Brain Research 881 (2000) 237–240
www.elsevier.com / locate / bres
Short communication
Anesthetic concentrations of riluzole inhibit neuronal nitric oxide
synthase activity, but not expression, in the rat hippocampus
¨
Hawa Keita, Jorge Boczkowski, Abdulaye Samb, Sophie Lanone, Loıc Lang-Lazdunski,
*
´
Danielle Rouelle, Jean-Marie Desmonts, Jean Mantz
´ ´ ´ ´
Institut National de la Sante et de la Recherche Medicale(INSERM) U 408, Faculte de Medecine Xavier Bichat, 16 rue Henri Huchard, 75018 Paris, France
Accepted 8 August 2000
Abstract
We hypothesized that anesthetic dose of riluzole, an inhibitor of glutamate neurotransmission, may affect the activity and / or expression
G
of neuronal NOS (nNOS). Riluzole, N -nitro-L-arginine-methyl ester (L-NAME) and 7-nitro indazole (7-NI) produced a
concentration-related inhibition of nNOS activity in vitro. Riluzole competed with 7-NI for inhibition of nNOS activity, but had no effect on nNOS or
21
endothelial NOS (eNOS) protein expression. Also, nNOS activity was significantly decreased in riluzole-anesthetized rats (40 mg kg i.p.,23266% from controls, P,0.05). Therefore, blockade of nNOS activity may be involved in the anesthetic effects of riluzole in vivo. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Second messengers and phosphorylation
Keywords: nNOS activity; nNOS protein expression; Riluzole; Hippocampus; Anesthesia
Riluzole (2-amino-6-trifluoromethoxy benzothiazole) is way could also be one of the potential targets of anes-a sedanes-ative / anes-anesthetic [18] anes-and neuroprotective anes-agent in thetics in the mammalian brain [14,19,20] We have vitro and in vivo [7,17]. Also, it prolongs survival in previously shown that riluzole, used at concentrations patients with neurodegenerative diseases such as amyot- greater than those for which neuroprotective properties are rophic lateral sclerosis [15]. Some of the target sites of observed, is an anesthetic agent [18]. Here, we hypoth-riluzole have been identified yet: hypoth-riluzole depresses gluta- esized that anesthetic concentrations of this agent could mate release in the striatum [4], it slows down conduction also directly affect nNOS activity. The aim of the present velocity of presynaptic glutamate fibers [16] and blocks study was first, to examine the effects of riluzole on some postsynaptic responses mediated by glutamate-gated hippocampal nNOS and eNOS protein expression, and ionic channels [5] as well as by voltage-gated calcium and second, on hippocampal nNOS activity both in vitro and in sodium channels [12,13]. It has also been recently shown riluzole-anesthetized rats.
1
to stimulate neuronal two P domain K channels [8]. All experiments were conducted on male Sprague–Daw-The mechanisms underlying the action of anesthetics in ley rats strictly according to the recommendations of the the central nervous system remain still to be clarified. European Communities Council Directive of 24 November Anesthetics are known to enhance inhibitory GABA -A 1986 and the NIH Guide for the Care and Use of mediated neurotransmission and depress the excitatory, Laboratory Animals.
glutamate-mediated neurotransmission [11]. Several lines In order to verify that constitutive NOS was predomi-of evidence suggest, however, that the NO–cGMP path- nantly represented by nNOS, the nNOS:eNOS ratio was determined in the preparation. Tissue samples were homogenized in a 1.5 ml lysis buffer (Tris–HCl 50 mM *Corresponding author. Tel.: 133-1-4025-8080; fax: 1
33-1-4228-(pH 7.4), EDTA 0.1 mM, Leupeptin 1 mM, PMSF 1mM, 9996.
