www.elsevier.com / locate / bres
Interactive report
Effects of neuroactive substances on the morphine-induced respiratory
1depression; an in vitro study
a,c ,2 b , ,2
*
b cKoichi Takita
, Eric Herlenius
, Yuji Yamamoto , Sten G.E. Lindahl
a
Department of Anesthesiology and Intensive Care, Hokkaido University School of Medicine, Kita-15, Nishi-7, Kita-ku, Sapporo 060, Japan
b
Department of Woman and Child Health, Karolinska Hospital and Karolinska Institutet, S171 76 Stockholm, Sweden
c
Department of Anesthesiology and Intensive Care, Karolinska Hospital and Karolinska Institutet, S171 76 Stockholm, Sweden
Accepted 19 September 2000
Abstract
Effects of different neuroactive substances on morphine-induced respiratory depression were studied in medullary respiration-related structures using in vitro brainstem–spinal cord preparation from 1 to 4-day-old rats. Application of morphine (10mM) reduced respiratory rhythm (fR) as measured by C4 ventral root activity. The depressant effects of morphine were reversed by acetylcholine (10 mM), substance P (50 nM), thyrotropin releasing hormone (TRH) (100 nM) and forskolin (10 mM). The adenosine receptor antagonist, theophylline (100mM), the dopamine receptors antagonist, haloperidol (10mM), the cyclooxygenase inhibitor, indomethacin (10mM) and the phospholipase A inhibitor, quinacrine (102 mM) had no effect on morphine-induced respiratory depression. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Opioids: anatomy, physiology, and behavior
Keywords: Brainstem; Respiration; Opioid; Acetylcholine, Substance P; TRH
Morphine causes a G-protein mediated respiratory de- over, in different cell types activation of G-protein coupled pression, a major drawback during its use as analgesic. opioid receptors have been shown to induce changes in Finding a way to reverse or counteract this depression of several second messenger-signalling systems. These in-respiration without canceling analgesia is an important and clude elevation of intracellular calcium, stimulation of clinically relevant goal. One of the putative action sites of inositol 1,4,5-trisphosphate (IP3) turnover, arachidonic morphine, in respiratory depression, is the medulla oblon- acid mobilization and cAMP decrease) [5,6,17]. IP3, gata [4,25,26]. Some of its pharmacological effects seem cAMP and arachidonic acid metabolites are involved in to be due to the presynaptic modulation of transmitter respiratory control [12,17]. However, it is not clear release of substances such as acetylcholine, substance P, whether these substances interact with morphine-induced dopamine and adenosine [9,18,21,24]. These neuroactive respiratory depression. Other drugs that have been reported substances have all been suggested to play an important to antagonize opioid-induced respiratory depression in-role for respiratory activity in the medulla oblongata. clude; benzodiazepine receptor antagonist and thyrotropin Acetylcholine and Substance P stimulate while adenosine releasing hormone (TRH) [10,15]. Hence, in the present and dopamine depress respiration [3,8,14,22,29]. More- study possible interactions in medullary respiration-related structures between morphine and these substances were examined in an in vitro brainstem–spinal cord preparation.
1
Published on the World Wide Web on 23 October 2000. The head and upper thorax of SD-BKL57 rats (1–4 days *Corresponding author. Department of Woman and Child Health, old, n587) were dissected under ether anesthesia. Brain-Astrid Lindgrens Hospital Q2:07, S171 76 Stockholm, Sweden. Tel.:
stem and spinal cord were isolated and perfused with
146-85-177-7357; fax:146-8-335-635.
artificial cerebrospinal fluid (aCSF) at 28.58C as described
E-mail address: [email protected] (E. Herlenius).
