www.elsevier.comrlocateranireprosci
Influence of the nutritional status on ovarian
development in female pigs
A. Prunier
), H. Quesnel
Unite Mixte de Recherches sur le Veau et le Porc, INRA, 35590 Saint-Gilles, France´
Abstract
In female pigs, undernutrition may influence growth of antral follicles from various size classes, decrease ovulation rate, delay puberty and return to oestrus after weaning. It could also affect the oocyte maturation and hence the number of viable embryos per litter. Inhibition of the gonadotrophin release due to undernutrition is presumably involved in these phenomena. Presence of receptors, as well as in vitro and in vivo studies suggest that insulin and hormones from the somatotrophic axis are able to alter folliculogenesis directly at the ovarian level. They should act as hormones controlling nutrition, proliferation, growth and differentiation of the cells andror as amplifiers of the action of gonadotrophins. Information are needed to determine whether their availability at the ovarian level may become insufficient or excessive in case of nutritional deficit. Increase in plasma concentrations of progesterone due to lower hepatic metabolic rate in underfed females probably contributes to inhibit folliculogenesis.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Gilt; Sow; Ovarian follicle; Nutrition; Physiology
1. Introduction
Numerous data from various mammalian species have shown that nutrition may
influence the reproductive function. Most experimental studies involving farm animals
have focussed on the negative consequences of undernutrition on reproductive
perfor-mance. This is particularly true in the pig species and is related to the fact that
nutritional deficit may occur spontaneously in gilts and adult sows under farm
condi-Ž
.
tions
Prunier and Quesnel, 2000 . Physiological mechanisms explaining the links
)Corresponding author. Tel.:q33-2-23-48-50-56; fax:q33-2-23-48-50-80.
Ž .
E-mail address: [email protected] A. Prunier .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
between nutrition and reproduction are partly known. It is clear that the GnRH pulse
Ž
generator system and the gonadotrophin release are inhibited by undernutrition for
.
review, see Booth, 1990; Cameron, 1996; Wade et al., 1996 . As a consequence, the
gonadotrophin support to folliculogenesis may become insufficient. In addition, metabolic
Ž
.
mediators hormones or metabolites may act directly at the ovarian level and amplify
Ž
the gonadotrophin-mediated effects of nutrition for review, see Downing and
Scara-.
muzzi, 1991; Cosgrove and Foxcroft, 1996; Prunier and Quesnel, 2000 . However,
doubts concerning the nature and the relative importance of the physiological
mecha-nisms explaining the effects of nutrition directly at the ovarian level are still existing.
Therefore, the present paper will focus on these potential mechanisms, after a review of
the consequences of undernutrition on folliculogenesis and ovulation. We will
concen-trate on the pig species since inadequate nutrition of gilts and sows is thought to play a
Ž
.
major role in reproductive disturbances Dourmad et al., 1994 .
2. Effects of nutrition on the ovarian activity
2.1. Folliculogenesis
To our knowledge, there are only two reports relating the effects of nutrition on the
overall population of antral follicles: one in prepubertal gilts from two different
Ž
.
genotypes Dufour et al., 1985 and the second in primiparous sows before and shortly
Ž
.
after weaning Quesnel et al., 1998a . Results failed to show any influence of feed
restriction on the repartitioning of healthy follicles between size classes in prepubertal
gilts from one genotype and in luteal-phase gilts. Contrarily, in prepubertal gilts from
Ž
.
the second genotype and in adult sows, the proportion of small 0.4 to 1.0 mm healthy
follicles to the total number of antral follicles was increased in feed-restricted females at
Ž
.
the expense of the proportion of bigger healthy follicles Fig. 1 . Regardless of its
influence on the repartitioning of the follicles between size classes and whatever the
Fig. 1. Average proportion of healthy antral follicles per size class to the total number of antral follicles in
Ž . )
Ž
physiological stage, nutrition had no effect on the rate of atresia prepubertal gilts:
Dufour et al., 1985; cyclic gilts: Quesnel et al., unpublished data; lactating and weaned
.
sows: Quesnel et al., 1998a .
The effects of feed restriction or of realimentation after feed restriction on
character-istics of the follicles present at the ovarian surface have been studied in prepubertal gilts
Ž
Cosgrove et al., 1992; Charlton et al., 1993; Booth et al., 1994, 1996 and in sows
.
Ž
.
around weaning Quesnel et al., 1998a . These follicles measured
G
2 mm in diameter
Ž
.
and were healthy or atretic. In one study Cosgrove et al., 1992 , gilts received a
progestagen that blocks LH pulsatility but may also alter follicular growth and
matura-Ž
.
tion directly at the ovarian level Guthrie and Bolt, 1990 . All these studies have shown
that growth of the surface follicles was influenced by the feeding regimen. For instance,
the mean diameter and volume of the 10 largest follicles were decreased in underfed
Ž
females, regardless of the physiological stage and the use of a progestagen Cosgrove et
.
al., 1992; Charlton et al., 1993; Quesnel et al., 1998a . However, the biochemical
characteristics of these follicles, such as the follicular fluid concentrations of oestradiol
Ž
and testosterone, the in vitro synthesis of oestradiol, the thecal binding of hCG human
.
Ž
chorionic gonadotrophin were not clearly modified by the pattern of feeding Cosgrove
.
et al., 1992; Charlton et al., 1993 .
