Platelet HDL
3binding sites are not related to integrin
a
IIbb
3(GPIIb – IIIa)
Javier Pedren˜o
a,*, Conxita de Castellarnau
b, Lluı´s Masana
aaFacultat de Medicina,Uni
6ersitat Ro6ira i Virgili,Unitat de Recerca en Lı´pids i Arteriosclerosi,Sant Llorenc¸21Reus,Tarragona,Spain bInstitut de Recerca del Hospital de la Santa Creu i Sant Pau de Barcelona,Barcelona,Spain
Received 1 October 1999; received in revised form 14 February 2000; accepted 18 February 2000
Abstract
Early studies considered that fibrinogen receptor (glycoprotein [GP] IIb – IIIa or platelet integrin aIIbb3) is the binding site for
low-density lipoprotein (LDL) and high-density lipoprotein type 3 (HDL3). Recent data, however, do not support the hypothesis
that the binding of LDL to human intact resting platelets is related to integrin aIIbb3. In this study we present evidence that
platelet integrinaIIbb3is also not involved in the interaction of HDL3and human intact resting platelets. Firstly, specific ligands
for platelet integrin aIIbb3, such as fibrinogen, vitronectin, von Willebrand factor and fibronectin, were unable to inhibit the
binding of HDL3to intact resting platelets. Secondly, the HDL3binding characteristics (Kd andBmaxvalues), the activation of
protein kinase C (PKC) and the inhibition of thrombin-induced inositoltriphosphate (IP3) formation and calcium (Ca2+)
mobilization mediated by HDL3 particles were similar in platelets from control subjects and patients with type I and type II
Glanzmann’s thrombasthenia, which are characterized by total and partial lack of GPIIb – IIIa and fibrinogen, respectively. In contrast, nitrosylation of tyrosine residues of HDL3by tetranitromethane fully abolished both the ability of particles to interact
with its specific binding sites and the functional effects. Thirdly, polyclonal antibodies against the GPIIb – IIIa complex (edu-3 and 5B12), human antiserums against platelet alloantigens (anti-Baka/Band anti-PLA1/2), anti-integrin subunits (anti-a
Vand anti-b3),
and a wide panel of monoclonal antibodies (mAbs) against well-known epitopes of GPIIb (M3, M4, M5, M6, M8 and M95-2b) and GPIIIa (P23-7, P33, P37, P40, and P97) did not affect the binding of HDL3particles to human intact resting platelets. Overall
results show that neither the GPIIb – IIIa complex nor GPIIb or GPIIIa individually are the membrane binding proteins for HDL3on intact resting platelets. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Platelets; HDL3; Glanzmann’s thrombasthenia; Integrin aIIbb3
www.elsevier.com/locate/atherosclerosis
1. Introduction
Elevated serum cholesterol is widely accepted as a systemic thrombogenic risk factor in thrombus forma-tion on ruptured atherosclerotic plaques [1]. Platelet reactivity is enhanced by low density lipoproteins (LDL), whereas high density lipoproteins (HDL) seem to have the opposite effect [2,3]. Specific platelet LDL and HDL receptors coupled to phosphatidylinositol hydrolysis have been implicated in the aggregatory
response mediated by lipoproteins [4 – 6]. Advances in characterizing the platelet LDL receptor [7 – 10] and in understanding both the mechanisms of action and the effects on platelet function have been rapid [11 – 13]. In contrast, little is known about the platelet HDL recep-tor [14,15]. Early studies argued that platelet HDL3and
LDL binding sites could be related to integrin aIIbb3.
Koller et al. [16], using ligand and western blotting techniques, identified platelet membrane GPs such as GPIIb (136 kDa) or aIIb subunit and GPIIIa (92 kDa)
orb3subunit, and also the intact GPIIb – IIIa complex,
as the main GPs implicated in the binding of HDL3.
Overall, these results indicated that fibrinogen and platelet lipoprotein binding sites for LDL and HDL3
could be related via platelet integrin aIIbb3. However,
several studies have recently demonstrated that GPIIb – Portions of this work were presented at the 11th International
Symposium on Atherosclerosis, Paris, France, October 1997, and published in abstract form (Atherosclerosis, 1997;138:184 – 185).
