW), FCR, NRE, CF, HSI and carcass components among the treatments, and mean compar-isons were examined using Tukey HSD test. Survival, SGR, NRE, HSI and body components were arcsine and logarithm transformed. Relationships between WG and fish meal inclusion level was examined using multiple linear regression. Significance was accepted atPb0.05.
3. Results
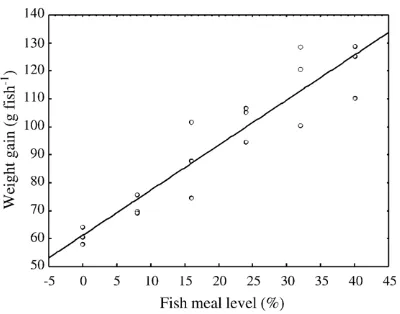
Survival of fish was greater than 94% and not affected by feed composition. Weight gain (WG: g) linearly declined with the decrease of fish meal inclusion level (FL: %), and the regression equation was expressed as: WG = 61.2 + 1.61 × FL (n= 18, r2= 0.87, Pb0.01, Fig. 1). There was no significant difference in feed intake among fish fed the control feed and feeds in which SBM replaced 20 to 80% of the fish meal (PN0.05, Table 4). Fish fed the fish meal free feed had higher feed intake than that of fish fed the control feed (Pb0.05).
Specific growth rate in fish fed the control feed was not significantly different from fish fed the feeds in which SBM replaced 20 to 40% of the fish meal (PN0.05), but was higher than fish fed the feeds in which SBM replaced 60 to 100% of the fish meal (Pb0.05). At the end of the trial, FBW in fish fed the control feed was not significantly different from fish fed the feed in which SBM replaced 20% of the fish meal, but was higher than fish fed the feeds in which SBM replaced 40 to 100% of the fish meal (Pb0.05). Fish fed the control feed had
lower FCR than fish fed the feeds in which SBM replaced 40 to 100% of the fish meal (Pb0.05), and
higher NRE than that of fish fed the feeds in which SBM replaced 60 to 100% of the fish meal (Pb0.05). Fish fed the fish meal free feed had the highest FCR and lowest NRE among the feed treatments.
At the end of the feeding trial, condition factor was 1.90 ± 0.08, 2.14 ± 0.09, 2.00 ± 0.10, 1.74 ± 0.21, 1.81 ± 0.12 and 1.71 ± 0.08 g cm−3(Mean ± S.E.,n= 3), while HSI was 1.25 ± 0.04, 1.21 ± 0.05, 1.21 ± 0.05, 1.16 ± 0.04, 1.15 ± 0.04 and 1.14 ± 0.01%, for fish fed the feeds containing 40, 32, 24, 16, 8 and 0% fish meal. Fish fed the control feed had higher CF and HSI than those of fish fed the feeds in which 60 to 100% of the fish meal was replaced (Pb0.05).
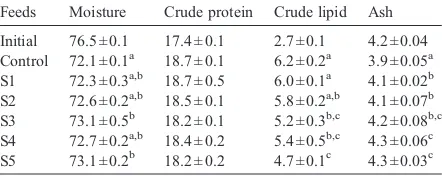
There was no significant difference in carcass protein content among fish fed the different feeds (PN0.05,
Table 5). Fish fed the control feed had lower carcass moisture content, but higher lipid content than those of fish fed the feeds in which 60 to 100% of the fish meal was replaced (Pb0.05). Fish fed the feeds in which SBM replaced 20 to 100% of the fish meal had higher carcass ash content than that of fish fed the control feed (Pb0.05).
4. Discussion
In the present study, the test feeds were formulated to contain 39% DP and 16 MJ kg−1 DE that had been Fig. 1. Relationship between weight gain and fish meal level included
in the test feeds.
Table 4
Body weight (g fish−1), feed intake (% d−1), specific growth (% d−1), feed conversion ratio (feed gain−1) and nitrogen retention efficiency (%) of
cuneate drum fed the test feeds (Mean ± S.E.,n= 3)
Feeds Initial weight Final weight Feed intake Specific growth rate Feed conversion ratio Nitrogen retention efficiency
Control 30.1 ± 0.9 152.2 ± 6.6a 2.66 ± 0.03a 2.86 ± 0.03a 1.12 ± 0.02a 39.3 ± 1.0a S1 30.3 ± 0.7 146.8 ± 9.0a,b 2.64 ± 0.06a 2.81 ± 0.07a 1.14 ± 0.02a,b 38.1 ± 2.7a S2 29.2 ± 0.6 131.3 ± 3.9b,c 2.73 ± 0.01a 2.69 ± 0.06a 1.21 ± 0.03b,c 36.2 ± 0.9a,b
S3 30.1 ± 0.6 118.1 ± 8.4c,d 2.62 ± 0.02a 2.43 ± 0.10b 1.24 ± 0.04c 33.6 ± 2.6b
S4 29.4 ± 0.6 101.0 ± 2.6d,e 2.72 ± 0.03a 2.20 ± 0.03b 1.39 ± 0.02d 31.5 ± 1.4b
S5 29.2 ± 0.9 90.2 ± 2.6e 2.89 ± 0.05b 2.01 ± 0.02b 1.60 ± 0.03e 26.4 ± 1.3c
The superscripts present results of Tukey HSD test among the feed treatments. The values within the same column with different superscripts are significantly different atPb0.05.
