www.elsevier.comrlocateranireprosci
Effect of stress-like concentrations of cortisol on the
feedback potency of oestradiol in orchidectomized
sheep
qC.A. Daley
1, H. Sakurai, B.M. Adams, T.E. Adams
)Department of Animal Science, UniÕersity of California, DaÕis, CA 95616, USA
Received 15 July 1999; received in revised form 24 February 2000; accepted 3 March 2000
Abstract
The effect of stress-like concentrations of cortisol on oestradiol-induced change in LH
Ž .
secretion and GnRH receptor expression was evaluated in orchidectomized sheep wethers . Twenty-four wethers were assigned at random to one of the four treatment groups in a 2=2
Ž . Ž .
factorial design ns6 wethersrgroup . Wethers received cortisol 90mgrkgrh; groups 2 and 4
Ž .
or a comparable volume of cortisol delivery vehicle groups 1 and 3 by continuous infusion for
Ž .
48 h. During the final 24 h of infusion, wethers received oestradiol 6 ngrkgrh; groups 3 and 4
Ž .
or oestradiol delivery vehicle groups 1 and 2 . The pattern of LH secretion was assessed during a 3-h period of intensive blood collection beginning 21 h after initiation of oestradiol infusion.
Ž .
Although neither cortisol nor oestradiol alone affected P)0.05 mean serum concentration of LH or LH pulse frequency, serum LH and the frequency of secretory episodes of LH were
Ž .
significantly reduced P-0.05 in wethers receiving cortisol and oestradiol in combination. Anterior pituitary tissue was collected at the end of the infusion period. Oestradiol increased
ŽP-0.05 tissue concentrations of GnRH receptor and GnRH receptor mRNA. Although cortisol.
Ž .
alone did not affect P)0.05 basal concentrations of receptor or receptor mRNA, the magnitude of oestradiol-induced increase in GnRH receptor and GnRH receptor mRNA was significantly reduced in wethers receiving cortisol and oestradiol concurrently. Conversely, steady-state
concen-Ž .
trations of mRNA encoding the LHb and FSHb subunits were increased P-0.05 in wethers receiving cortisol. These observations demonstrate that stress-like concentrations of cortisol act in concert with oestradiol to suppress LH secretion. In addition, cortisol blocks oestradiol-dependent
q
Supported by USDA Grant 93-37203-9111 and the California Agriculture Experiment Station. )Corresponding author. Tel.:q1-530-752-1266; fax:q1-530-752-0175.
Ž .
E-mail address: [email protected] T.E. Adams .
1
Current address: School of Agriculture, California State University, Chico, CA 95929, USA. 0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
increase in pituitary tissue concentrations of GnRH receptor and GnRH receptor mRNA.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Cortisol; Sheep; Oestradiol; Gonadotropin secretion; GnRH receptor mRNA
1. Introduction
Marked and persistent increase in the serum concentration of glucocorticoids is a component of the endocrine response to many physical or psychological stressors in
Ž . Ž
primates Chen et al., 1992; Norman et al., 1994 , rodents Cook et al., 1973; Suzuki et
. Ž
al., 1986 , and domestic species Caraty et al., 1990; Guillaume et al., 1992; Komesaroff .
and Funder, 1994 . This increase in glucocorticoids may be causally linked to the inhibition of reproductive function that is another physiological correlate that often accompanies stress. Indeed, extended administration of natural or synthetic
glucocorti-Ž
coids has an anti-gonadal effect in rodents Smith et al., 1971; Baldwin and Sawyer,
. Ž .
1974 , primates Cunningham et al., 1978; Hayashi and Moberg, 1990 and sheep ŽDaley et al., 1999a ..
The anti-gonadal effect of stress andror glucocorticoids is due, at least in part, to the Ž
decrease in gonadotropin secretion that occurs during stress Chen et al., 1992; Norman
. Ž
et al., 1994 or prolonged administration of glucocorticoids Fonda et al., 1984; Dubey .
and Plant, 1985 . This stress- or glucocorticoid-dependent reduction in gonadotropin secretion may be the consequence of reduced hypothalamic activity andror decreased pituitary sensitivity. Interestingly, the reduction in gonadotropin secretion induced by
Ž . Ž .
stress Chen et al., 1992 or exogenous glucocorticoid Vreeburg et al., 1984, 1988 is accentuated by gonadal steroids. Indeed, Vreeburg et al. have suggested that glucocorti-coids enhance the negative feedback potency of gonadal steroids. This postulate is consistent with our recent observation that the negative feedback response to oestradiol
Ž .
in sheep is amplified after chronic administration of cortisol Daley et al., 1999b . In the work presented, we extend our earlier studies by examining the feedback response to oestradiol in castrated male sheep after short-term exposure to stress-like concentrations of cortisol. Continuous infusion of cortisol was used to establish a stable serum concentration of cortisol that approximated the serum concentration noted in sheep during exposure to potent stressors, such as bacterial endotoxin or profound
Ž
hypoglycemia Coleman et al., 1993; Komesaroff and Funder, 1994; Battaglia et al., .