* Corresponding author. Tel.: +34-977-759370; fax: + 34-977-759322.
E-mail address:[email protected] (J. Pedren˜o).
IIIa apparently does not act as a platelet LDL binding site [7,12,13,17] and that other receptors, as CD36 [18,19] are implicated in the LDL-platelet interaction. Overall, these new data provide conflicting results, and further studies are needed to explore the implication of GPIIb – IIIa in platelet HDL3 binding. In fact, HDL3
binding characteristics clearly differ from those of fibrinogen binding [4 – 6]. Moreover, rather than induc-ing inhibition, HDL3particles did not alter the binding
of fibrinogen to activated platelets [7]. The aim of this study was to investigate the hypothetical involvement of platelet integrin aIIbb3 on the binding of HDL3 to
intact human resting platelets.
2. Materials and methods
2.1. Materials
125I-Na from New England Nuclear (Boston, MA);
Iodo-Gen was purchased from Sigma Chemical Co. (St Louis, MO), and highly purified (B95% by SDS – PAGE) vitronectin and fibronectin, from Calbiochem Corp and Chemicon Intl. Polyclonal antibodies against the GPIIb – IIIa complex (edu-3 and 5B12 [the latter from Dako] and human antiserums against platelet alloantigens (anti-Baka/B
[=Leka/b
, HPA-3, or anti-GPIIb]) and anti-PLA1/2 [ZWa/b, HPA-1, or
anti-GPI-IIa]) were a gift from Dr E. Mun˜iz (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain). A panel of pure murine mAbs against GPIIb (M3, M4, M5, M6, M8 and M95-2b) and against GPIIIa (P23-7, P33, P37, P40, and P97), which recognize different epitopes of GPIIb and GPIIIa, respectively, were a gift from Dr J. Gonzalez-Rodriguez (Instituto de Quı´mica Fı´sica ‘Ro-casolano’ CSIC, Madrid, Spain); polyclonal antibodies against aV (a-vitronectin receptor) and b3 (anti-IIIa)
integrin subunits and their corresponding nonimmune rabbit sera were kindly donated by Dr E. Dejana (Vascular Biology Laboratory, Mario Negri Institut, Milan, Italy). A mouse immunoglobulin G used as a negative control in binding studies was from Menarini Diagnostics.
2.2. Isolation of lipoproteins
HDL3(density range, 1.125 – 1.210 g/ml) was isolated
from pooled sera of normolipidemic volunteers and separated by sequential ultracentrifugation [20] follow-ing the procedure previously reported [6]. Serum was treated with NaN3(7.5 mmol/l), gentamicin sulfate (0.1
mmol/l), PMSF (1 mmol/l), EDTA (3 mmol/l), chlo-ramphenicol (0.25 mmol/l) and aprotinin (0.25 mmol/l). Lipoproteins were washed during their flotation in a potassium bromide solution of the respective lipo-protein density containing EDTA (1 mmol/l), NaN3 (3
mmol/l), gentamicin sulfate (0.1 mmol/l), chloram-phenicol (0.25 mmol/l), glutathione (0.65 mmol/l) and NaCl (150 mmol/l). HDL3 was dialyzed against buffer
A (150 mmol/l NaCl, 20 mmol/l Tris, and 0.3 mmol/l EDTA, pH 7.4) and filtered through a Millipore filter (0.22 mm) before use. The concentration of HDL3 was
expressed in terms of their protein content (g/l) mea-sured by Bradford’s method [21] using BSA as stan-dard. The purity, composition and specific mobility of HDL3 were assessed by previously reported methods
[6].
2.3. Iodination of HDL3
Freshly isolated HDL3 were labeled with Na 125I by
the Iodo-Gen method [22] and the solution was subse-quently treated to remove unbound iodine as previously described [6]. In all cases, more than 75% of trichlo-racetic acid-precipitable radioactivity was associated with the protein component of lipoproteins. The 125
I-HDL3 was used immediately at a specific radioactivity
of 1.790.1 Bq/ng protein. The chromatographic profi-les of HDL3 before and after iodination, obtained in a
fast protein liquid chromatography device [6], indicated that this iodination method did not cause aggregation or breakdown products of HDL3.