2
Has contributed equally. previously [23,25]. Respiratory activity was measured at
202 K. Takita et al. / Brain Research 884 (2000) 201 –205
the C4 or C5 ventral root using suction electrodes. tolerance was observed 30–60 min after the application. Recorded signals were amplified and band-pass filtered (10 As a few preparations responded weakly to morphine, the Hz to 5 kHz, differential AC amplifier model 1700, A–M analysis of reversibility of the different test agents were systems Inc.). The C4 / C5 activity was rectified and performed on experiments where a clear-cut morphine integrated with a time constant of 100 ms. These activities induced respiratory depression was seen (.20% fR reduc-were simultaneously monitored via an analogue–digital tion) (67 / 80). Also the effect of a test agent on respiratory converter (Digidata 1200, Axon Instruments) and data activity / morphine induced respiratory depression was acquisition software (Axotape, Axon Instruments). Data evaluated 30 min after its application. The average values were stored on a computer for later off-line analysis. were calculated from the bursts recorded during 1–2 min and results expressed as percentage of the controls. All Experimental procedure: After a 20 min control and data are presented as means6standard error of the mean stabilization period in which the preparation was super- (S.E.M.). The influence of test agents on the morphine-fused with aCSF alone, morphine (10 mM, Pharmacia, induced respiratory depression were analyzed with one-Stockholm, Sweden) was applied for 30 min. Subsequently way ANOVA repeated measures design with Scheffe’s F morphine (10 mM) and a test agent (acetylcholine, sub- post hoc test. The effects of morphine, atropine and stance P, forskolin, theophylline, indomethacin, quinacrine flumazenil on respiratory activity were analyzed with (all from Sigma, St. Louis, MO, USA), haloperidol (Jan- Student’s paired t-test. Probability values (P) below 0.05 ssen Phamaceutica, Beerse, Belgium), flumazenil (Roche, were considered as statistically significant.
Basel, Switzerland) or TRH (Fluka Chemi, Buchs, Swit- The main findings are summarized in Table 1. Morphine zerland) were co-applied for 30 min. In 5 preparations that (10 mM) caused a significant reduction of fR (P,0.01) were used as control, morphine (10 mM) was applied for and RMA (P,0.05) while the effect on respiratory peak 60 min after a 20 min control period. This was followed by amplitude (Int. C4) was inconsistent. Morphine effects the simultaneous application of morphine (10 mM) and were completely reversed by naloxone (1 mM) (%fR: naloxone (1 mM). According to previous investigations 53.56 13.9% to 99.265.3%, % Int. C4: 111.9610.9% to using this in vitro preparation or brain slices, the con- 105.7612.4% and %RMA: 56.7611.7% to 116.3611.7%, centrations of test agents were determined as follows: n55).
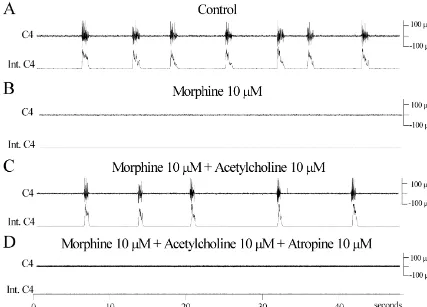
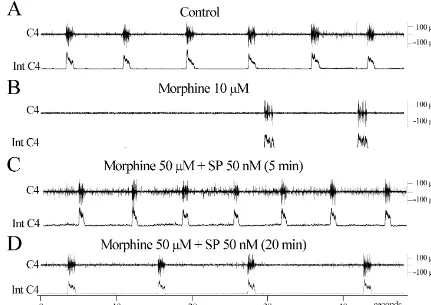
acetylcholine, 10 mM [14]; atropine, 10 mM [14]; sub- Acetylcholine (10 mM) (Fig. 1), substance P (50 nM) stance P, 50 nM [14,29] haloperidol, 10 mM [14]; theo- (Fig. 2), TRH (100 nM) and forskolin (10 mM) could phylline, 100mM [8]; forskolin, 10mM [1]; indomethacin, partly reverse the morphine-induced fR reduction (Table 10 mM [19]; quinacrine, 10 mM [19]; flumazenil 1 mM 1). The fR stimulant effect of acetylcholine was abolished [27]; TRH, 100 nM [14]. C4 ventral root activity was used by atropine 10mM (n53 / 3) (Fig. 1) while the effects of to calculate respiratory frequency (fR) and peak amplitude substance P and TRH were not affected by atropine (n54). of integrated C4 discharge (Int C4). Respiratory minute Atropine (10 mM) alone had no respiratory effects (%fR activity (RMA) was calculated using the product of fR and 99.868.6% and %Int C4 99.563.7%, n54). Apart from Int C4. The measurements for control values were taken 2 reversing the effects of morphine, Substance P, forskolin min before morphine application and 30 min after mor- and TRH increased tonic motor activity of C4 and in-phine application when steady-state effect of morin-phine had creased Int C4 (see Fig. 2).