1997 . Oestrogenic effects were assessed in sheep receiving exogenous oestradiol at a rate sufficient to establish serum concentrations of oestradiol of 2–3 pgrml. During the breeding season, this concentration of oestradiol does not affect the mean serum concentration of LH or the episodic character of LH secretion in unstressed wethers ŽSakurai et al., 1995 . However, this level of oestradiol does induce a marked increase in. the concentration of GnRH receptor and GnRH receptor mRNA in pituitary tissue of
Ž .
2. Materials and methods
2.1. Animals
Spring-born crossbred Suffolk lambs were castrated within 2 weeks of birth. All
Ž .
orchidectomized lambs wethers were housed in an open-sided barn under natural lighting with free access to water and alfalfa pellets supplemented with cereal grains and
Ž
vitamin and mineral premix. Wethers were 6–8 months of age bodyweights50–60 .
kg at the time of experimentation. These studies were conducted during October and early November, a period of high reproductive activity in female sheep at this latitude Ž388N . All experimental procedures involving the use of animals were conducted in.
Ž .
accordance with National Institutes of Health NIH Guidelines and were reviewed and approved by the Animal Use and Care Committee for the University of California.
2.2. Cannulation
Ž
Prior to experimentation, two polyethylene cannulae Intramedic PE 190, Clay .
Adams, Parsippany, NJ were inserted into the left jugular vein to serve as hormone-de-livery cannulae. A third cannula was inserted into the contralateral vein and used for blood collection. All cannulae were passed through a protective plastic tubing sheath along the halter and lead rope to the exterior of the animal holding area. Animals were freely mobile at the end of a 1-m lead. The cannulae were inserted 3 days prior to initiation of treatment to permit acclimation to the conditions of experimentation.
2.3. Hormone deliÕery
Cannulae for the delivery of cortisol or oestradiol were connected to syringes placed
Ž .
in Harvard infusion pumps Model 2265, Harvard Bioscience, South Natick, MA .
Ž . Ž .
Cortisol Sigma, St. Louis, MO in 50% ethanol–saline cortisol delivery vehicle
Ž . Ž .
andror oestradiol Sigma in 10% ethanol–saline oestradiol delivery vehicle were delivered by continuous infusion.
2.4. Experimental procedure
The effect of cortisol and oestradiol on gonadotropin secretion and pituitary concen-trations of GnRH receptor and GnRH receptor mRNA was examined using 24 wethers
Ž assigned at random to one of the four treatment groups in a 2=2 factorial design ns6
. Ž .
wethersrgroup . Twelve wethers received cortisol 90mgrkgrh; groups 2 and 4 or a
Ž .
comparable volume of vehicle groups 1 and 3 by continuous infusion for 48 h. Preliminary studies demonstrated that intravenous delivery of cortisol at this rate resulted in stable serum concentrations of cortisol of 75–90 ngrml. During the final 24
Ž .
h of infusion, wethers also received oestradiol 6 ngrkgrh; groups 3 and 4 or a
Ž .
pulses of LH were assessed in blood samples collected during a 3-h period of intensive
Ž .
blood collection 10-min collection interval beginning 21 h after initiation of oestradiol infusion. At the end of infusion, animals were stunned by means of a captive bolt pistol and killed by exsanguination. Anterior pituitary tissue was quickly excised, halved by a mid-sagittal cut and immediately frozen in liquid nitrogen and stored aty808C for later analysis. Blood was allowed to clot on ice and serum was harvested within 24 h of sample collection. Serum samples were rapidly frozen and stored aty208C for later endocrine analysis.
2.5. Endocrine analysis
Tissue andror serum concentrations of LH, FSH, oestradiol and cortisol were Ž
determined by use of previously validated procedures Adams et al., 1975, 1988; Sakurai
. Ž . Ž
et al., 1992; Daley et al., 1999b . The LH oLH-26 and FSH NIAMDD-.
oFSH-RP-1 reference standards were gifts from the National Hormone and Pituitary
Ž .