2.4. Modification of HDL3 particles
Tyrosine (Tyr) residues of HDL3 were modified by
tetranitromethane (TNM) nitrosylation according to the method described by Chacko et al. [23]. HDL3 at a
protein concentration of 2 mg/ml was incubated with 5 ml/ml of a freshly prepared solution of TNM 0.6 M in ethanol for 1 h at room temperature. TNM – HDL3was
then dialyzed and filtered before use (0.45 mm, Millipore)
2.5. Platelets
washed under our experimental conditions showed ‘swirling’ as a strong indication that discoid shape was maintained and fully responded to agonists in the pres-ence of fibrinogen.
2.6. Ligand binding studies and competition experiments
Washed platelets (108) from healthy volunteers were
incubated at room temperature for 25 min with 125
I-HDL3 (up to 1.5 g/l) in a total volume of 0.25 ml of
incubation buffer. 125
I-HDL3 binding to platelets was
determined as described previously [6] and nonspecific binding was defined as binding that was not displaced by a 100-fold excess of unlabeled HDL3. Displacement
of 125I-HDL
3 (0.05 g/l) in the presence of varying
protein concentrations of unlabeled HDL3 particles
(native and TNM – HDL3) or the main GPIIb – IIIa
ligands (fibrinogen, fibronectin, von Willebrand factor and vitronectin) was measured as described above. Cal-culation of the bound lipoprotein was based on the specific activity of the labeled ligand, and the results were expressed as nanograms of protein bound per 108
platelets dissociation constants (Kd) for the competing ligands (nmol/l) were determined according to the method of Cheng and Prussof [25]. Displacement curves were analyzed and the NHobtained [26]. Finally, Bmax
andKdwere calculated by Scatchard analysis [27] of the specific binding data using the KINETIC/EBDA/LIGAND program [28]. To investigate whether platelet GPIIb and GPIIIa are implicated in the binding of HDL3 to
intact resting platelets, we used the following ap-proaches: (1) the specificity of HDL3 binding sites for
platelet integrin aIIbb3 was evaluated by displacement
experiments of 125I-HDL
3 by several purified GPIIb –
IIIa ligands, such as fibrinogen, fibronectin, von Wille-brand factor and vitronectin, as mentioned above; (2) with a human model of inherited lack of GPIIb – IIIa, the relationship between HDL3 binding sites and
platelet integrin aIIbb3 was investigated on intact
platelets from GT patients (type I and II) by binding and functional studies (HDL-mediated activation of PKC and the HDL-mediated inhibition of thrombin-in-duced inositoltriphosphate formation and calcium [Ca2+] mobilization); and (3) immunological
ligand-binding assays were performed after washed human platelets had been preincubated with the indicated con-centrations of a wide panel of polyclonal antibodies and mAbs or their respective negative antisera for 10 min at room temperature before the addition of 125
I-HDL3 (0.25 g/l). The polyclonal antibodies tested were
the following: anti-GPII – IIIa complex (edu-3 and 5B12), anti-integrin subunits (anti-aV or anti-a
-vit-ronectin receptor, and anti-b3), and human antiserums
(anti-Baka/B
and anti-PLA1/2
). We also tested pure murine mAbs that recognize different epitopes of
GPIIb (M3, M4, M5, M6, M8 and M95-2b) and GPI-IIa (P23-7, P33, P37, P40 and P97) and strongly inhibit both platelet aggregation and fibrinogen binding; some of them (P40, P37, P23-7 and M5) cross react with b -anda-subunits of the vitronectin receptor in endothelial cells [29].