Feed intake may influence the ability of the oocytes to be fertilized and
r
or to
Ž
.
develop into viable embryos as suggested by Zak et al. 1997a . These authors have
shown that the percentage of corpora lutea represented by an embryo at day 28 of
Ž
.
gestation
s
embryo survival was low in sows submitted to feed restriction during their
last week of lactation and inseminated at their first oestrus after weaning. This effect of
undernutrition may be due to an impaired quality of the oocytes which may, itself, be
Ž
.
related to a poor ability of the follicles to support oocyte maturation Zak et al., 1997b .
These hypotheses were tested in vitro by comparing the rate of maturation of oocytes
harvested in sows submitted or not to feed restriction during their last week of lactation,
as well as the ability of the follicular fluid, recovered from the same sows, to support
Ž
.
oocyte maturation Zak et al., 1997b . Data showed advantages for both oocytes and
follicular fluid recovered from preovulatory follicles of non-restricted sows. However, it
should be noted that the duration between weaning and ovarian collection was different
in the two groups of sows, assuming that oestrus and preovulatory surge of LH may
Ž
.
occur 12 h later in non-restricted than in restricted sows Zak et al., 1997a . Moreover,
the reduction in embryo survival associated with feed restriction during the last week of
lactation was no more observed in a more recent experiment from the same laboratory
Ž
Mao et al., 1999 .
.
Therefore, it seems that nutrition may influence growth of antral follicles from
various sizes but consequences on their ability to further mature or to become atretic are
still not clear. Similarly, experiments are needed to definitively conclude on the
consequences of undernutrition on the oocyte maturation.
2.2. O
Õ
ulation occurrence
is generally inferred from oestrus detection with a mature boar, the occurrence of
‘‘silent’’ ovulation and of oestrus without ovulation being observed in only a minority of
Ž
.
gilts Eliasson, 1991 .
In prepubertal gilts, feed restriction delays the occurrence of first ovulation in most
Ž
.
Ž
studies Table 1 . In cyclic gilts, very low level of feeding daily maintenance levels of
essential amino acids and of protein but less than 25% of the daily energy requirements
.
Ž
for maintenance was able to cause cessation of the ovulatory oestrous cycles Armstrong
.
and Britt, 1987; Rozeboom et al., 1993 . However, gilts experienced three cycles in
average before becoming anoestrus. Duration of these cycles was normal and
proges-terone levels during the luteal phase were within a common range. A less severe level of
Ž
.
energy restriction about 75% of the energy requirements for maintenance applied
Ž
during six to seven oestrous cycles was not sufficient to induce anoestrus Rozeboom et
.
al., 1993 .
In most studies, feed restriction during lactation delays the occurrence of oestrus in
Ž
.
sows after weaning Table 2 . Similarly, protein restriction during lactation prolongs the
Ž
weaning-to-oestrus interval in primiparous sows King and Williams, 1984a; Jones and
.
Ž
Stahly, 1999 . In contrast, feed restriction after weaning does not delay oestrus Table
.
2 . However, it should be noted that, in this latter situation, feed supply covers more
than 100% of the requirements for maintenance in both control and restricted sows.
Table 1
Influence of feed restriction during growth on the age at puberty of gilts† ‡
Ž .
Reference Feed supply %
300 to 250 250 to 200 200 to 150 150 to 100
( )
Age at first oestrus or at first oÕulation days
Friend et al., 1981
Den Hartog and Noordewier, 1984 234 237 244 –
a b )
Beltranena et al., 1991 170 187 – –
( ) Percentage of cyclic gilts at a fixed age %
Prunier et al., 1987 – 30 20 – ns
a a b )
Newton and Mahan, 1992 91 89 77 –
a b )
Prunier et al., 1993 40 – 0 –
† Ž
Data published before 1980 were discarded since characteristics of female pigs reproductive and growth
.
performance, metabolic body reserves have greatly changed from that period.
‡ Ž .
Feed supply % is the estimated ratio between metabolic energy intake and requirements for maintenance
ŽMAINT . When this ratio was not indicated in the publication, it was necessary to calculate it. The following.
Ž .
equations derived from Noblet et al. 1999 were used: metabolic energy intakes0.95=digestible energy
Ž . Ž .0.60
intake; MAINT MJs1.0=liveweight kg . )
P-0.05, ns P)0.05 within a row, between feeding levels. a,bWithin a row, means with a different superscripts differ at P
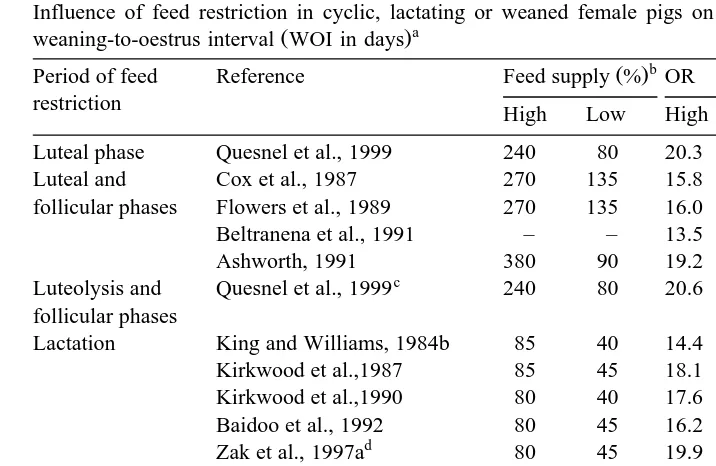
Table 2
Ž .
Influence of feed restriction in cyclic, lactating or weaned female pigs on the ovulation rate OR and the
Ž .a
weaning-to-oestrus interval WOI in days
b Ž .