Program Baltimore, MD . In all cases, intra- and interassay coefficients of variation were -10%. The minimum sensitivity of the LH, FSH, oestradiol, and cortisol assays was 0.1 ngrml, 0.25 ngrml, 0.6 pgrml, and 1.0 ngrml, respectively.
The affinity and tissue concentration of GnRH receptor were determined by use of
Ž .
the procedure previously described Sakurai and Adams, 1991; Sakurai et al., 1993 . Tissue concentrations of mRNA for the LHb and FSHb subunits and GnRH receptor mRNA were determined by use of the solution hybridization RNase protection
proce-Ž .
dures described previously Sakurai et al., 1993; Adams et al., 1996 . Plasmids
Ž . Ž
containing cDNA inserts for the bovine LHb Maurer, 1985 and FSHb Maurer and
. Ž
Beck, 1986 subunits were provided by Dr. R. Maurer Department of Cell Biology and .
Anatomy, Oregon Health Sciences University, Portland, OR . A plasmid containing a
Ž .
cDNA insert for the ovine GnRH receptor Brooks et al., 1993 was kindly provided by
Ž .
Dr. J. Brooks MRC Reproductive Biology Unit, Edinburgh . The sense and antisense cRNAs were generated by in vitro transcription using either T7 RNA or SP6 RNA
Ž
polymerase and the Riboprobe Gemini System II reagent system Promega, Madison, .
WI .
2.6. Statistical analyses
Wethers were assigned to one of the four cells in a 2=2 factorial experiment, with the main effects being cortisol and oestradiol infusion. Data were analyzed by ANOVA ŽGill, 1978 . Where significant treatment effects were noted, mean comparisons were. made using Duncan’s Multiple Range test. Data are presented in the text, table and figures as means"SEM. The frequency and amplitude of secretory episodes of LH
Ž .
3. Results
3.1. Serum concentrations of cortisol
Continuous delivery of cortisol at a rate of 90mgrkgrh increased serum concentra-tion of cortisol to 80"2 ngrml within 4 h of initiation of infusion. Serum concentration of cortisol was maintained at this level for the remainder of cortisol delivery. In contrast, serum concentration of cortisol in wethers receiving vehicle alone did not differ from
Ž .
pretreatment concentrations 20"1 ngrml . Serum concentrations of cortisol at the end of the infusion period were 77"8 and 18"5 ngrml in wethers receiving cortisol or vehicle, respectively.
3.2. Serum concentrations of oestradiol
Ž .
Although oestradiol was not detectable -0.6 pgrml in wethers receiving vehicle alone, continuous infusion of oestradiol at 6 ngrkgrh increased serum concentration of oestradiol to 2.5"0.1 pgrml 4 h after beginning oestradiol administration. Serum concentration of oestradiol was maintained at this level throughout the remainder of the oestradiol infusion period. Serum concentration of oestradiol in oestradiol-treated wethers
Ž .
was not affected P)0.05 by concurrent infusion of cortisol or cortisol delivery vehicle.
3.3. Serum concentrations of LH and FSH
Ž .
Serum concentration of LH during the first 24 h of infusion did not differ P)0.05 in wethers receiving cortisol or the cortisol delivery vehicle. Similarly, at the beginning
Ž .
of the oestradiol infusion period serum concentrations of LH were comparable P)0.05
Ž . Ž
in wethers receiving cortisol 3.3"0.6 ngrml or vehicle alone 3.9"0.3 ngrml; Fig. .
1 . Continued infusion of cortisol or vehicle for an additional 24-h period did not
Ž . Ž
Fig. 1. Serum concentrations of LH in orchidectomized sheep wethers receiving cortisol 90mgrkgrh;`,
. Ž .
v or a comparable volume of cortisol delivery vehicle 50% ethanol–saline;n,' during a 48-h infusion
period. Twelve wethers were included in both the cortisol and vehicle infusion groups. During the final 24 h of
Ž
infusion, six wethers from each of the cortisol and vehicle infusion groups received concurrent oestradiol 6
. Ž .
ngrkgrh;v,' or oestradiol delivery vehicle 10% ethanol–saline;`,^ by continuous infusion. Values
Ž . Ž .
significantly affect serum concentration of LH. Similarly, mean serum concentration of LH in wethers was not significantly affected by infusion of oestradiol alone. However, concurrent infusion of cortisol and oestradiol significantly decreased mean serum concentration of LH relative to LH levels in control animals receiving vehicle alone. Indeed, at the end of the infusion period the serum concentration of LH in wethers
Ž . Ž .
receiving cortisol and oestradiol 2.2"0.2 ngrml was significantly reduced P-0.05
Ž .
relative to the final serum concentration of LH in control wethers 4.5"0.7 ngrml . In
contrast, the final serum concentration of LH in wethers receiving cortisol or oestradiol
Ž . Ž .
alone 3.7"0.6 and 3.7"0.4 ngrml, respectively did not differ P)0.05 from the final LH concentration in control animals. Conversely, the final serum concentration of
Ž . Ž .