2.7. Measurement of cytosolic free Ca2+ concentration
Basal platelet cytosolic free Ca2+ concentration
[Ca2+]
i using the Ca
2+-sensitive fluorophore Fura 2
(Fura 2/AM) was determined in control and thrombas-thenic platelets. Briefly, fura 2/AM (2 mmol/l) in DMSO and PGE1 (5 mmol/l) were added to 10 ml of
PRP. Next, PRP was incubated for 1 h at 37°C and centrifuged at 1100×g for 15 min. The supernatant plasma was discarded and the pellet was resuspended in 1 ml HEPES – Tyrode’s buffer containing 5 mmol/l PGE1 and 5 mmol/l EGTA. The platelet count was
adjusted to 1×108/ml using HEPES – Tyrode’s buffer
without PGE1 and EGTA. In some experiments,
washed platelet suspension was loaded with 2 mmol/l fura 2/AM and data were similar to those of the PRP loaded method (results not shown). Aliquots of 1.6 ml were incubated at 37°C for 5 – 10 min, in the presence and absence of thrombin (0.1 U/ml), HDL3 (1.0 g/l)
and both agents, in a thermostatized quarz cuvette with constant stirring. Fluorescence intensity was measured with excitation set at 340 and 380 nm and emission at 510 nm.
2.8. Determination of inositol 1,4,5-triphosphate (IP3)
release
After stimulating control and thrombasthenic platelets with thrombin (0.1 U/ml), HDL3(1.0 g/l), and
both agents, the reaction was stopped by adding 1 g/l TCA 1:5 (v/v). The precipitate was removed by cen-trifugation and the water soluble-phase was neutralized. Finally, IP3 release was determined by a commercial
radioreceptor assay (NEN, Barcelona, Spain).
2.9. Platelet membrane isolation and protein kinase
(PKC) assay
was investigated following the method described previ-ously [6]. The PKC enzyme activity assay was assayed using a commercially available kit (Amersham).
2.10. Statistical tests
The results are expressed as mean9S.E.M. Statisti-cal analysis was performed with Student’s t-test for paired data with PB0.05 considered significant.
3. Results
3.1. Binding of 125I-HDL
3 to washed human platelets
To determine whether platelet GPIIb – IIIa or fibrino-gen receptors are implicated in the binding of HDL3to
platelets, we carried out binding studies of125
I-HDL3to
platelets from type I (I AIo) and type II (A Dom) thrombasthenic patients with abnormal levels of GPIIb – IIIa and fibrinogen. We found that both type I and type II GT platelets bound 125I-HDL
3 and
pre-sented similar values (2978 binding sites per platelet with a Kd of 236 nmol/l and 3356 binding sites per
platelet with a Kd of 189 nmol/l, respectively) to those of control platelets (27739432 binding sites with a Kd
of 3559120 nmol/l).
3.2. [Ca2+]
i transients in human platelets
The basal value of cytosolic free [Ca2+]
i in resting
control and thrombasthenic platelets incubated in a low-Ca2+ medium was 115916 and 126921 nmol/l
(mean9S.E.M., n=3), respectively. This response was not changed when platelet cytosolic free [Ca2+]
i was
determined in a medium containing 1 mmol/l external Ca2+ (111914 and 119917 nmol/l; mean9S.E.M., n=3). In a low-Ca2+ medium, HDL
3 (1.0 g/l) did not
change the basal cytosolic free [Ca2+]
i, in control and
thrombasthenic platelets. Thrombin (0.1 U/ml) induced in control and thrombasthenic platelets a significant increase in cytosolic free [Ca2+] in a low-Ca2+ medium
(489978 and 501976 nmol/l; mean9S.E.M., n=3, respectively) and in 1 mmol/l [Ca2+] medium (8059
101 and 824989 nmol/l; mean9S.E.M.,n=3, respec-tively). When control and thrombasthenic platelets were pretreated with HDL3 (1,0 g/l for 2 min at 37°C), the
thrombin-induced rise in [Ca2+]
idecreased significantly
to 65 and 72%, respectively (PB0.001). This inhibitory effect in the presence and absence of external Ca2+ was
dose-dependent (results not shown). Finally, TNM – HDL3 (1.0 g/l) had no effect and cytosolic free [Ca
2+] i
mobilization induced by thrombin was not significantly different from that observed in the absence of native HDL3.