Period of feed Reference Feed supply % OR WOI
restriction High Low High Low High Low
Luteal phase Quesnel et al., 1999 240 80 20.3 20.3 – – )
Luteal and Cox et al., 1987 270 135 15.8 13.4 – –
)
follicular phases Flowers et al., 1989 270 135 16.0 9.4 – – )
Beltranena et al., 1991 – – 13.5 11.1 – – )
Ashworth, 1991 380 90 19.2 14.2 – –
c )
Luteolysis and Quesnel et al., 1999 240 80 20.6 16.9 – – follicular phases
) Lactation King and Williams, 1984b 85 40 14.4 13.4 7.6 19.9
) Kirkwood et al.,1987 85 45 18.1 18.6 4.3 5.8
) Kirkwood et al.,1990 80 40 17.6 17.7 6.0 8.9 Baidoo et al., 1992 80 45 16.2 16.7 5.9 7.5
d ) )
Zak et al., 1997a 80 45 19.9 15.4 3.7 5.6
Quesnel and Prunier, 1998 90 60 19.2 20.7 5.7 5.9 )
After weaning Den Hartog and – – 15.2 14.8 9.1 8.2
van der Steen, 1981
)
King and Williams, 1984b 285 115 14.6 13.2 13.4 14.1 Baidoo et al., 1992 245 155 16.6 16.2 6.0 5.9
)
P-0.05 between control and feed-restricted groups, within criterium.
a Ž
Data published before 1980 were discarded since characteristics of female pigs reproductive and growth
.
performance, metabolic body reserves have greatly changed from that period.
b Ž .
Feed supply % is the estimated ratio between metabolic energy intake and requirements for maintenance
ŽMAINT in prepubertal, mature and pregnant gilts, and in sows restricted after weaning or for maintenance. q
Ž .
milk production MAINTqMILK, in lactating sows . When this ratio was not indicated in the publication, it
Ž .
was necessary to calculate it. The following equations derived from Noblet et al. 1990, 1999 were used:
Ž . Ž .0.60
metabolic energy intakes0.95=digestible energy intake; MAINT MJs1.0=liveweight kg for
prepu-Ž . Ž .0.75
bertal and mature gilts; MAINT MJs0.44=liveweight kg for pregnant gilts and weaned sows;
Ž . Ž .0.75 Ž .
MAINTqMILK MJs0.46=liveweight kg q28.59=daily gain of the litter kgy0.52 for lactating sows.
c
Number of follicles larger than 5 mm, 5 days after induced luteolysis. d
Feed restriction was limited to the last week of lactation.
e Ž .
Sows with delayed return to oestrus after weaning )10 days were discarded.
In summary, oestrous cycles can be disrupted in cyclic gilts only in case of very
severe and prolonged feed restriction. Delayed ovulation at puberty and after weaning
may occur in less drastic conditions. Therefore, under farm conditions, inadequate
appetite is suspected to have negative effects on the age at puberty and on the
weaning-to-oestrus interval.
2.3. O
Õ
ulation rate
Feed restriction of growing gilts before puberty has no marked effect on the ovulation
Ž
.
Ž
.
Ž
.
any significant difference between feeding levels, whereas King 1989 observed a lower
ovulation rate in underfed females. In cyclic gilts, imposing feed restriction only during
the luteal phase seems to have no effect on the number of corpora lutea at the
Ž
.
subsequent ovulation Table 2 . Contrarily, feed restriction starting during the luteal
phase or at luteolysis and maintained during the follicular phase reduces the ovulation
rate at next oestrus. In this case, feed restriction occurs, at least, during recruitment and
selection of the preovulatory follicles.
In reproductive sows, most researchers have determined the effects of feeding level
during lactation on ovulation rate after weaning. Therefore, they have imposed the
nutritional deficit before recruitment of the preovulatory follicles. This might explain
Ž
.
why, in most studies, feed restriction has no clear effect on ovulation rate Table 2 .
Ž
.
Likewise, King and Williams 1984a showed similar ovulation rates in primiparous
sows submitted or not to protein restriction during lactation. Few studies have evaluated
Ž
the effects of feed intake between weaning and oestrus
s
during and after recruitment
.
and selection . Out of the three studies reported, only one showed an altered ovulation
Ž
.
rate in the restricted group Table 2 .
Therefore, it seems that, in cyclic gilts, the critical period for the ovulation rate is at
luteolysis and during the follicular phase, i.e., during recruitment and selection of the
preovulatory follicles. In reproductive sows, more studies are necessary to elucidate
whether nutrition may also influence the ovulation rate by focussing experiments around
Ž
.
weaning day of weaning and the following days .
3. Physiological mechanisms mediating the effects of nutrition directly at the
ovarian level
Besides the effects of nutrition on the release of gonadotrophins and their
conse-quences on folliculogenesis, metabolic mediators may act directly at the ovarian level. In
the pig as in other species, undernutrition leads to a decrease in circulating insulin,
Ž
.
insulin-like growth factor-I IGF-I and leptin and a rise in circulating growth hormone
Ž
GH
. Ž
Prunier and Quesnel, 2000 . Contrarily, undernutrition does not significantly
.
reduce systemic glycemia, a tightly controlled parameter in the pig. Nutritional deficit
Ž
.
may decrease plasma concentration of binding proteins for the IGFs IGFBPs but
Ž
variations are less marked than for IGF-I
Thissen et al., 1994; Louveau et al.,
.
unpublished data .
Nutrition may also influence the ovarian activity via other pathways. For instance,
feed intake may affect the hepatic clearance rate of steroid hormones and hence the
circulating concentrations of these hormones which, themselves, may act on
folliculoge-nesis.