FSH in control wethers 9.7"1.1 ngrml did not differ P)0.05 from values noted in
Ž . Ž .
wethers receiving cortisol 9.6"1.3 ngrml or oestradiol 7.8"1.0 ngrml alone, or in
Ž .
combination 9.2"0.9 ngrml .
3.4. Pattern of LH secretion
Although the frequency of secretory episodes of LH in wethers receiving cortisol or
Ž .
oestradiol alone did not differ P)0.05 from pulse frequency in control wethers, LH pulse frequency was reduced in animals receiving cortisol and oestradiol concurrently ŽTable 1 . Similarly, the amplitude of secretory episodes of LH was not affected by. intravenous delivery of cortisol or oestradiol alone. However, pulse amplitude was significantly augmented in wethers receiving cortisol and oestradiol in combination.
Ž .
Conversely, basal or nadir concentrations of LH were decreased P-0.05 during concurrent infusion of cortisol and oestradiol.
3.5. Pituitary concentrations of GnRH receptor and GnRH receptor mRNA
Concentrations of GnRH receptor and GnRH receptor mRNA in pituitary tissue of wethers receiving oestradiol were significantly increased relative to tissue levels of
Table 1
Ž .
The effect of continuous infusion of cortisol 90 mgrkgrh or a comparable volume of cortisol delivery
Ž . Ž
vehicle 50% ethanol–saline for 48 h on the pattern of LH secretion in wethers receiving oestradiol 6
. Ž .
ngrkgrh or oestradiol delivery vehicle 10% ethanol–saline during the final 24 h of the infusion period
Treatment n LH pulse Pulse Basal LH
a Ž .
The pattern of LH secretion was assessed during a 3-h window of intensive blood collection 10-min
.
collection interval beginning 21 h after initiation of oestradiol infusion. Pulse amplitude is defined as the concentration of LH at the peak less the concentration at the preceding nadir.
b,c Ž .
Values means"SEM in a column that do not share a common superscript differ significantly
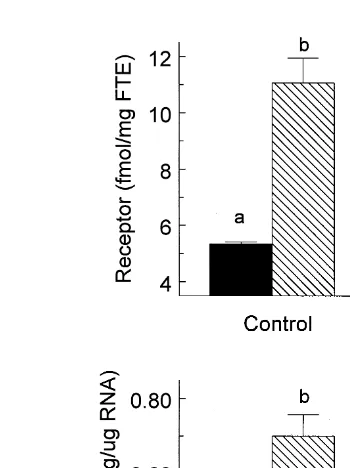
Fig. 2. Effect of cortisol andror oestradiol on steady state concentrations of GnRH receptor and GnRH
Ž .
receptor mRNA in pituitary tissue of orchidectomized sheep wethers . Wethers received either cortisol
ŽCortisol; 90 mgrkgrh; ns12. or a comparable volume of cortisol delivery vehicle ŽControl; 50%
.
ethanol–saline; ns12 during a 48-h infusion period. During the final 24 h of infusion, six wethers from each
Ž .
of the Control and Cortisol infusion groups received concurrent oestradiol Oestradiol; 6 ngrkgrh or
Ž .
oestradiol delivery vehicle Vehicle; 10% ethanol–saline by continuous infusion. Anterior pituitary tissue was collected at the end of the infusion period. Tissue concentrations of GnRH receptor or GnRH receptor mRNA
Ž .
that do not share a letter designation differ significantly P-0.05 . FTE, fresh tissue equivalent.
Ž . GnRH receptor and receptor mRNA noted in vehicle-infused control animals Fig. 2 . Although continuous infusion of cortisol did not significantly affect basal concentrations of GnRH receptor or GnRH receptor mRNA, the oestradiol-induced augmentation of GnRH receptor and receptor mRNA was significantly reduced in wethers receiving cortisol and oestradiol in combination.