E-mail address: [email protected] (J. Mantz). aprotinin 1mM) and incubated with riluzole (200mM, 60
238 H. Keita et al. / Brain Research 881 (2000) 237 –240
min) or without any pharmacological agent (control). (pH 5.5) / 2 mM EDTA, and the total volume was applied to Dowex-50W X8 column (Bio-Rad) preequilibrated with Proteins (75mg per lane) and the tissue homogenates were
3
the same buffer. L-[ H]Arginine was retained on the
denatured by boiling for 5 min in sample buffer (0.5 M
3
Tris–HCl (pH 6.8), 10% (w / v) SDS, 10% (v / v) glycerol, column, whereas L-[ H]citrulline was eluted with 2 ml
3
5% (v / v) 2-b-mercaptoethanol, 0.05% (w / v) bromophenol deionized water. L-[ H]Citrulline concentrations were
de-blue) and separated by electrophoresis on precasted 7.5% termined by liquid scintillation counting after subtracting sodium dodecyl sulfate–polyacrylamide gel (Bio-Rad, the blank values, which gave the non-specific radioactivity Richmond, CA). They were transferred overnight at 48C to in the absence of the enzyme. Various concentrations of
26 23
a PVDF membrane (Bio-Rad) in 25 mM CAPSO buffer riluzole (10 –10 M) or its vehicle dimethylsulfoxide
G
(pH 10) and 20% methanol. Subsequent steps were (DMSO), 7-nitro indazole (7-NI) and N -nitro-L
-arginine-27 23
performed at room temperature. The membranes were then methyl ester (L-NAME) (10 –10 M) were added to the
blocked with 10% non-fat dry milk in Tris-buffered saline incubation buffer. Concentration–response curves, IC50
(25 mM Tris (pH 7.5), 150 mM NaCl)10.05% (v / v) values and Hill coefficients were computer-generated using Tween-20 (TBST solution). After washing in TBST, the GraphPAD software (Intuitive Software for Science, San membranes were incubated 1 h with a monoclonal anti- Diego, CA).
nNOS or anti-eNOS antibody used at 1:1000 dilution Animals anesthetized with riluzole were given either 21
(Transduction Laboratories, Lexington, KY). After wash- riluzole (40 mg kg ) dissolved in 0.5 ml in DMSO or ing, the membranes were incubated for 1 h with 1:3000 DMSO intraperitoneally. Anesthesia was defined by the dilution of a goat anti-mouse IgG or a goat anti-mouse loss of righting reflex, which is the inability of the animal conjugated to alkaline phosphatase (Bio-Rad). The blots to right itself when being placed in a lateral recumbent were washed with TBST, followed by detection of im- position. Animals were killed 30 min after the i.p. in-munoreactive proteins by the enhanced chemiluminescence jection, since this delay corresponds to the time of method using AMMPD (Tropix) as substrate (Bio-Rad). maximal riluzole plasma concentrations achieved follow-Results were expressed as the nNOS /b actin ratio. ing i.p. injection [2].
NOS activity was measured by the conversion of L- Results were considered reliable only if they had been
3 3
[ H]arginine to L-[ H]citrulline [3]. Briefly, a 25-ml reproduced in at least four independent experiments (each
homogenate of hippocampal tissue corresponding to 50mg run in triplicate). Data are presented as mean6S.D. total cellular proteins was incubated for 60 min at 378C in Normality of distributions was first assessed by the Fisher the reaction buffer (150 ml final volume) containing 50 test for equality of variances. Statistics were performed by mM Hepes (pH 7.40), 0.5 mM NADPH, 5 mM FAD, and analysis of variance followed by post hoc Scheffe’s test. A 5 mM tetrahydrobiopterin (B. Schricks Laboratories), 1.25 P value of less than 0.05 was considered the threshold for