been achieved. Neither progression of morphine effects nor Haloperidol (10mM, n55) did not reverse or potentate
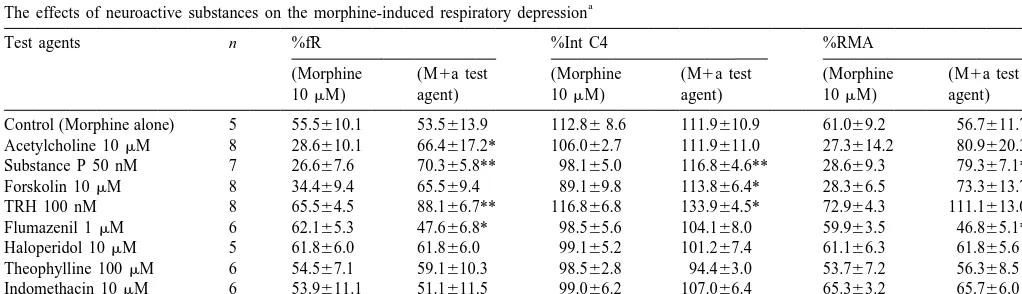
Table 1
a
The effects of neuroactive substances on the morphine-induced respiratory depression
Test agents n %fR %Int C4 %RMA
(Morphine (M1a test (Morphine (M1a test (Morphine (M1a test
10mM) agent) 10mM) agent) 10mM) agent)
Fig. 1. The effects of acetylcholine on the morphine-induced respiratory depression. (A) C4 activity (upper traces) and integrated C4 activity (lower traces) recorded from the in vitro preparation in standard solution. (B) 30 min after perfusion with morphine (10mM). (C) 30 min after perfusion with morphine (10 mM) and acetylcholine (10mM). (D) 10 min after perfusion with morphine (10mM), acetylcholine (10mM) and atropine (10mM).
morphine induced respiratory depression. Flumazenil (1 have been shown to induce depolarization in respiratory
mM), a benzodiazepine-antagonist, alone had no respirato- neurons (TRH, Ach, forskolin and SP) [7,11,16,20]. Thus, ry effects (%fR 96.363.3% and %Int. C4 95.966.1%) a possible explanation to our data could be that morphine (n53). However, flumazenil potentate morphine induced induced postsynaptic hyperpolarization of brainstem respi-fR reduction. Theophylline (100 mM), indomethacin (10 ratory neurons (Shinhiro Takeda et al., unpublished data)
mM) and quinacrine (10mM) did not reverse or potentate may be reversed by agents / modulators depolarizing the the morphine-induced respiratory depression (Table 1). affected neurons.
We show in the present study that TRH, Ach, SP and Opioids act presynaptically to inhibit the release of Forskolin may partly reverse the respiratory depression acetylcholine and substance P, and enhance the release of induced by morphine action in the medulla oblongata. dopamine and adenosine [9,12,18]. Our present results do Several in vivo studies have shown that anticholinesterases not support the idea that the morphine-induced fR reduc-and TRH can antagonize or reverse opioid-induced respira- tion was mainly due to inhibition of substance P and tory depression see ex. [10,28]. Our data are in agreement acetylcholine release. Because firstly, atropine alone did with these in vivo findings and indicate that their effects not affect fR and Int C4; and secondly, acetylcholine and are mediated through direct action in the medulla oblon- substance P could not completely reverse the morphine-gata. We furthermore suggest that activating SP-receptors induced fR reduction. Neither does enhancement of the may partly reverse respiratory depression induced by release of adenosine and dopamine seem to play a role in
morphine. the respiratory depression induced by morphine because an
Muscarin, Tachykinin and TRH receptors are all cou- adenosine receptor antagonist like theophylline and a pled to G-proteins.m-opioid and NK -receptors are present1 dopamine receptors antagonist like haloperidol did not on respiratory neurons in the proposed respiratory rhythm reverse the morphine-induced respiratory depression. generating centers in rostral ventrolateral medulla oblon- Hence, it is not likely that the morphine effects found in gata [7]. Also, postsynaptic Muscarin and TRH receptors this study were due to presynaptic effects.