3.1. The influence of the IGF system
The stimulating role of IGF-I on folliculogenesis, alone or in synergy with
go-Ž
nadotrophins, has been well demonstrated by in vitro experiments for review, see
.
system during follicular maturation. In sows, concentrations of IGF-I and the ratio of
IGFBP-3 to BP-2 and BP-4 increase during follicular growth, whereas concentrations of
IGFBP-2 and BP-4 increase during atresia. These changes in follicular IGFBPs, which
could be explained by changes in their ovarian expression and
r
or in the proteolytic
Ž
.
activity degrading them, are likely to modulate the IGF-I effects Besnard et al., 1997 .
Feed restriction decreases plasma IGF-I concentrations, by ‘‘uncoupling’’ systemic
GH and IGF-I secretions. Refeeding restricted gilts increases systemic concentrations of
Ž
.
IGF-I without changing follicular levels
Charlton et al., 1993 . In contrast, feed
restriction during lactation simultaneously decreases systemic and intrafollicular levels
Ž
.
of IGF-I Quesnel et al., 1998a,b . Low concentrations of IGF-I in plasma and
r
or in
follicular fluid have been associated with reduced ovulation rate or impaired
folliculoge-Ž
.
nesis Cosgrove et al., 1992; Booth et al., 1994; Quesnel et al., 1998a . Indeed, as IGF-I
amplifies FSH action, low levels of IGF-I in feed-restricted sows may alter follicular
recruitment. However, further studies are needed to check the influence of nutrition on
ovarian IGFBPs and proteases to clearly estimate the bioavailability of IGF-I according
to the metabolic status.
3.2. The influence of insulin
In mammals, insulin stimulates in vitro uptake and utilization of nutrients, granulosa
Ž
cell proliferation and differentiation, alone or in synergy with gonadotrophins for
.
review, see Poretsky and Kalin, 1987 . It also prevents in vitro apoptosis in pig
Ž
.
granulosa cells Rein and Schomberg, 1982; Purvis et al., 1997 . Recently, binding sites
Ž
.
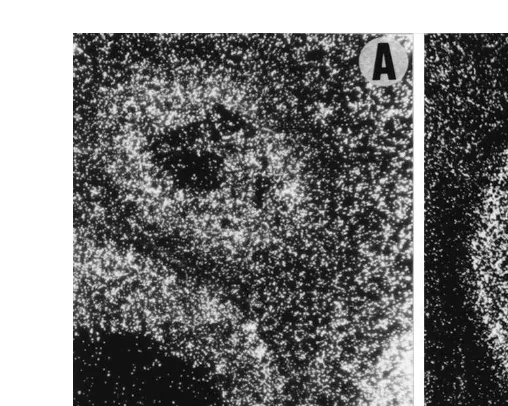
for insulin have been detected in pig ovaries Quesnel, 1999; Fig. 2 . In addition to these
in vitro studies, numerous in vivo experiments have provided evidence of a stimulating
role of insulin in folliculogenesis. For instance, increasing plasma insulin, by treatment
Ž
.
or overfeeding flushing starting in the late luteal phase or the early follicular phase,
Ž
.
increased ovulation rate irrespective of changes in plasma LH Cox et al., 1987 . This
may be related to the ability of insulin to decrease atresia in small- and medium-sized
Ž
.
follicles Matamoros et al., 1990, 1991 . Gilts with streptozotocin-induced diabetes
exhibited a high percentage of atretic follicles and low levels of IGF-I in large follicles,
Ž
compared with normo-glycemic gilts Meurer et al., 1991; Cox et al., 1994; Edwards et
.
al., 1996 . Moreover, withdrawal of insulin therapy in diabetic gilts resulted in an
increased rate of atresia, lowered IGF-I levels in follicles and altered pattern of IGFBPs,
Ž
.
without decrease in gonadotrophin secretion Cox et al., 1994; Edwards et al., 1996 .
Taken together, these studies suggest that insulin may increase ovulation rate by
reducing atresia through enhanced levels of intrafollicular IGF-I.
However, treating primiparous sows with long-acting insulin during the last days of
Ž
lactation has been shown to be without effect on ovulation rate Quesnel and Prunier,
.
1998 , whereas treatment starting the day before weaning resulted in a lower ovulation
Ž
.
rate Rojkittikhun et al., 1993b . Moreover, insulin treatment during the 5 days after
weaning had no effect on the number of preovulatory follicles and on atresia in
Ž
.
primiparous sows Whitley et al., 1998a,b . This treatment stimulated steroidogenesis
Ž
.
without altering ovarian IGF-I and IGFBPs in one experiment Whitley et al., 1998a ,
but resulted in lowered levels of oestradiol and IGF-I and increased levels of small
Ž
.
Ž
.
Ž . Ž .
Fig. 2. Localization of binding sites for insulin A and GH B in swine ovary by in situ binding and
Ž .
autoradiography. Binding of radiolabelled insulin white grains is observed in granulosa and thecal cells of
Ž . Ž .
healthy antral follicles -1 mm: upper; 2 mm: lower, left and atretic antral follicle lower, right , and also in
Ž .
stromal cells. Binding of radiolabelled GH white grains is observed in the oocyte, granulosa cells and theca
Ž . Ž .
interna cells of a preantral follicle and, although less intense, in stromal cells.=70 A ,=120 B .
during 5 days starting at the induction of luteolysis in gilts had no effect on the number
Ž
of preovulatory follicles and on their levels of IGF-I and oestradiol Quesnel et al.,
.
unpublished data .