3
mM CaCl , 10 mM calmodulin and 39 nM2 L-[ H]arginine significance. nNOS was the predominant NOS isoform
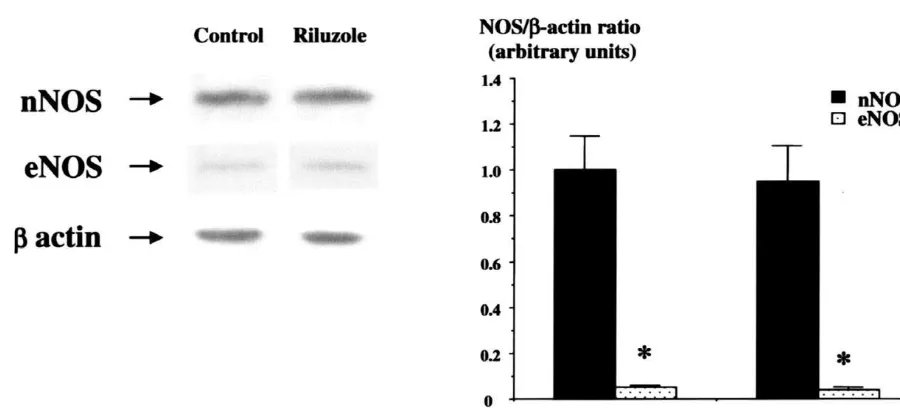
(specific activity 35.7 Ci / mmol). The enzymatic reaction present in the hippocampal homogenates (Fig. 1). Indeed, was stopped by the addition of 2 ml ice-cold mM Hepes 92% of the expression of NOS was represented by nNOS,
Fig. 1. Western blot analysis of hippocampal nNOS (neuronal NOS) and eNOS (endothelial NOS) in homogenates of the rat hippocampus. (Left) Representative Western blot analysis of nNOS, eNOS andb-actin expression. (Right) Densitometric analysis of nNOS, eNOS andb-actin bands showing
21
H. Keita et al. / Brain Research 881 (2000) 237 –240 239
while eNOS was only 8%. Riluzole (200 mM) was not stitutive NOS, since it was clearly dependent on external found to significantly alter either nNOS or eNOS protein calcium. Theoretically, both nNOS and eNOS were likely expression (Fig. 1). nNOS basal activity was 16.163.2 to contribute to the NOS signal, since these two isoforms
21 21
pmol (mg protein) min . in the hippocampus,. Remov- are present in both the hippocampus [10]. Our results ing CaCl2 from the buffer resulted an almost complete clearly indicate that the main isoform of NOS expressed in (more than 90%) reduction in nNOS activity in both the hippocampus was the nNOS. Both eNOS and nNOS structures. Both L-NAME and 7-NI induced a potent, are sensitive to competitive inhibitors of the class of
concentration-related, inhibition of the calcium-dependent arginine analogues, such as L-NAME. However, 7-NI
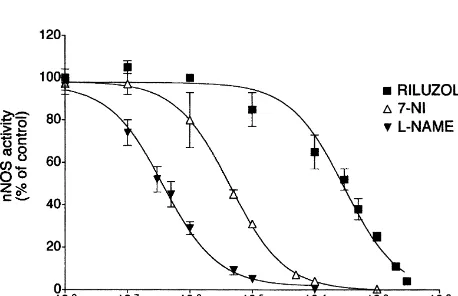
NOS activity, the IC50 values being 0.5 mM for L-NAME appears to be a potent and somewhat selective inhibitor of
and 4mM for 7-NI (Fig. 2). The best fit of the curves to nNOS [1]. We found that bothL-NAME and 7-NI potently
the data was always obtained when the Hill coefficient was inhibited NOS activity in the hippocampus. The efficacy of forced to 1. Riluzole caused a concentration-related inhibi- 7-NI at blocking NOS in the present study supports that tion of nNOS activity in the hippocampus (IC505200mM, nNOS played a major role in the NOS activity measured in Fig. 2). The vehicle, DMSO (5.mM) did not produce any our study. 7-NI exhibited a lower potency in our study than change in nNOS activity. The kinetic profile of nNOS in the cerebellum in vitro while L-NAME had the same
inhibition induced by riluzole was characterized by a lack IC50 as that reported previously in this area [1]. This might of change in Vmax (24936131 vs. 26676102 pmol (mg be due to the presence of a different nNOS:eNOS ratio in
21 21
protein) min ) and a significant increase in Km the cerebellum in comparison with the hippocampus. (9.560.9 vs. 6.460.8mM, P,0.05). In the presence of an The ability of riluzole to block nNOS activity has not IC50 concentration of 7-NI (4 mM) there was a marked been reported yet and was consistent with a competitive decrease in the potency of riluzole inhibition as assessed profile. The kinetic parameters associated with riluzole by a more than 1000-fold increase in the IC50 value effects were supportive of a competitive action, since no (500 000 mM), while no change in IC50 value was change in Vmax was observed whilst a significant decrease observed for L-NAME under these experimental condi- in riluzole affinity for the enzyme was reported.