3.6. Tissue concentrations of LH and FSH and gonadotropin subunit mRNA
Although infusion of oestradiol alone did not significantly affect pituitary tissue concentrations of LH and LHb mRNA, both measures of gonadotrope function were
Ž .
significantly increased after 48 h of cortisol infusion Table 2 . In contrast, tissue concentrations of LH and LHb mRNA in wethers receiving cortisol and oestradiol
Ž .
concurrently did not differ P)0.05 from values noted in control wethers. Similarly, pituitary stores of FSH in wethers receiving cortisol or oestradiol alone, or in
combina-Ž .
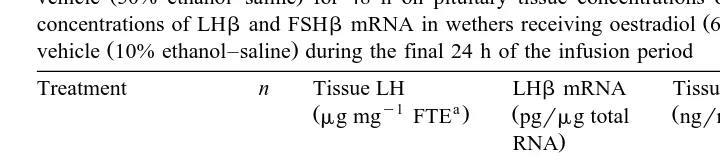
Table 2
Ž .
The effect of continuous infusion of cortisol 90 mgrkgrh or a comparable volume of cortisol delivery
Ž .
vehicle 50% ethanol–saline for 48 h on pituitary tissue concentrations of LH and FSH and steady-state
Ž .
concentrations of LHband FSHbmRNA in wethers receiving oestradiol 6 ngrkgrh or oestradiol delivery
Ž .
vehicle 10% ethanol–saline during the final 24 h of the infusion period
Treatment n Tissue LH LHbmRNA Tissue FSH FSHbmRNA
y1 a a
Cortisolqoestradiol 6 0.60"0.03 21.1"3.0 77.8"2.0 4.8"0.7 a
FTE, fresh tissue equivalent.
b,c Ž .
Values means"SEM in a column that do not share a common superscript differ significantly
ŽP-0.05 ..
Ž .
receiving oestradiol alone did not differ P)0.05 from values noted in control animals. Conversely, continuous infusion of cortisol for 48 h significantly increased tissue concentrations of FSHb mRNA.
4. Discussion
In the work presented here, we examine the effect of short-term infusion of cortisol on gonadotrope function. Although neither cortisol nor oestradiol alone affected the pattern of LH secretion, concurrent administration of oestradiol and stress-like concen-trations of cortisol resulted in a significant reduction in serum concenconcen-trations of LH and suppression of LH pulse frequency. These observations are consistent with the response
Ž .
noted in wethers after more prolonged infusion of cortisol Daley et al., 1999b . A Ž
similar glucocorticoid-induced response has also been reported in rodents Vreeburg et .
al., 1984, 1988 . Collectively, these studies suggest that both short-term and chronic administration of stress-like concentrations of cortisol increase the negative feedback effect of oestradiol. We postulate that the reduced fertility associated with stress may reflect, at least in part, cortisol-dependent enhancement of the negative feedback potency of oestradiol, which results in reduced activity of the hypothalamic GnRH pulse generating system and decreased gonadotropin secretion.
This postulate is supported by recent reports indicating that the activity of GnRH-con-taining neurons and episodic release of GnRH are suppressed during periods of stress ŽChen et al., 1992; Battaglia et al., 1997; Nappi and Rivest, 1997 . It is interesting to. note that this response to stress is particularly acute during the follicular phase of the
Ž .
reproductive cycle and less prominent in ovariectomized animals Chen et al., 1992 or
Ž .
animals in the luteal phase Norman et al., 1994 . Moreover, ovariectomized animals are made increasingly sensitive to the suppressive effects of stress by concurrent treatment
Ž .
Tissue concentration of GnRH receptor is generally considered to be one, of several, Ž
key determinants of gonadotrope responsiveness Conn et al., 1987; Stojilkovic et al., .
1994 and oestrogen-dependent augmentation of tissue concentration of GnRH receptor
Ž .
is associated with enhanced responsiveness Sakurai and Adams, 1991 . The results presented here demonstrate that basal concentrations of GnRH receptor and GnRH receptor mRNA are not affected by cortisol. This is consistent with the response noted in
Ž .
rodents Suter et al., 1988; Rosen et al., 1991 . In contrast, the observations reported Ž .
here indicate that short-term 48 h exposure to stress-like concentrations of cortisol significantly reduced the magnitude of oestradiol-dependent increase in tissue levels of GnRH receptor and GnRH receptor mRNA. This glucocorticoid-dependent reduction in GnRH receptor expression appears to be dependent on the magnitude and duration of oestradiol stimulation. Indeed, our recent studies demonstrate that cortisol-dependent suppression of the oestrogenic response is attenuated by increasing the duration or level
Ž .
of oestrogenic stimulation Adams et al., 1999; Daley et al., 1999b . Although the mechanism underlying this glucocorticoid-dependent response cannot be precisely de-fined using our in vivo model system, recent in vitro studies indicate that glucocorticoids
Ž
decrease gene transcription and reduce mRNA stability in pituitary tissue Gothard et al., .