are present on respiration-related neurons in the medulla The increased noise in the interburst period observed
oblongata [16,20]. during TRH, SP and forskolin application is in accordance
204 K. Takita et al. / Brain Research 884 (2000) 201 –205
Fig. 2. The effects of substance P on the morphine-induced respiratory depression. (A) C4 activity (upper traces) and integrated C4 activity (lower traces) recorded from the in vitro preparation in standard solution. (B) 30 min after perfusion with morphine (10mM). (C) 5 min after perfusion with morphine (10mM) and substance P (50 nM). D: 20 min after perfusion with morphine (10mM) and substance P (50 nM). Note the rapid reversal of morphine effect and the increased non-respiratory ventral root activity in C.
depolarization of motor neurons [16] and SP is well known Acknowledgements to be able to similarly induce such increased excitation
also in non-rhythm generating and non-respiratory neurons This work was supported by grants from the Swedish
in the spinal cord. Medical Research Council (SMFR 17X-10401, 19X-5234
Arachidonic acid metabolites are involved in respiratory and 14X-0907), the Swedish Society for Medical Re-control [2]. Administration of an intravenous cyclooxy- search, Swedish Freemason Children’s house foundation genase inhibitor, ketoprofen, may have an antagonistic and the Fraenckel Foundation for Medical Research. action on the morphine-induced respiratory depression in
humans [13]. Based on the results in the present study it was not possible to support these earlier results. Thus,
References arachidonic acid metabolites as prostaglandins and
leuko-trienes are probably not mediators of respiratory
depres-[1] A. Arata, H. Onimaru, I. Homma, Effects of cAMP on respiratory
sion via opioid receptors in the medulla oblongata of rhythm generation in brainstem–spinal cord preparation from
new-newborn rat. born rat, Brain Res. 605 (1993) 193–199.
Our present data of the inability of benzodiazepine [2] K. Ballanyi, P.M. Lalley, B. Hoch, D.W. Richter, Camp-dependent reversal of opioid- and prostaglandin-mediated depression of the
antagonist to reverse the effects of morphine are in
isolated respiratory network in newborn rats, J. Physiol. (Lond.) 504
accordance with a recent study showing that GABA-A
(1997) 127–134.
receptors are not crucially involved inm-receptor induced [3] Z. Chen, J. Hender, T. Hender, Local effects of substance P on depression of respiratory frequency [7]. respiratory regulation in the rat medulla oblongata, J. Appl. Physiol.
In summary; agents like acetylcholine, substance P, TRH 68 (1989) 693–699.
´ ´
[4] J. Florez, M.A. Hurle, Opioids in respiration and vomiting, in: A.
and forskolin, which are known to depolarize
respiration-Herz (Ed.), Hand book of Experimental pharmacology, Opioids II,
related neurons, reversed morphine-induced fR reduction.
Springer-Verlag, New York, 1993, pp. 263–292.
Adenosine, dopamine agonists / antagonists, a cyclooxy- [5] E.A. Frey, J.W. Kebabian, A mu-opiate receptor in 7315c tumor genase and a phospholipase A inhibitor did not reverse2 tissue mediates inhibition of immunoreactive prolactin release and
[6] K. Fukuda, S. Kato, H. Morikawa, T. Shoda, K. Mori, Functional [18] J. Sawynok, M.I. Sweeney, T.D. White, Adenosine release may coupling of the delta-, mu-, and kappa-opioid receptors to mitogen- mediate spinal analgesia by morphine, Trends Pharmacol. Sci. 10 activated protein kinase and arachidonate release in Chinese hamster (1989) 186–189.
ovary cells, J. Neurochem. 67 (1996) 1309–1316. [19] P. Schweitzer, S. Madamba, G.R. Siggins, Arachidonic acid metabo-[7] P.A. Gray, J.C. Rekling, C.M. Bocchiaro, J.L. Feldman, Modulation lites as mediators of somatostatin-induced increase of neuronal
of respiratory frequency by peptidergic input to rhythmogenic M-current, Nature 346 (1990) 464–467. ¨
neurons in the Pre-botzinger complex, Science 286 (1999) 1566– [20] X.M. Shao, J.L. Feldman, Acetylcholine modulates respiratory ¨
1568. pattern: effects mediated by M3- like receptors in preBotzinger
[8] E. Herlenius, H. Lagercrantz, Y. Yamamoto, Adenosine modulates complex inspiratory neurons, J. Neurophysiol. 83 (2000) 1243– inspiratory neurons and the respiratory pattern in the brainstem of 1252.