3.3. Inositol 1,4,5-triphosphate(IP3) formation
On stimulation with HDL3 (1.0 g/l) the amount of
IP3did not change the resting level (16.394.1 pmol/10 9
platelets) in both control and thrombasthenic platelets (15.695.1 and 18.393.3 pmol/109 platelets,
respec-tively). Thrombin (0.1 U/ml) rapidly enhanced IP3
for-mation in control and thrombasthenic platelets, reaching the maximum effect within 15 s (5896.6 and 65912 pmol/109platelets, respectively). IP
3returned to
basal levels within 45 s and no increase was observed thereafter. When platelets were preincubated in the presence of HDL3 (1.0 g/l), the thrombin-induced IP3
formation in control and thrombasthenic platelets was inhibited to 80 and 75%, respectively. In contrast, in the presence of TNM – HDL3 (1.0 g/l), the
thrombin-in-duced IP3 formation was unaltered in control and
thrombasthenic platelets (63.497.1 59.696.6 pmol/ 109 platelets, mean9S.E.M., n=3, respectively).
3.4. Protein kinase C acti6ation
In resting control and thrombasthenic platelets, 159
8 and 1795% of total PKC activity was membrane associated. When platelets were preincubated for 2 min in the presence of HDL3(1.0 g/l) the total PKC activity
associated with membranes rapidly increased (449
3.1% in control platelets and 4793% in thrombas-thenic platelets), with no return to basal level within the 10-min stimulation period. Exposure to TNM – HDL3
(1.0 g/l) produced no significant effect in control and thrombasthenic platelets.
3.5. Competition experiments
To determine whether increasing concentrations of unlabeled HDL3 and specific GPIIb – IIIa ligands
(fibrinogen, vitronectin, von Willebrand factor and fibronectin) interfere with 125I-HDL
3, we carried out
competition experiments of HDL3 binding to intact
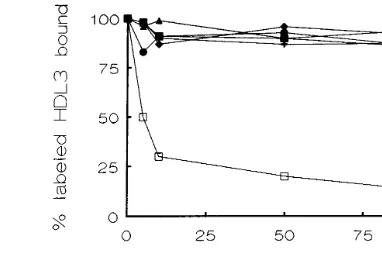
resting platelets at 22°C for 25 min. Fig. 1 shows that the binding of125
I-HDL3(0.05 g of protein/l) was fully
inhibited by unlabeled HDL3. Half-maximal (IC50) and
inhibition constant (Ki) values were 0.04 g/l and 34 mg/ml, respectively. The Hill coefficient, calculated from the slope of displacement curves analyzed by Hill plots, was −1.05 which suggests that a single set of binding sites is involved. In contrast, purified ligands of GPIIb – IIIa, such as fibrinogen (up to 10 g of protein/ml), fibronectin (up to 10mmol/l), von Willebrand factor (up to 10mmol/l) and vitronectin (up to 10mmol/l) were not effective competitors of125I-HDL
3and there was only a
slight unspecific decrease in the total binding. Finally, TNM – HDL3 (up to 1.5 g/l) had no effect, indicating
that modification of Tyr residues by nitrosylation fully abolished the ability of HDL3particles to interact with
Fig. 1. Inhibition of125I-HDL
3binding to intact resting platelets by specific ligands of GPIIb – IIIa. Platelets were preincubated for 25 min at room temperature with125I-HDL
3 (0.05 g/l) both in the presence and in the absence of unlabeled HDL3(", up to 0.5 g/l), fibrinogen (, up to 10 mg/l), fibronectin (, up to 10mmol/l), von Willebrand factor ( , up to 10 mmol/l) and vitronectin (+, up to 10mmol/l). Specific binding was determined as indicated in Section 2. Results are expressed as the mean of four independent experiments per duplicate (S.E.M. never exceeds more than 10% of the mean).
Fig. 3. Plot showing the effect of human antiserums against platelet alloantigens. (+, anti-Baka/B[=Leka/b, HPA-3, or anti-GPIIb]); , anti-PLA1/2[ZWa/b, HPA-1, or anti-GPIIIa]) and polyclonal antibod-ies against integrin subunits (, anti-b3;, anti-aV) or against the GPIIb – IIIa complex;", 5B12). Unlabeled HDL3() was used as a positive inhibitor in all experiments. Results are the mean of four independent experiments (S.E.M. never exceeds more than 10% of the mean).
showed any specific inhibitory effect at any protein concentration. However, with some mAbs against GPIIb, a maximum inhibition (35%) was found with low concentrations of P37. A similar unspecific effect was found with antiserums against platelet alloantigens and polyclonal antibodies against anti-aV (anti-a
-vit-ronectin receptor), and anti-b3 (anti-GPIIIa) integrin
subunits. All these experiments clearly indicate that neither the GPIIb – IIIa complex nor GPIIb or GPIIIa individually are the GPs implicated in the binding of HDL3 to intact resting platelets.