demonstrated adequate for growth of cuneate drum (Wang et al., 2006a). To make the feeds isonitrogenous and isocaloric, contents of blood meal (0.1 to 6.2%), wheat middlings (27.2 to 6.5%) and fish oil (6.7 to 10%) were adjusted, and these changes had been demonstrated to be acceptable for cuneate drum (Wang et al., 2006a). Specific growth rate (2.86% d−1) and NRE (39%) of fish fed the control feed were higher than those of the 28 g fish fed frozen Sardinella sp (SGR = 2.40% d−1, NRE = 23%, Guo et al., in press), suggesting cuneate drum fed a herring meal-based feed with 39% DP and 16 MJ kg−1 DE grew well, and had lower nitrogen-waste relative to the fish fed raw fish.
The present study reveal the negative effects of replacing 40 to 100% of the fish meal by inclusion of SBM withDL-methionine supply on SGR, WG, FCR and NRE, suggesting at least 32% fish meal needed in feed formulation for cuneate drum. By inclusion of SBM, 40% to 42% fish meal was still needed in the feeds for Japanese flounder, Korean rockfish and olive flounder (Kikuchi, 1999; Lim et al., 2004; Choi et al., 2004), and 31% for Atlantic salmon (Refstie et al., 1998), and 25% to 27% for Asian seabass, silver seabream, hybrid striped bass and cobia (El-Sayed, 1994; Gallagher, 1994; Boonyaratpalin et al., 1998; Chou et al., 2004), and 5% for red drum (McGoogan and Gatlin, 1997). Therefore, cuneate drum has much lower ability in using SBM as fish meal substitute than that of red drum (McGoogan and Gatlin, 1997).
The poor growth and feed utilization of fish fed the feeds containing SBM protein may be due to the presence of anti-nutritional factors (Wilson and Poe, 1985; van den Ingh et al., 1996; Bureau et al., 1998; Refstie et al., 1998; Peres et al., 2003), low protein digestibility (Refstie et al., 1998), and essential amino acid deficiency (Chong et al., 2003; Tantikitti et al., 2005) in the SBM feeds. The SBM used in the present study was a widely used commercial
product without any additional treatment. Protein digest-ibility of the test feeds was not measured in the feeding trial, thus we could not evaluate the effects of anti-nutritional factors and protein digestibility from inclusion SBM on growth and feed utilization of cuneate drum. Methionine is generally the limiting amino acid of SBM (Hertrampf and Piedad-Pascual, 2000), and methionine deficiency of SBM feeds has been observed in other studies (Chong et al., 2003; Chou et al., 2004). In the present study, methionine content of the test feeds decreased gradually from 1.29% of the control feed to 0.88% of the feeds containing 56.3% SBM, although 0.5%DL-methionine was added in the feed formulations. This indicates that methionine deficiency may be one of the reasons responsible for low growth performance and poor feed utilization of cuneate drum fed the feeds that contain high level of SBM.
In the present study, fish fed the fish meal free feed had higher feed intake than that of fish fed the control feed, and there was no significant difference in feed intake among fish fed the control feed and feeds in which SBM replaced 20 to 80% of the fish meal. This indicates that poor growth performance of cuneate drum fed the feeds containing high levels of SBM is not due to feed palatability. Inconsistent results still exist on how SBM inclusion affects feed palatability. Red drum refused to accept feed in which fish meal was completely replaced with SBM (Reigh and Ellis, 1992), or had lowed feed intake when fed feeds containing low fish meal (Davis et al., 1995). Feed intake of Asian seabass and discus fed feeds containing high level of SBM was significantly lower (Boonyaratpalin et al., 1998; Chong et al., 2003; Tantikitti et al., 2005). However, red drum fed a feed in which 90% of the protein came from SBM had higher feed intake than that of the fish fed a fish meal feed (McGoogan and Gatlin, 1997). In the present study, cuneate drum showed active feeding behavior when fed SBM containing feeds, this is consistent with the conclusion that palatability was not a major factor responsible for the lower feed consumption of SBM-based feed than the fish meal-SBM-based feed (Refstie et al., 1997, 1998).