In summary, despite stimulatory effects of insulin on nutrition, growth and
develop-ment of follicular cells, the consequences of high circulating levels of insulin on
follicular biochemistry and ovulation rate are controversial and vary between studies
from positive to negative. Variation in the metabolic and hormonal background may
explain the discrepancies between studies. Finally, direct evidence is still missing to
demonstrate that low level of insulin in feed-restricted animals is responsible for low
ovulation rate.
3.3. The influence of GH
In vitro studies have shown that GH stimulates mitogenesis and amplifies the effects
of FSH on the induction of LH receptors and on steroidogenesis by granulosa cells in
Ž
.
numerous species for review, see Booth, 1990 . Binding sites for GH have been
Ž
.
recently detected in pig ovaries Fig. 2 , being very abundant in preantral and small
Ž
antral, less abundant in larger follicles and undetectable in atretic follicles Quesnel,
.
1999 . In vivo manipulation of GH levels has led to contradictory results. Treatment of
cyclic gilts with exogenous GH suppressed oestrus or increased ovulation rate in gilts
Ž
.
Ž
.
positive effects on the number of medium-sized follicles 4–6.9 mm and on the
Ž
concentration of IGF-I in plasma and follicular fluid of prepubertal gilts Echternkamp
.
et al., 1994 . In contrast, over expression of GH in transgenic prepubertal gilts decreased
Ž
.
the number of oestrogenic follicles
)
5 mm and their ability to synthetize oestradiol
Ž
Guthrie et al., 1993 , and GH treatment for 5 days after weaning did not influence the
.
Ž
.
number of oestrogenic follicles Whitley et al., 1998a . Most of these treatments induced
Ž
.
an increase in plasma and probably follicular IGF-I, in one hand, and, in the other
hand, a rise in both plasma insulin and glucose, suggesting an insulin resistance. It may
be hypothesized that stimulatory influence of GH on ovulation rate is mediated by high
IGF-I, whereas inhibitory influence is related to insulin resistance at the ovarian level.
However, the mechanisms whereby one of these ways predominates are not known.
3.4. The in
Õ
ol
Õ
ement of leptin
To our knowledge, data concerning the effects of leptin at the ovarian level are
lacking in the female pig. Leptin receptor mRNA has been identified in human ovary
Ž
.
tissue Cioffi et al., 1996 . However, this mRNA codes for the short form of the leptin
receptor and doubts exist concerning the efficiency of this truncated form to transduce
Ž
.
the hormonal signal for review, see Houseknecht et al., 1998 . Leptin inhibits in vitro
secretion of steroids by granulosa or theca cells stimulated by insulin, IGF-I or
Ž
gonadotrophins human ovary: Zachow and Magoffin, 1997; bovine ovary: Spicer and
.
Francisco, 1997, 1998 . Such in vitro inhibitory effects of leptin at the ovarian level
contrast with the in vivo and in vitro stimulatory influence of leptin on gonadotrophin
Ž
.
release demonstrated in rodents for review, see McCann et al., 1998 .
3.5. The progesterone hypothesis
It has been demonstrated that hepatic portal blood flow and metabolic clearance rate
of progesterone are decreased in ovariectomized gilts submitted to feed restriction
Ž
Prime and Symonds, 1993 . Moreover, higher concentrations of progesterone have been
.
Ž
observed in feed-restricted than in well-fed gilts at the beginning of gestation Dyck et
.
al., 1980; Dyck and Kennedy, 1995; Jindal et al., 1996 . Decline in plasma progesterone
Ž
at prostaglandin-induced luteolysis is lengthened in feed-restricted gilts Prunier et al.,
.
1999 . High levels of progesterone may impair folliculogenesis through the inhibition of
LH pulsatility, but also through inhibition of aromatase activity, and thus of follicular
Ž
.
maturation, directly at the ovarian level Guthrie and Bolt, 1990; Flowers et al., 1991 .
4. Conclusion
also reduce the ovulation rate in weaned sows and alter the oocyte maturation in
ovulatory females and hence their potential litter size. Metabolic hormones such as
insulin, GH and IGF-I belong to the hormonal milieu that controls the activity of the
follicular cells. Determining whether their availability at the ovarian level may become
insufficient or excessive in case of nutritional deficit is necessary. Nutritionally 1–1
related variations in blood supply to the ovaries as well as in the hepatic clearance rate
of sexual steroids probably modify the hormonal environment of the follicular cells. The
relative importance of these physiological mechanisms is not known and requires further
studies.
References
Adashi, E.Y., Resnick, C.E., D’Ercole, A.J., Svoboda, M.E., Van Wyk, J.J., 1985. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr. Rev. 6, 400–420.
Armstrong, J.D., Britt, J.H., 1987. Nutritionally induced anestrus in gilts: metabolic and endocrine changes associated with cessation and resumption of estrous cycles. J. Anim. Sci. 65, 508–523.
Ashworth, C.J., 1991. Effect of pre-mating nutritional status and post-mating progesterone supplementation on embryo survival and conceptus growth in gilts. Anim. Reprod. Sci. 26, 311–321.
Baidoo, S.K., Aherne, F.X., Kirkwood, R.N., Foxcroft, G.R., 1992. Effect of feed intake during lactation on sow reproductive performance. Can. J. Anim. Sci. 72, 911–917.
Beltranena, E., Foxcroft, G.R., Aherne, F.X., Kirkwood, R.N., 1991. Endocrinology of nutritional flushing in gilts. Can. J. Anim. Sci. 71, 1063–1071.