1996; Iredale and Duman, 1997 . One, or both, of these glucocorticoid mediated responses may account for the reduction in oestrogen-induced increase in GnRH receptor mRNA in wethers receiving stress-like concentrations of cortisol.
Although cortisol alone did not affect the basal concentration of GnRH receptor mRNA, stress-like concentrations of cortisol significantly increased the steady state concentration of FSHb mRNA. A similar increase in tissue concentrations of FSHb
Ž
mRNA has been noted in rodents after glucocorticoid stimulation Ringstrom et al., .
1991; McAndrews et al., 1994 . This apparently reflects a direct effect of
glucocorti-Ž .
coids on gonadotrope cells to increase gene transcription Kilen et al., 1996 .
In addition to increasing pituitary concentrations of FSHb mRNA, we noted that stress-like concentrations of cortisol increased pituitary stores of LH and steady-state concentrations of LHb mRNA. Cortisol-dependent augmentation of tissue concentra-tions of LH and LHb mRNA was not evident in wethers receiving cortisol and oestradiol in combination. This suggests that oestradiol may suppress the glucocorticoid-induced response. This response appears to be affected by the duration of glucocorticoid stimulation since prolonged cortisol administration results in increased
Ž
tissue stores of LH even in the face of concurrent oestradiol stimulation Daley et al., .
1999b . Although glucocorticoids do not affect tissue concentrations of LHb mRNA in
Ž .
the rodent model Ringstrom et al., 1991; Kilen et al., 1996 , an increase in pituitary
Ž .
stores of LH was noted in cortisol-treated rodents Ringstrom et al., 1991 . The glucocorticoid-dependent augmentation of tissue concentrations of LHb and FSHb mRNA noted in our study is consistent with the postulate that stress-induced secretion of glucocorticoids enhances gonadotropin synthesis and storage in preparation for the
Ž
resumption of reproductive activity after return of the stress-free condition McAndrews .
et al., 1994 .
Ž
Stressful stimuli mobilize an array of endocrine factors Coleman et al., 1993; .
establish a specific casual link between stress and infertility. In the studies described here, we attempt to minimize this problem by confining our focus to the impact of cortisol on reproductive function. The important role of cortisol in stress-induced infertility is indicated by our recent observation that continuous delivery of stress-like
Ž .
concentrations of cortisol prevents or delays ovulation in sheep Daley et al., 1999a . The observations reported here indicate that stress-like concentrations of cortisol may act at both hypothalamic and pituitary sites to exert this anti-gonadal effect. Cortisol, acting at hypothalamic sites, appears to enhance the negative feedback response induced by oestradiol. Similarly, cortisol acts at hypophyseal sites to reduce oestrogen-dependent increase in tissue concentrations of GnRH receptor and receptor mRNA. The combined effect of glucocorticoid action at both hypothalamic and hypophyseal sites would likely decrease gonadotropin secretion and depress fertility.
References
Adams, T.E., Kinder, J.E., Chakraborty, P.K., Estergreen, V.L., Reeves, J.J., 1975. Ewe luteal function influenced by pulsatile administration of synthetic LHRHrFSHRH. Endocrinology 97, 1460–1467. Adams, T.E., Quirke, J.F., Hanrahan, J.P., Adams, B.M., Watson, J.G., 1988. Gonadotrophin secretion during
the preovulatory period in Galway and Finnish Landrace ewes and Finnish Landrace ewes selected for high ovulation rate. J. Reprod. Fertil. 83, 575–584.
Ž .
Adams, B.M., Sakurai, H., Adams, T.E., 1996. Concentrations of gonadotropin-releasing hormone GnRH receptor messenger ribonucleic acid in pituitary tissue of orchidectomized sheep: effect of estradiol and GnRH. Biol. Reprod. 54, 407–412.
Adams, T.E., Sakurai, H., Adams, B.M., 1999. Effect of stress-like concentrations of cortisol on estradiol-de-pendent expression of gonadotropin-releasing hormone receptor in orchidectomized sheep. Biol. Reprod. 60, 164–168.