Cuneate drum fed the feeds contain high levels of SBM had lowed carcass lipid content in the present study. Similar findings have been observed in red drum (McGoogan and Gatlin, 1997), discus (Chong et al., 2003) and Asian seabass (Tantikitti et al., 2005), but not in cobia (Chou et al., 2004), Korean rockfish (Lim et al., 2004) and olive flounder (Choi et al., 2004). Cuneate drum fed formulated feeds had higher carcass lipid content than that of the fish fed raw fish (Wang et al., 2006a). Lower carcass lipid content and high moisture Table 5
Proximate composition (%) in carcass of cuneate drum fed the test feeds (Mean ± S.E.,n= 3)
Feeds Moisture Crude protein Crude lipid Ash
Initial 76.5 ± 0.1 17.4 ± 0.1 2.7 ± 0.1 4.2 ± 0.04
The superscripts present results of Tukey HSD test among the feed treatments. The values within the same column with different super-scripts are significantly different atPb0.05.
and ash contents of fish fed the feeds in which SBM was included to replace the fish meal, in the present study, is attributed to the reduced growth of these fish. The lower carcass lipid content of fish fed the SBM-based feed is responsible for the highest feed intake of the fish, because feed intake of fish is regulated by their body lipid storage. In our previous studies, the fish meal content in cuneate drum feeds could be reduced to 17.5% by inclusion of PBM (Wang et al., 2006b) or to 7% by inclusion of combinations of PBM, MBM, FEM and BM (Guo et al., in press) without significantly negative effect on growth performance. In the present study, signifi-cantly lowed FBW and increased FCR occurred when fish meal content was reduced to 24%. It is clear that cuneate drum prefer to accept PBM, relative to SBM, as a fish meal alternate protein.
Acknowledgements
This project was funded by grants from the National Natural Science Foundation of China (30471340) and Aquaculture Division in E-Institute of Shanghai Uni-versities. We thank Yu Yu for his help in procurement of the rendered animal ingredients, and Kai Li,. Zhouxing Zheng, Wei-zhou Chen, Ze-wei Sun and Yuan-xi Lin for their help with various aspects of this work.
References
Boonyaratpalin, M., Suraneiranat, P., Tunpibal, T., 1998. Replacement of fish meal with various types of soybean products in diets for the Asian seabass,Lates calcarifer. Aquaculture 161, 67–78. Bureau, D.P., Harris, A.M., Cho, C.Y., 1998. The effects of purified
alcohol extracts from soy products on feed intake and growth of chinook salmon (Oncorhynchus tshawytscha) and rainbow trout (Oncorhynchus mykiss). Aquaculture 161, 27–43.
Cho, C.Y., Bayley, H.S., Slinger, S.J., 1974. Partial replacement of herring meal with soybean meal and other changes in a diet for rainbow trout (Salmo gairdneri). J. Fish. Res. Board Can. 31, 1523–1528.
Choi, S.M., Wang, X., Park, G.J., Lim, S.R., Kim, K.W., Bai, S.C., Sin, I.S., 2004. Dietary dehulled soybean meal as a replacement for fish meal in fingerling and growing olive flounder Paralichthys olivaceus
(Temminck et Schlegel). Aquac. Res. 35, 410–418.
Chong, A., Hashim, R., Ali, A., 2003. Assessment of soybean meal in diets for discus (Symphysodon aequifasciata HECKEL) farming through a fishmeal replacement study. Aquac. Res. 34, 913–922.
Chou, R.L., Her, B.Y., Su, M.S., Hwang, G., Wu, Y.H., Chen, H.Y., 2004. Substituting fish meal with soybean meal in diets of juvenile cobiaRachycentron canadum. Aquaculture 229, 325–333. Dabrowski, K., Poczyczynski, P., Köck, G., Berger, B., 1989. Effect of
partially or totally replacing fish meal protein by soybean meal protein on growth, food utilization and proteolytic enzyme activities in rainbow trout (Salmo gairdneri). Aquaculture 77, 29–49.
Davis, D.A., Jirsa, D., Arnold, C.R., 1995. Evaluation of soybean proteins as replacements for menhaden fish meal in practical diets for red drum,Sciaenops ocelltus. J. World Aquac. Soc. 26, 48–58. El-Sayed, A.F.M., 1994. Evaluation of soybean meal, spirulina meal
and chicken offal meal as protein sources for silver seabream (Rhabdosargus sarba) fingerlings. Aquaculture 127, 169–176.