4. Discussion
Previous studies demonstrated that LDL and HDL3
binding sites are present on platelet membranes [4 – 6]. Koller et al. [16] reported that integrin aIIbb3, the
3.6. Platelet IntegrinaIIbb3 and 125I-HDL3 binding to
platelets
To investigate further whether the intact GPIIb – IIIa complex is involved in the binding of 125I-HDL
3 to
intact resting platelets [either individually (GPIIb and GPIIIa) or as a Ca2+-dependent heterodimer (GPIIb –
IIIa)], we carried out ligand-binding studies in the absence and in the presence of a large panel of antibod-ies against platelet integrin aIIbb3 and known platelet
alloantigens. Fig. 2 represents typical line plots that show no inhibition of 125I-HDL
3 binding to intact
resting platelets by mAbs against GPIIIa (panel A), GPIIb, and the GPIIb – IIIa complex (panel B). Fig. 3 shows similar negative inhibitory effects of human anti-serums and polyclonal antibodies on125
I-HDL3binding
to intact resting platelets. No experiment (n=7)
Fig. 2. Plots showing the effect of several antibodies against the GPIIb – IIIa complex or, individually, GPIIb and GPIIIa on HDL3binding to intact resting platelets. Percentages of specifically bound125I-HDL
inducible fibrinogen receptor, may be related to the binding of LDL and HDL3 particles to human
platelets. However, the idea that platelet integrinaIIbb3
is related to platelet LDL binding sites has recently been discarded [7,12,13,17]. Moreover, platelet HDL3
binding characteristics clearly differ from those of fibrinogen [4 – 6]. Furthermore, instead of inducing inhi-bition, HDL3 particles did not modify the binding of
fibrinogen to ADP-activated platelets [7]. Evidence indi-cating that platelet HDL3 binding sites are coupled to
phospholipase C through pertussis toxin-sensitive GTP binding proteins has been described [14]. Moreover, the inhibition of phosphatidylinositol hydrolysis and the formation of phophoinositide-derived second messen-gers via protein kinase C (PKC) have been involved in the anti-aggregatory effect mediated by HDL3 [6,14].
Indeed, both the number of binding sites [6] and their coupled biological response [14] are downregulated and desensitized by a PKC-dependent mechanism when platelets are incubated with high concentrations of HDL3. Overall data suggest that platelet HDL3binding
sites may be unrelated to integrin aIIbb3. To explore
these controversial results, we have investigated the implication of platelet GPIIb – IIIa or integrinaIIbb3 in
the binding of HDL3 to intact platelets.
Platelet integrin aIIbb3 (the GPIIb – IIIa complex) is
the most prominent member of the integrin family of platelet adhesive receptors that, upon activation, medi-ates platelet aggregation by binding of fibrinogen and recognizes with high affinity several adhesive proteins with the Arg – Gly – Asp (RGD) sequence [30]. The con-version of platelet membrane GPIIb and GPIIIa from the latent state to the GPIIb – IIIa complex is a calcium-dependent mechanism. The binding of fibrinogen there-fore requires this divalent ion, and chelating agents such as EDTA cause the dissociation of the complex at 37°C. This fully inhibits both fibrinogen binding and platelet aggregation [30]. It is well known that platelet
125
I-HDL3 binding does not require calcium ions and
that EDTA at 37°C does not inhibit the binding of HDL3 to intact platelets [6]. In this study, the possible
involvement of platelet integrinaIIbb3in the binding of
HDL3 and in the functional HDL3-mediated effects
were investigated with platelets from type I and type II GT and by ligand and immunological binding studies. Our results provide evidence that platelet integrinaIIbb3
is not related to the binding of HDL3 to intact resting
platelets. First, HDL3 binding characteristcs (Kd and Bmax), HDL3-mediated activation of PKC and HDL3
-mediated inhibition of thrombin-induced IP3formation
and Ca2+ mobilization in platelets from type I and
type II GT patients were similar to those of control intact resting platelets. The specificity of these effects was corroborated using a well-characterized model of modified HDL3 particles [23]. Under our experimental
conditions, displacement studies demonstrated that125
I-HDL3 binding to intact resting platelets was inhibited
with the same affinity by unlabeled HDL3 and the Hill
coefficient determined from Hill plots showed that a single set of binding sites is involved. However, modifi-cation of Tyr residues of HDL3 particles by
nitrosyla-tion [23] fully abolished both the capacity of particles to interact with its specific platelet binding sites and the functional HDL3-mediated effects (activation of PKC
and inhibition of the thrombin-induced IP3 formation
and Ca2+ mobilization).