Baldwin, D.M., Sawyer, C.H., 1974. Effects of dexamethasone on LH release and ovulation in the cyclic rat. Endocrinology 94, 1397–1403.
Battaglia, D.F., Bowen, J.M., Krasa, H.B., Thrun, L.A., Viguie, C., Karsch, F.J., 1997. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophy-seal portal and peripheral blood. Endocrinology 138, 4273–4281.
Battaglia, D.F., Brown, M.E., Krasa, H.B., Thrun, L.A., Viguie, C., Karsch, F.J., 1998. Systemic challenge with endotoxin stimulates corticotropin-releasing hormone and arginine vasopressin secretion into hy-pophyseal portal blood: coincidence with gonadotropin-releasing hormone suppression. Endocrinology 139, 4175–4181.
Brooks, J., Taylor, P.L., Saunders, P.T., Eidne, K.A., Struthers, W.J., McNeilly, A.S., 1993. Cloning and sequencing of the sheep pituitary gonadotropin-releasing hormone receptor and changes in expression of its mRNA during the estrous cycle. Mol. Cell. Endocrinol. 94, R23–R27.
Caraty, A., Grino, M., Locatelli, A., Guillaume, V., Boudouresque, F., Conte-Devoix, B., Oliver, C., 1990. Insulin-induced hypoglycemia stimulates corticotropin-releasing factor and arginine vasopressin secretion into hypophyseal portal blood of conscious, unrestrained rams. J. Clin. Invest. 85, 1716–1721.
Chen, M.D., O’Byrne, K.T., Chiappini, S.E., Hotchkiss, J., Knobil, E., 1992. Hypoglycemic stress and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology 56, 666–673.
Coleman, E.S., Elsasser, T.H., Kemppainen, R.J., Coleman, D.A., Sartin, J.L., 1993. Effect of endotoxin on pituitary hormone secretion in sheep. Neuroendocrinology 58, 111–122.
Conn, P.M., Huckle, W.R., Andrews, W.V., McArdle, C.A., 1987. The molecular mechanism of action of
Ž .
Cook, D.M., Kendall, J.W., Greer, M.A., Kramer, R.M., 1973. The effect of acute and chronic ether stress on plasma ACTH concentration in the rat. Endocrinology 93, 1019–1024.
Cunningham, G.R., Goldzieher, J.W., de la Pena, A., Oliver, M., 1978. The mechanism of ovulation inhibition by triamcinolone acetonide. J. Clin. Endocrinol. Metab. 46, 8–14.
Daley, C.D., Macfarlane, M.S., Sakurai, H., Adams, T.E., 1999a. Effect of stress-like concentrations of cortisol on follicular development and the preovulatory surge of LH in sheep. J. Reprod. Fertil. 117, 11–16.
Daley, C.A., Sakurai, H., Adams, B.M., Adams, T.E., 1999b. Effect of stress-like concentrations of cortisol on gonadotroph function in orchidectomized sheep. Biol. Reprod. 60, 158–163.
Dubey, A.K., Plant, T.M., 1985. A suppression of gonadotropin secretion by cortisol in castrated male rhesus
Ž .
monkeys Macaca mulatta mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol. Reprod. 33, 423–431.
Fonda, E.S., Rampacek, G.B., Kraeling, R.R., 1984. The effect of adrenocorticotropin or hydrocortisone on serum luteinizing hormone concentrations after adrenalectomy andror ovariectomy in the prepubertal gilt. Endocrinology 114, 268–273.
Gill, J.L., 1978. Design and Analysis of Experiments in the Animal and Medical Sciences. Iowa State Univ. Press, Ames, IA.
Goodman, R.L., Karsch, F.J., 1980. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107, 1286–1290.
Gothard, L.Q., Hibbard, J.C., Seyfred, M.A., 1996. Estrogen-mediated induction of rat prolactin gene transcription requires the formation of chromatin loop between the distal enhancer and proximal promoter regions. Mol. Endocrinol. 10, 185–195.
Guillaume, V., Conte-Devolx, B., Magnan, E., Boudouresque, F., Grino, M., Cataldi, M., Muret, L., Priou, A., Deprez, P., Figaroli, J.C., Oliver, C., 1992. Effect of chronic active immunization anti-corticotropin-releas-ing factor on the pituitary–adrenal function in the sheep. Endocrinology 130, 2291–2298.
Hayashi, K.T., Moberg, G.P., 1990. Influence of the hypothalamic–pituitary–adrenal axis on the menstrual
Ž .
cycle and the pituitary responsiveness to estradiol in the female rhesus monkey Macaca mulatta . Biol. Reprod. 42, 260–265.