Co-tions. operative mechanisms with conformational changes of the
All rats treated with riluzole i.p. (n58) had loss of enzyme are unlikely, since the best fit of the curves to the righting reflex, while this was not observed in the DMSO- data was always obtained when Hill slope was forced to treated animals (control group, n58). The onset time of one. Further, we observed that the potency of riluzole riluzole action after injection was 4.762.3 min. nNOS effect on nNOS was dramatically decreased in the presence activity was significantly decreased in rats given riluzole of 7-NI. In contrast, the same 7-NI concentration failed to (40 mg / kg i.p.) vs. controls (23266%, P,0.05). significantly affect L-NAME potency for NOS inhibition.
The present study indicates that riluzole blocks nNOS These observations, together with the lack of effect of activity, but not protein expression, in homogenates of the riluzole on nNOS protein expression, are supportive of a rat hippocampus. Riluzole competes with 7-NI, a NOS competition between riluzole and 7-NI for the substrate inhibitor structurally distinct fromL-arginine analogues, for site of nNOS, sinceL-NAME is an irreversible inhibitor of
nNOS inhibition. These findings may have potential phys- nNOS [6]. Since 7-NI is known to bind to the heme site on iological relevance, since hippocampal nNOS activity is NOS, thereby hindering access of both the arginine and the significantly decreased in riluzole-anesthetized rats as well. tetrahydrobiopterin to their respective bindings sites, ex-NOS activity measured in the present study was con- plaining the apparent competitive kinetics for 7-NI at both sites, it might be speculated that riluzole likely acts on the same heme moiety.
The concentrations for which nNOS inhibition by riluzole was observed (IC505200 mM) were higher than those reported to be neuroprotective. We did not directly measure plasma concentrations of riluzole at the moment of sacrificing the rats for processing of nNOS activity measurement. However, owing to the pharmacokinetic properties of riluzole and the anesthetic dose selected, such concentrations were very likely to be present in the plasma of anesthetized animals [18]. In addition to its properties on glutamate transmission, the inhibitory effect of riluzole on nNOS activity may be relevant to the mechanisms whereby riluzole induces anesthesia. Decreasing the activi-ty of the NO–cGMP pathway has been proposed to Fig. 2. Dose–response curve of the inhibition of nNOS activity by
contribute to the effects of anesthetics on the mammalian L-NAME, 7-NI and riluzole in the hippocampus. Results (mean6S.D.) are
phenom-240 H. Keita et al. / Brain Research 881 (2000) 237 –240
[7] A.G. Estevez, J.M. Stutzmann, L. Barbeito, Protective effect of enon, any reported neurobiological action likely to
contrib-riluzole on excitatory amino acid-mediated neurotoxicity in ute to the production of unconsciousness should not induce
motoneuron-enriched cultures, Eur. J. Pharmacol. 280 (1995) 47– irreversible damage. Both the competitive profile of 53.
riluzole-induced nNOS inhibition and the lack of effect of [8] M. Fink, F. Lesage, F. Duprat, C. Heurteaux, R. Reyes, M. Fosset,
1
riluzole on expression of nNOS supports that riluzole does M. Lazdunski, A neuronal two P domain K channel stimulated by arachidonic acid and polyunsaturated fatty acids, EMBO J. 17 not irreversibly alter the structure of the enzyme. The fact
(1998) 3297–3308. that nNOS activity was inhibited both in vitro by riluzole
[9] H. Flohr, U. Glade, D. Motzko, The role of the NMDA synapse in and in rats anesthetized with this agent strongly supports general anesthesia, Toxicol. Lett. 100 (1998) 23–29.
physiological relevance of the reported effects. Finally, it [10] U. Forstermann, J.P. Boissel, H. Kleinert, Expressional control of the¨ has been recently proposed that processes controlled by the constitutive isoforms of nitric oxide synthase (NOSI and NOSIII),
FASEB J. 12 (1998) 773–790. cortical NMDA synapse (and thereby the NO–cGMP
[11] N.P. Franks, W.R. Lieb, Molecular and cellular mechanisms of pathway) play a crucial role for consciousness and are the
general anaesthesia, Nature 367 (1994) 607–614.
final common pathway of anesthetic action [9]. Our [12] T. Hebert, P. Drapeau, L. Pradier, R.J. Dunn, Block of the rat brain findings are consistent with this hypothesis. IIA sodium channel a subunit by the neuroprotective drug riluzole,
In summary, we have shown a concentration-related, Mol. Pharmacol. 45 (1994) 1055–1060.