Gallagher, M.L., 1994. The use of soybean meal as a replacement for fish meal in diets for hybrid striped bass (Morone saxatilis×M. chrysops). Aquaculture 126, 119–127.
Guo, J., Wang Y., Bureau, D.P., in press. Inclusion of rendered animal ingredients as fish meal substitutes in practical diets for cuneate drum,Nibea miichthioides(Chu, Lo et Wu). Aquacult. Nutr. Hertrampf, J.W., Piedad-Pascual, F., 2000. Handbook on Ingredients
for Aquaculture Feeds. Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 482–483.
Kaushik, S.J., Cravedi, J.P., Lalles, J.P., Sumpter, J., Fauconneau, B., Laroche, M., 1995. Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout,Oncorhynchus mykiss. Aquaculture 133, 257–274. Kikuchi, K., 1999. Use of defatted soybean meal as a substitute for fish
meal in diets of Japanese flounder (Paralichthys olivaceus). Aquaculture 179, 3–11.
Lim, S.R., Choi, S.M., Wang, X.J., Kim, K.W., Shin, I.S., Min, T.S., Bai, S.C., 2004. Effects of dehulled soybean meal as a fish meal replacer in diets for fingerling and growing Korean rockfish Se-bastes schlegeli. Aquaculture 231, 457–468.
McGoogan, B.B., Gatlin III, D.M., 1997. Effects of replacing fish meal with soybean meal in diets for red drumSciaenops ocelltusand potential for palatability enhancement. J. World Aquac. Soc. 28, 374–385.
Oliva-Telesa, A., Gouveia, A.J., Gomesb, E., Rema, P., 1994. The effect of different processing treatments on soybean meal utilization by rainbow trout,Oncorhynchus mykiss. Aquacu1ture 124, 343–349. Peres, H., Lim, C., Klesius, P.H., 2003. Nutritional value of
heat-treated soybean meal for channel catfish (Ictalurus punctatus). Aquaculture 225, 67–82.
Pongmaneerat, J., Watanabe, T., 1992. Utilization of soybean meal as protein source in diets for rainbow trout. Nippon Suisan Gakkaishi 58, 1983–1990.
Refstie, S., Helland, S.J., Trond, S., 1997. Adaptation to soybean meal in diets for rainbow trout,Oncorhynchus mykiss. Aquaculture 153, 263–272.
Refstie, S., Trond, S., Roem, A.J., 1998. Feed consumption and conversion in Atlantic salmon (Salmo salar) fed diets with fish meal, extracted soybean meal or soybean meal with reduced content of oligosaccharides, trypsin inhibitors, lectins and soya antigens. Aquaculture 162, 301–312.
Reigh, R.C., Ellis, S.C., 1992. Effects of dietary soybean and fish-protein ratios on growth and body composition of red drum (Sciaenops ocellatus) fed isonitrogenous diets. Aqcaculture 104, 279–292.
Shiau, S., Kwok, C., Kwang, J., Chen, C., Lee, S., 1989. Replacement of fishmeal with soybean meal in male tilapia (Oreochromis niloticus×O. aureus) fingerling diets at a suboptimal protein level. J. World Aquac. Soc. 20, 230–235.
Shiau, S., Lin, S., Yu, S., Lin, A., Kwok, C., 1990. Defatted and full-fat soybean meal as partial replacements for fishmeal in tilapia (Oreochromis niloticus×O. aureus) diets at low protein level. Aquaculture 86, 401–407.
nitrogen and phosphorus excretion in Asian seabass (Lates calcarifer). Aquaculture 248, 41–50.
van den Ingh, T.S.G.A.M., Olli, J., Krogdahl, A., 1996. Alcohol-soluble components in soybeans cause morphological changes in the distal intestine of Atlantic salmon,Salmo salarL. J. Fish Dis. 19, 47–53.
Wang, Y., Guo, J., Bureau, D.P., Cui, Z., 2006a. Effects of dietary protein and energy levels on growth, feed utilization and body composition of cuneate drum,Nibea miichthioides. Aquaculture 252, 421–428.
Wang, Y., Guo, J., Li, K., Bureau, D.P., 2006b. Replacement of fish meal with rendered animal ingredients in feeds for cuneate drum,
Nibea miichthioides. Aquaculture 252, 476–483.
Webster, C.D., Tidwell, J.H., Goodgame, L.S., Yancey, D.H., Mackey, L., 1992. Use of soybean meal and distillers grains with solubles as partial or total replacement of fish meal in diets for channel catfish,
Ictalurus punctatus. Aquaculture 106, 301–309.