Besnard, N., Pisselet, C., Monniaux, D., Monget, P., 1997. Proteolytic activity degrading insulin-like growth factor-binding protein-2,-3,-4, and-5 in healthy growing and atretic follicles in the pig ovary. Biol. Reprod. 56, 1050–1058.
Booth, P.J., 1990. Metabolic influences on hypothalamic-pituitary-ovarian function in the pig. J. Reprod.
Ž .
Fertil. Suppl. 40 , 89–100.
Booth, P.J., Craignon, J., Foxcroft, G.R., 1994. Nutritional manipulation of growth and metabolic and reproductive status in prepubertal gilts. J. Anim. Sci. 72, 2415–2424.
Booth, P.J., Cosgrove, J.R., Foxcroft, G.R., 1996. Endocrine and metabolic responses to realimentation in feed-restricted prepubertal gilts: associations among gonadotropins, metabolic hormones, glucose, and uteroovarian development. J. Anim. Sci. 74, 840–848.
Cameron, J.L., 1996. Regulation of reproductive hormone secretion in primates by short-term changes in nutrition. Rev. Reprod. 1, 117–126.
Charlton, S.T., Cameron, B.J., Glimm, D.R., Foxcroft, G.R., Kennelly, J.J., 1993. Insulin-like growth factor 1
ŽIGF-1 gene expression in porcine ovarian tissue. Can. J. Anim. Sci. 73, 253–257..
Cioffi, J.A., Shafer, A.W., Zupancic, T.J., Smith-Gbur, J., Mikhail, A., Platika, D., Snodgrass, H.R., 1996. Novel B219rOB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat. Med. 2, 585–588.
Cosgrove, J.R., Foxcroft, G.R., 1996. Nutrition and reproduction in the pig: ovarian aetiology. Anim. Reprod. Sci. 42, 131–141.
Cosgrove, J.R., Tilton, J.E., Hunter, M.G., Foxcroft, G.R., 1992. Gonadotropin-independent mechanisms participate in ovarian responses to realimentation in feed-restricted prepubertal gilts. Biol. Reprod. 47, 736–745.
Cox, N.M., Stuart, M.J., Althen, T.G., Bennett, W.A., Miller, H.W., 1987. Enhancement of ovulation rate in gilts by increasing dietary energy and administering insulin during follicular growth. J. Anim. Sci. 64, 507–516.
Den Hartog, L.A., van der Steen, H.A.M., 1981. Reproductive traits in primiparous sows in relation to feeding level. Neth. J. Agric. Sci. 29, 285–286.
Den Hartog, L.A., Noordewier, G.W., 1984. The effect of energy intake on age at puberty in gilts. J. Agric. Sci. 32, 263–280.
Dourmad, J.Y., Prunier, A., Etienne, M., Le Jossec, P., 1990. Influence des apports energetiques et proteiques´ ´ ´
sur les performances de croissance, la composition corporelle et le developpement corporel de jeunes truies´
destinees a la reproduction. Journ. Rech. Porcine Fr. 23, 251–258.´ `
Dourmad, J.Y., Etienne, M., Prunier, A., Noblet, J., 1994. The effect of energy and protein intake of sows on their longevity: a review. Livest. Prod. Sci. 40, 87–97.
Downing, J.A., Scaramuzzi, R.J., 1991. Nutrient effects on ovulation rate, ovarian function and metabolic
Ž .
hormones in sheep. J. Reprod. Fertil. Suppl. 43 , 209–227.
Dufour, J.J., Fahmy, M.H., Flipot, P.M., 1985. Follicular development during the prepuberal period of different morphological types of ovaries in Hampshire and Yorkshire gilts fed two planes of nutrition. J. Anim. Sci. 61, 1201–1210.
Dyck, G.W., 1989. Influence of sire, dietary intake and housing facilities on the attainment of puberty in crossbred gilts. Can. J. Anim. Sci. 69, 939–946.
Dyck, G.W., Kennedy, A.D., 1995. The effect of level of diet intake after mating on the serum concentration of thyroxine, triiodothyronine, growth hormone, insulin and glucose, and embryonic survival in the gilt. Can. J. Anim. Sci. 75, 315–325.
Dyck, G.W., Palmer, W.M., Simaraks, S., 1980. Progesterone and luteinizing hormone concentration in serum of pregnant gilts on different levels of feed consumption. Can. J. Anim. Sci. 60, 877–884.
Echternkamp, S.E., Spicer, L.J., Klindt, J., Vernon, R.K., Yen, J.T., Buonomo, F.C., 1994. Administration of porcine somatotropin by a sustained-release implant: effects on follicular growth, concentrations of steroids and insulin-like growth factor-I, and insulin-like growth factor binding protein activity in follicular fluid of control, lean and obese gilts. J. Anim. Sci. 72, 2431–2440.
Edwards, J.L., Hughey, T.C., Moore, A.B., Cox, N.M., 1996. Depletion of insulin in
streptozocin-induced-di-Ž .
abetic pigs alters estradiol, insulin-like growth factor IGF -I and IGF binding proteins in cultured ovarian follicles. Biol. Reprod. 55, 775–781.
Eliasson, E., 1991. Relationships between puberty and production traits in the gilt: 2. Oestrous symptoms at puberty. Anim. Reprod. Sci. 25, 255–264.
Etienne, M., Camous, S., Cuvillier, A., 1983. Effets de restrictions alimentaires pendant la croissance des truies sur leur maturite sexuelle et leur reproduction ulterieure. Reprod. Nutr. Dev. 23, 309–319.´ ´
Flowers, B., Martin, M.J., Cantley, T.C., Day, B.N., 1989. Endocrine changes associated with a
dietary-in-Ž .
duced increase in ovulation rate flushing in gilts. J. Anim. Sci. 67, 771–778.