[13] J.P. Hubert, J.C. Delumeau, J. Glowinski, J. Premont, A. Doble, competitive, inhibition of basal hippocampal nNOS
activi-Antagonism by riluzole of entry of calcium evoked by NMDA and ty by riluzole in the rat hippocampus. This action may
veratridine in rat cultured granule cells: evidence for dual mecha-contribute to the anesthetic effect of riluzole in vivo. nism of action, Br. J. Pharmacol. 113 (1994) 261–267.
[14] F. Ichinose, P.L. Huang, W.M. Zapol, Effects of targeted neuronal
G
nitric oxide synthase gene disruption and nitro -L-arginine methyles-ter on the threshold for isoflurane anesthesia, Anesthesiology 83 References
(1995) 101–108.
[15] (for the Amyotrophic Lateral Sclerosis Study Group-II), L. Lacom-[1] R.C. Babbedge, P.A. Bland-Ward, S.L. Hart, P.K. Moore, Inhibition blez, G. Bension, P.N. Leigh, P. Guillet, V. Meininger, Dose-ranging of nitric oxide synthase by 7-nitro indazole and related substitute study of riluzole in amyotrophic lateral sclerosis, Lancet 347 (1996) indazoles, Br. J. Pharmacol. 110 (1993) 225–228. 1425–1431.
[2] J. Benavides, J.C. Camelin, N. Mitrani, F. Flamand, A. Uzan, J.J. [16] M.B. MacIver, S.M. Amagasu, A.A. Mikulec, F.A. Monroe, Legrand, C. Gueremy, G. Le Fur, 2-amino-6-trifluoromethoxy Riluzole anesthesia: use-dependent block of presynaptic glutamate benzothiazole, a possible inhibitor of excitatory aminoacid neuro- fibers, Anesthesiology 85 (1996) 626–634.
transmission. II. Biochemical properties, Neuropharmacology 26 [17] C. Malgouris, M. Daniel, A. Doble, Neuroprotective effects of (1985) 1085–1092. riluzole on N-methyl-D-aspartate or veratridine-induced neurotoxici-[3] D. S Bredt, S.H. Snyder, Nitric oxide mediates glutamate-linked ty in rat hippocampal slices, Neurosci Lett. 177 (1994) 95–99.
enhancement of cGMP levels in the cerebellum, Proc. Natl. Acad. [18] J. Mantz, A. Cheramy, A.M. Thierry, J. Glowinski, J.M. Desmonts, Sci. USA 86 (1989) 9030–9033. Anesthetic properties of riluzole (54274 RP) a new inhibitor of [4] A. Cheramy, L. Barbeito, G. Godeheu, J. Glowinski, Riluzole glutamate neurotransmission, Anesthesiology 76 (1992) 844–848.
inhibits the release of glutamate in the caudate nucleus of the cat in [19] J.R. Tobin, L.D. Martin, M.J. Breslow, R.J. Traystman, Selective vitro, Neurosci. Lett. 147 (1992) 209–212. anesthetic inhibition of brain nitric oxide synthase, Anesthesiology [5] M.W. Debono, J. Le Guern, T. Canton, A. Doble, L. Pradier, 81 (1994) 1264–1269.
Inhibition by riluzole of electrophysiological responses mediated by [20] Z. Zuo, A. Tichotsky, R.A. Johns, Halothane and isoflurane inhibit rat kainate and NMDA receptors expressed in Xenopus oocytes, Eur. vasodilation due to constitutive, but not inducible nitric oxide J. Pharmacol. 235 (1993) 283–289. synthase. Implications for the site of anesthetic inhibition of the [6] M.A. Dwyer, D.S. Bredt, S.H. Snyder, Nitric oxide synthase: nitric oxide / guanylyl cyclase signalling pathway, Anesthesiology 84
G