Second, assuming that the GPIIb – IIIa complex may binds HDL3, then its main specific ligands (fibrinogen,
vitronectin, von Willebrand factor and fibronectin) should inhibit the binding of HDL3 to platelets.
How-ever, our results demonstrate that none of them have any inhibitory effect. These results are in agreement with previous data [7] showing that HDL3 was unable
to modify fibrinogen binding in activated platelets. Third, in contrast to Koller et al. [16], an ample panel of polyclonal, human platelet antiserum, and pure murine mAbs at concentrations that strongly inhibit both platelet aggregation and platelet fibrinogen bind-ing [29], did not block the bindbind-ing of 125I-HDL
3 to
intact resting platelets.
In conclusion, we present evidence that neither the GPIIb – IIIa complex nor GPIIb or GPIIIa individually are the ligand binding proteins for HDL3 on intact
resting platelets. Further studies are required to identify platelet HDL3 binding sites.
Acknowledgements
This work was supported by grants from the Comi-sio´n Interministerial de Ciencia y Tecnologı´a (SAF94-0191, SAF98-0081) and from the EC Biomed-2 project BMH4-CT 96-0134. Dr. Pedren˜o was the recipient of the Daria Haust Fellowship Award 1995 of the Interna-tional Atherosclerosis Society.
References
[1] Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogen-esis of coronary artery disease and the acute coronary syn-dromes. N Engl J Med 1992;326:310 – 8.
[2] Carvalho ACA, Colman RW, Lees RS. Platelet function in hyperlipoproteinemia. N Engl J Med 1974;290:434 – 8.
[3] Higashihara M, Kinoshita K, Kume S, Teramoto M, Kurokawa K. The role of apoE in inhibitory effects of apoE-rich HDL on platelet function. FEBS Lett 1991;18:1331 – 6.
[4] Koller E, Koller F, Doleschel W. Specific binding sites on human blood platelets for plasma lipoproteins. Hoppe-Seyler’s Z Physiol Chem 1982;363:395 – 405.
[6] Pedren˜o J, Vila M, Masana L. Mechanisms for regulating platelet high density lipoprotein type 3 binding sites. Evidence that binding sites are down-regulated by a protein kinase C-de-pendent mechanism. Thromb Res 1999;94:33 – 44.
[7] van Willigen G, Gorter G, Akkerman J-W. LDLs increase the exposure of fibrinogen binding sites on platelet and secretion of dense granules. Arterioscler Thromb 1994;14:41 – 6.
[8] Pedren˜o J, de Castellarnau C, Cullare C, Sanchez J, Go´mez J, Ordo J, Gonalez F. LDL binding sites on platelets differ from the ‘classical’ receptor of nucleated cells. Arterioscler Thromb 1992;12:1353 – 62.
[9] Pedren˜o J, de Castellarnau C, Cullare´ C, Ortin R, Sanchez J, Llopart R, Gonalez F. Platelet LDL receptor recognizes with the same apparent affinity both oxidized and native LDL. Evidence that the receptor – ligand complexes are not internalized. Arte-rioscler Thromb 1994;14:401 – 8.
[10] Pedren˜o J, Ferna´ndez R. Proteolytic susceptibility of platelet LDL receptor. Lipids 1995;30:927 – 33.