Flowers, B., Cantley, T.C., Martin, M.J., Day, B.N., 1991. Episodic secretion of gonadotrophins and ovarian steroids in jugular and utero-ovarian vein plasma during the follicular phase of the oestrous cycle in gilts. J. Reprod. Fertil. 91, 101–112.
Friend, D.W., Lodge, G.A., Elliot, J.I., 1981. Effects of energy and dry matter intake on age, body weight and backfat at puberty and on embryo mortality in gilts. J. Anim. Sci. 53, 118–124.
Giudice, L.C., 1992. Insulin-like growth factors and ovarian follicular development. Endocr. Rev. 13, 641–669.
Guthrie, H.D., Bolt, D.J., 1990. Changes in plasma follicle-stimulating hormone, luteinizing hormone, estrogen and progesterone during growth of ovulatory follicles in the pig. Domest. Anim. Endocrinol. 7, 83–91.
Guthrie, H.D., Pursel, V.G., Bolt, D.J., Cooper, B.S., 1993. Expression of a bovine growth hormone transgene inhibits pregnant mare’s serum gonadotropin-induced follicle maturation in prepubertal gilts. J. Anim. Sci. 71, 3409–3413.
Houseknecht, K.L., Baile, C.A., Matteri, R.L., Spurlock, M.E., 1998. The biology of leptin: a review. J. Anim. Sci. 76, 1405–1420.
Jindal, R., Cosgrove, J.R., Aherne, F.X., Foxcroft, G.R., 1996. Effect of nutrition on embryonal mortality in gilts: association with progesterone. J. Anim. Sci. 74, 620–624.
King, R.H., 1989. Effect of liveweight and body composition of gilts at 24 weeks of age on subsequent reproductive efficiency. Anim. Prod. 49, 109–115.
King, R.H., Williams, I.H., 1984a. The effect of nutrition on the reproductive performance of first-litter sows: 2. Protein and energy intakes during lactation. Anim. Prod. 38, 249–256.
King, R.H., Williams, I.H., 1984b. The effect of nutrition on the reproductive performance of first-litter sows: 1. Feeding level during lactation, and between weaning and mating. Anim. Prod. 38, 241–247.
Kirkwood, R.N., Baidoo, S.K., Aherne, F.X., Sather, A.P., 1987. The influence of feeding level during lactation on the occurrence and endocrinology of the postweaning estrus in sows. Can. J. Anim. Sci. 67, 405–415.
Kirkwood, R.N., Thacker, P.A., Gooneratne, A.D., Guedo, B.L., Laarveld, B., 1988. The influence of exogenous growth hormone on ovulation rate in gilts. Can. J. Anim. Sci. 68, 1097–1103.
Kirkwood, R.N., Thacker, P.A., Guedo, B.L., Laarveld, B., 1989. The effect of exogenous growth hormone on the endocrine status and the occurrence of estrus in gilts. Can. J. Anim. Sci. 69, 931–937.
Kirkwood, R.N., Baidoo, S.K., Aherne, F.X., 1990. The influence of feeding level during lactation and gestation on the endocrine status and reproductive performance of second parity sows. Can. J. Anim. Sci. 70, 1119–1126.
McCann, S.M., Kimura, M., Walczewska, A., Karanth, S., Rettori, V., Yu, W.H., 1998. Hypothalamic control of gonadotropin secretion by LHRH, FSHRH, NO, cytokines, and leptin. Domest. Anim. Endocrinol. 15, 33–344.
Mao, J., Zak, L.J., Cosgrove, J.R., Shostak, S., Foxcroft, G.R., 1999. Reproductive, metabolic, and endocrine responses to feed restriction and GnRH treatment in primiparous, lactating sows. J. Anim. Sci. 77, 724–735.
Matamoros, I.A., Moore, A.B., Cox, N.M., 1990. Exogenous insulin and additional dietary energy affect follicular distribution, follicular steroid concentrations and granulosa cell human chorionic gonadotropin binding in swine. Biol. Reprod. 43, 1–7.
Matamoros, I.A., Cox, N.M., Moore, A.B., 1991. Effects of exogenous insulin and body condition on metabolic hormones and gonadotropin-induced follicular development in prepubertal gilts. J. Anim. Sci. 69, 2081–2091.
Meurer, K.A., Cox, N.M., Matamoros, I.A., Tubbs, R.C., 1991. Decreased follicular steroids and insulin-like growth factor-I and increased atresia in diabetic gilts during follicular growth stimulated with PMSG. J. Reprod. Fertil. 91, 187–196.
Newton, E.A., Mahan, D.C., 1992. Effect of feed intake during late development on pubertal onset and resulting body composition in crossbred gilts. J. Anim. Sci. 70, 3774–3780.
Noblet, J., Dourmad, J.Y., Etienne, M., 1990. Energy utilization in pregnant and lactating sows: modelling of energy requirements. J. Anim. Sci. 68, 562–572.
Noblet, J., Karege, C., Dubois, S., van Milgen, J., 1999. Metabolic utilization of energy and maintenance requirements in growing pigs: effect of sex and genotype. J. Anim. Sci. 77, 1208–1216.
Ogle, R.B., Dalin, A.M., 1989. The Effect of food intake in the rearing period on the reproductive performance of heavy and light gilts. Anim. Prod. 49, 305–310.