neonatal rats, Pediatric Res. 42 (1997) 46–53. [21] R. Spanagel, A. Herz, T.S. Shippenberg, The effects of opioid [9] T.M. Jessell, L.L. Inversen, Opiate analgesics inhibit substance P peptides on dopamine release in the nucleus accumbens: an in vivo
release from rat trigeminal nucleus, Nature 268 (1977) 549–551. microdialysis study, J. Neurochem. 55 (1990) 1734–1740. [10] D.A. Kharkevich, B.A. Chizh, S.A. Kasparov, Stimulant effect of [22] M. Srinivasan, H. Lagercrantz, Y. Yamamoto, A possible
dopa-thyrotropin-releasing hormone and its analog, RGH 2202, on the minergic pathway mediating hypoxic depression in neonatal rabbits, diaphragm respiratory activity, and their antagonism with morphine: J. Appl. Physiol. 67 (1989) 1271–1276.
possible involvement of the N-methyl-D-asparatate receptors, Brain [23] T. Suzue, Respiratory rhythm generation in the in vitro brainstem– Res. 551 (1991) 110–115. spinal cord preparation of the neonatal rat, J. Physiol. (Lond.) 354 [11] P.M. Lalley, O. Pierrefiche, A.M. Bischoff, D.W. Richter, cAMP- (1984) 173–183.
Dependent protein kinase modulates expiratory neurons in vivo, J. [24] K. Taguchi, Y. Hagiwara, Y. Suzuki, T. Kubo, Effects of morphine Neurophysiol. 77 (1997) 1119–1131. on release of acetylcholine in the rat striatum: an in vivo mi-[12] R. Lydic, J.C. Keifer, H.A. Baghdoyan, L. Becker, Microdialysis of crodialysis study, Naunyn-Schmiedebergs Arch. Pharmacol. 347
the pontine reticular formation reveals inhibition of acetylcholine (1993) 9–13.
release by morphine, Anesthesiology 79 (1993) 1003–1012. [25] K. Takita, E. Herlenius, S. Lindahl, Y. Yamamoto, Actions of [13] J. Moren, T. Francois, Y. Blanloeil, M. Pinaud, The effects of a opioids on respiratory activity via activation of brainstemm-,d- and
nonsteroidal antiinflammatory drug (ketoprofen) on morphine respi- k-receptors; an in vitro study, Brain Res. 778 (1997) 233–241. ratory depression: a double-blind, randomized study in volunteers, [26] K. Takita, E. Herlenius, S. Lindahl, Y. Yamamoto, Age and Anesthesia & Analgesia 85 (1997) 400–405. temperature dependent effects of opioids on medulla oblongata [14] T. Murakoshi, T. Suzue, S. Tamai, A pharmacological study on respiratory activity: An in vitro study in newborn rat, Brain Res. 800
respiratory rhythm generation in the in isolated brainstem–spinal (1998) 308–311.
cord preparation of the newborn rat, Br. J. Pharmacol. 86 (1985) [27] P.M. Verbanck, V. Seutin, L. Massotte, A. Dresse, Differential effects
95–104. of picrotoxin and RO 15-1788 on high and low ethanol
con-[15] P. Paakkari, I. Paakkari, P. Landes, A.L. Siren, G. Feuerstein, centrations on rat locus coeruleus in vitro, Eur. J. Pharmacol. 211 Respiratory mu-opioid and benzodiazepine interactions in the unre- (1992) 15–21.
strained rat, Neuropharmacology 32 (1993) 323–329. [28] R.N. Willette, B.M. Doorley, H.N. Sapru, Activation of cholinergic [16] J. Rekling, J. Champagnat, M. Denavit-Saubie, Thyrotropin-releas- mechanisms in the medulla oblongata reverse intravenous opioid-ing hormone (TRH) depolarizes a subset of inspiratory neurons in induced respiratory depression, J. Pharmacol. Exp. Ther. 240 (1987) the newborn mouse brain stem in vitro, J. Neurophysiol. 75 (1996) 352–358.