[11] Le Quan Sang K-H, Levenson J, Megnien J-L, Simon A, De-vynck M-A. Platelet cytosolic Ca2+and membrane dynamics in
patients with primary hypercholesterolemia. Effects of pravas-tatin. Arterioscler Thromb Vasc Biol 1995;15:759 – 64.
[12] Hackeng CM, Huigsloot M, Pladet MW, Nieuwenhuis HK, van Rijn HJ, Akkerman JW. Low-density lipoprotein enhances platelet secretion via integrin-alphaIIbbeta3-mediated signaling. Arterioscler Thromb Vasc Biol 1999;19:239 – 47.
[13] Hackeng CM, Pladet MW, Akkerman JW, van Rijn HJ. Low density lipoprotein phosphorylates the focal adhesion-associated kinase p125(FAK) in human platelets independent of integrin. J Biol Chem 1999;274:384 – 9.
[14] Nazih H, Nazih-Sanderson F, Magret V, Caron B, Goudemand J, Fruchart JC, Delbart C. Protein kinase C-dependent desensi-tization of HDL3-activated phospholipase C in human platelets. Arterioscler Thromb 1994;14:1321 – 9.
[15] Nofer JR, Walter M, Kehrel B, Wierwille S, Tepel M, Seedorf U, Assmann G. HDL3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol 1,4,5-triphosphate. Arterioscler Thromb Vasc Biol 1998;18:861 – 9.
[16] Koller E, Koller F, Binder B. Purification and identification of the lipoprotein-binding proteins from human blood platelet membrane. J Biol Chem 1989;264:12412 – 8.
[17] Pedren˜o J, Ferna´ndez R, Cullare´ C, Barcelo´ A, Elorza MA, de Castellarnau C. Platelet integrinaIIbb3 (GPIIb – IIIa) is not
im-plicated in the binding of LDL to intact resting platelets. Arte-rioscler Thromb Vasc Biol 1997;17:156 – 63.
[18] Endemann G, Stanton L, Madden K, Bryant K, While RT, Protter A. CD36 is a receptor for oxidized low density lipo-proteins. J Biol Chem 1993;268:1181 – 6.
[19] Pedren˜o J, Hurt-Camejo E, Wiklund O, Masana L. Platelet CD36 is a specific receptor for native and modified low-density lipoproteins. Atherosclerosis (Abstr) 1997;138:184.
[20] Havel RJ, Eder Ha, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in hu-man serum. J Clin Invest 1955;34:1345 – 53.
[21] Bradford MA. A rapid and sensitive method for the quantifica-tion of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 1976;72:248 – 54.
[22] Salacinski PR, McLean C, Sykes JE, Clement-Jones VV, Lowry PJ. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetracholoro-3,6-diphenylgly-coluril (iodogen). Anal Biochem 1981;117:136 – 46.
[23] Chacko GK. Modification of human high density lipoprotein (HDL3) with tetranitromethane and the effect on its binding to isolated rat liver membranes. J Lipid Res 1985;26:745 – 54. [24] James NG, Caen JP, Nurden AT. Glanzmann’s thrombasthenia:
the spectrum of clinical disease. Blood 1990;75:1383 – 95. [25] Cheng YC, Prusoff WH. Relationship between the inhibition
constant (Ki) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem Pharma-col 1973;22:3099 – 108.
[26] Bennett JP, Yamamura HI. Neurotransmitter, hormone, or drug receptor binding methods. In: Yamamura HI, Enna SJ, Kuhar MJ, editors. Neurotransmitter Receptor Binding. New York: Raven Press Publishers, 1985:57 – 90.
[27] Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci 1949;51:660 – 72.
[28] McPherson GA. Analysis of radioligand binding experiments: a collection of computer programs for IBM PC. J Pharmacol Methods 1985;14:213 – 28.
[29] de Castellarnau C, Cullare´ C, Alvarez MV, Mun˜iz-Diez E, Calzada J, Gonzalez-Rodriguez J. Functional characterization of GPIIb- and GPIIIa-specific monoclonal antibodies. Further evi-dence for the existence of agonist-specific activated states of the platelet fibrinogen receptor. Platelets 1997;8:243 – 53.
[30] Shatill SJ. Function and regulation of the beta3 integrins in hemostasis and vascular biology. Thromb Haemost 1995;74:149 – 55.