Poretsky, L., Kalin, M.F., 1987. The gonadotropic function of insulin. Endocr. Rev. 8, 132–141.
Prime, G.R., Symonds, H.W., 1993. Influence of the plane of nutrition on portal blood flow and the metabolic
Ž .
clearance rate of progesterone in ovariectomized gilts. J. Agric. Sci. Cambridge 121, 389–397. Prunier, A., Bonneau, M., Etienne, M., 1987. Effects of age and live weight on the sexual development of gilts
and boars fed two planes of nutrition. Reprod. Nutr. Dev. 27, 689–700.
Prunier, A., Martin, C., Mounier, A.M., Bonneau, M., 1993. Metabolic and endocrine changes associated with undernutrition in the peripubertal gilt. J. Anim. Sci. 71, 1887–1894.
Prunier, A., Quesnel, H., 2000. Nutritional influences on the hormonal control of reproduction in females pigs. Livest. Prod. Sci. 63, 1–16.
Prunier, A., Quesnel, H., Quiniou, N., Le Denmat, M., 1999. Effets du niveau d’alimentation sur la
w
concentration plasmatique de progesterone et sur la mortalite embryonnaire chez la cochette. Influence of´ ´ x
dietary intake on plasma progesterone and embryo mortality in gilts Journ. Rech. Porcine Fr. 31, 17–22. Purvis, J., Skelton, J.O., Quirk, M.N., Whitley, N.C., Cox, N.M., 1997. Influence of insulin and insulin-like
Ž . Ž .
Quesnel, H., 1999. Localization of binding sites for IGF-I, insulin and GH in the sow ovary. J. Endocrinol. 163, 363–372.
Quesnel, H., Pasquier, A., Mounier, A.M., Prunier, A., 1998a. Influence of feed restriction during lactation on gonadotropic hormones and ovarian development in primiparous sows. J. Anim. Sci. 76, 856–863. Quesnel, H., Pasquier, A., Mounier, A.M., Louveau, I., Prunier, A., 1998b. Influence of feed restriction in
primiparous lactating sows on body condition and metabolic parameters. Reprod. Nutr. Dev. 38, 261–274. Quesnel, H., Pasquier, A., Prunier, A., 1999. Is ovulation rate of gilts influenced by feed restriction during the luteal phase or during the follicular phase? Third Conference of the European Society for Domestic Animal Reproduction, November 26–27th. Le Lion d’Angers, France.
Quesnel, H., Prunier, A., 1998. Effect of insulin administration before weaning on reproductive performance in feed-restricted primiparous sows. Anim. Reprod. Sci. 51, 119–129.
Rein, M.S., Schomberg, D.W., 1982. Characterization of insulin receptors on porcine granulosa cells. Biol.
Ž .
Reprod. 26 Suppl. 1 , 113.
Rojkittikhun, T., Einarsson, S., Zilinskas, H., Edqvist, L.E., Uvnas-Moberg, K., Lundeheim, N., 1993. Effects¨
of insulin administration at weaning on hormonal patterns and reproductive performance in primiparous sows. J. Vet. Med., Ser. A 40, 161–168.
Rozeboom, D.W., Pettigrew, J.E., Dial, G.D., Moser, R.L., Cornelius, S.G., Wheaton, J.E., 1993. Estrous characteristics of postpubertal gilts approaching anestrus due to limited dietary energy intake. J. Anim. Sci. 71, 436–441.
Spicer, L.J., Francisco, C., 1997. The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology 138, 3374–3379.
Spicer, L.J., Francisco, C., 1998. Adipose obese gene product, leptin, inhibits bovine ovarian thecal cell steroidogenesis. Biol. Reprod. 58, 207–212.
Thissen, P., Ketelslegers, J.-M., Underwood, L.E., 1994. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 15, 80–101.
van den Brandt, H., Dieleman, S.J., Soede, N.M., Kemp, B., 2000. Dietary energy source at two feeding levels during lactation of primiparous sows: I. Effects on glucose, insulin, and LH and on follicle development, weaning-to-estrus-interval and ovulation rate. J. Anim. Sci. 78, 396–404.
Wade, G.N., Schneider, J.E., Li, H.Y., 1996. Control of fertility by metabolic cues. Am. J. Physiol.:
Ž .
Endocrinol. Metab. 270 33 , E1–E19.
Whitley, N.C., Moore, A.B., Cox, N.M., 1998a. Comparative effects of insulin and porcine somatotropin on postweaning follicular development in primiparous sows. J. Anim. Sci. 76, 1455–1462.
Whitley, N.C., Quirk-Thomas, M.N., Skelton, J.O., Moore, A.B., Purvis, J., Qui, Y., Cox, N.M., 1998b.
Ž .
Influence of insulin on follicular development and the intrafollicular insulin-like growth factor I IGF-I system in sows after weaning. J. Reprod. Fertil. 112, 175–184.
Zachow, R.J., Magoffin, D.A., 1997. Direct intraovarian effects of leptin: impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17b production by rat ovarian granulosa cells. Endocrinology 138, 847–850.
Zak, L.J., Cosgrove, J.R., Aherne, F.X., Foxcroft, G.R., 1997a. Pattern of feed intake and associated metabolic and endocrine changes differentially affect postweaning fertility in primiparous lactating sows. J. Anim. Sci. 75, 208–216.
Zak, L.J., Xu, X., Hardin, R.T., Foxcroft, G.R., 1997b. Impact of different patterns of feed intake during lactation in the primiparous sow on follicular development and oocyte maturation. J. Reprod. Fertil. 110, 99–106.