Iredale, P.A., Duman, R.S., 1997. Glucocorticoid regulation of corticotropin-releasing factor receptor expres-sion in pituitary-derived AtT-20 cells. Mol. Pharmacol. 51, 794–799.
Kilen, S.M., Szabo, M., Strasser, G.A., McAndrews, J.M., Ringstrom, S.J., Schwartz, N.B., 1996. Corticos-terone selectively increases follicle-stimulating hormoneb-subunit messenger ribonucleic acid in primary anterior pituitary cell culture without affecting its half-life. Endocrinology 137, 3802–3807.
Komesaroff, P.A., Funder, J.W., 1994. Differential glucocorticoid effects on catecholamine responses to stress. Am. J. Physiol. 266, E118–E128.
Maurer, R.A., 1985. Analysis of several bovine lutropin b subunit cDNAs reveals heterogeneity in the nucleotide sequence. J. Biol. Chem. 260, 4684–4687.
Maurer, R.A., Beck, A., 1986. Isolation and nucleotide sequence analysis of a cloned cDNA encoding the b-subunit of bovine follicle-stimulating hormone. DNA 5, 363–369.
McAndrews, J.M., Ringstrom, S.J., Dahl, K.K.D., Schwartz, N.B., 1994. Corticosterone in vivo increases
Ž .
pituitary follicle-stimulating hormone FSH -b messenger ribonucleic acid content and serum FSH bioactivity selectively in female rats. Endocrinology 134, 158–163.
Nappi, R.E., Rivest, S., 1997. Effect of immune and metabolic challenges on the luteinizing hormone-releasing hormone neuronal system in cycling female rats: an evaluation at the transcriptional level. Endocrinology 138, 1374–1384.
Norman, R.L., McGlone, J., Smith, C.J., 1994. Restraint inhibits luteinizing hormone secretion in the follicular phase of the menstrual cycle in rhesus macaques. Biol. Reprod. 50, 16–26.
Ringstrom, S.J., McAndrews, J.M., Rahal, J.O., Schwartz, N.B., 1991. Cortisol in vivo increases FSHb mRNA selectively in pituitaries of male rats. Endocrinology 129, 2793–2795.
Rosen, H., Dalkin, A., Haisenleder, D., DeMott Friberg, R., Ortolano, G., Barkan, A., 1991. Dexamethasone
Ž .
Sakurai, H., Adams, T.E., 1991. Gonadotrope responsiveness in orchidectomized sheep: I. Effect of continuous infusion of estradiol. Biol. Reprod. 45, 804–810.
Ž .
Sakurai, H., Adams, B.M., Adams, T.E., 1992. Pattern of gonadotropin-releasing hormone GnRH -like stimuli sufficient to induce follicular growth and ovulation in ewes passively immunized against GnRH. Biol. Reprod. 47, 177–184.
Sakurai, H., Adams, B.M., Oberbauer, A.M., Adams, T.E., 1993. Gonadotrope responsiveness in orchidec-tomized sheep: II. Effect of gonadotropin-releasing hormone amplitude shift during continuous infusion of estradiol. Biol. Reprod. 48, 683–691.
Sakurai, H., Adams, B.M., Adams, T.E., 1995. Gonadotroph responsiveness in orchidectomized sheep: IV. Effect of estradiol infusion during the breeding and anestrous seasons. Biol. Reprod. 52, 382–389. Smith, E.R., Johnson, J., Weick, R.F., Levine, S., Davidson, J.M., 1971. Inhibition of the reproductive system
in immature rats by intracerebral implantation of cortisol. Neuroendocrinology 8, 94–106.
Stojilkovic, S.S., Reinhart, J., Catt, K.J., 1994. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr. Rev. 15, 462–499.
Suter, D.E., Schwartz, N.B., Ringstrom, S.J., 1988. Dual role of glucocorticoids in regulation of pituitary content and secretion of gonadotropins. Am. J. Physiol. 254, E595–E600.
Suzuki, S., Oh, C., Nakano, K., 1986. Pituitary-dependent and -independent secretion of CS caused by bacterial endotoxin in rats. Am. J. Physiol. 250, E470–E474.
Vreeburg, J.T.M., DeGreef, W.J., Ooms, M.P., Van Wouw, P., Weber, R.F.A., 1984. Effects of adrenocorti-cotropin and corticosterone on the negative feedback action of testosterone in the adult male rat. Endocrinology 115, 977–983.