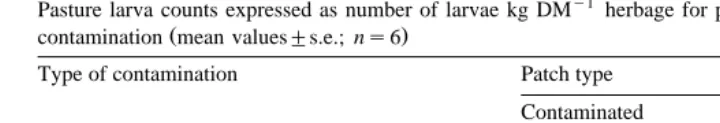www.elsevier.comrlocaterapplanim
Strategies for the avoidance of faeces by grazing
sheep
Jane Cooper
a,b,), Iain J. Gordon
a, Alan W. Pike
ba
Macaulay Land Use Research Institute, Craigiebuckler, Aberdeen AB15 8QH, UK
b
Department of Zoology, UniÕersity of Aberdeen, Tillydrone AÕenue, Aberdeen, AB24 2TZ, UK
Accepted 28 February 2000
Abstract
Experiments were conducted to investigate which environmental cues were used by sheep when discriminating against patches of pasture contaminated with faeces. The influence of the spatial distribution of contaminated patches and the parasite infection status of sheep on avoidance of contaminated patches and ingestion of parasite larvae was also investigated. In experiment 1, sheep infected with the parasite Ostertagia circumcincta were given the opportunity to graze in uncontaminated or aggregated contaminated patches. Patch contamination comprised of either faeces from sheep infected with O. circumcincta larvae, faeces from uninfected sheep, or O.
circumcincta larvae only. Infected sheep discriminated against faeces from parasite-infected
animals and faeces from uninfected animals equally. Sheep did not discriminate against patches contaminated with parasite larvae only. In experiment 2, sheep infected with O. circumcincta and uninfected sheep grazed experimental plots with differing spatial patterns of faecal-contaminated patches, allowing animals the opportunity to forage in contaminated or uncontaminated patches of herbage. Plots were also grazed by infected and uninfected animals that were fistulated at the oesophagus to enable the collection of ingested herbage. Sheep spent a greater proportion of their time foraging in uncontaminated patches than in contaminated patches. Where patches were highly aggregated, infected animals spent a greater proportion of total grazing time in uncontaminated patches than did uninfected animals, and grazed uncontaminated patches for longer on each sampling occasion. On grazing plots where all patches were contaminated, the difference between the numbers of larvae isolated from pasture herbage and ingested herbage was greatest for infected animals. In this situation, infected animals avoided parasites most. On grazing plots consisting of both contaminated and uncontaminated patches, the difference between the numbers of larvae isolated from pasture herbage and ingested herbage was greatest for uninfected animals. In this
)Corresponding author. Institute for Animal Health, Compton, Newbury, Berkshire, RG20 7NN, UK. Tel.: q44-1635-578-411, ext. 2613; fax:q44-1635-577-303.
Ž .
E-mail address: [email protected] J. Cooper .
0168-1591r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
situation, uninfected animals were most effective at parasite avoidance as they consumed fewer parasite larvae relative to what was available on pasture.q2000 Elsevier Science B.V. All rights
reserved.
Keywords: Sheep; Feeding and nutrition; Foraging behaviour; Grazing behaviour; Parasitism; Spatial distribu-tion
1. Introduction
The need to avoid parasites acts as a major selection pressure in the evolution of
Ž . Ž .
animal behaviour Hart, 1990 . Elements of migratory Folstad et al., 1991 , social
ŽLoehle, 1995 , sexual Kavaliers and Colwell, 1995b and grooming Mooring 1995. Ž . Ž .
behaviours of hosts all play a role as parasite defence strategies. Surprisingly, there have been few studies that investigate the role that an animal’s foraging strategy can play in behavioural defence against parasites. It has been established that negative factors such
Ž . Ž .
as the risk of predation Edwards, 1983 , competition Millikan et al., 1985 and
Ž .
consuming plant toxins Provenza et al., 1998 can have a constraining influence on a herbivore’s foraging strategy. Foraging animals should also be constrained by the risk of
Ž .
parasite infection Lozano, 1991 . Recent studies suggest that this is indeed the case
ŽHutchings et al., 1998, 1999 , although further evidence is required..
Gastrointestinal parasites can have detrimental effects on the fitness of an animal
ŽHart, 1990 . For example, when animals are under nutritional stress, gastrointestinal.
helminths may increase in number within the host and can be a contributing factor in
Ž .
host mortality Hart, 1990; Gulland, 1992 . Parasites can also incur a less apparent cost to host fitness by having detrimental effects on the location of resources and spatial
Ž
learning, reproduction, predator avoidance and territorial defence Godin and Sproul, 1988; Milinski, 1990; Moore and Gotelli, 1990; Zuk, 1992; Moller et al., 1993;
.
Kavaliers and Colwell 1995a . Hence, minimising the number of parasites which are ingested whilst foraging should be a driving force in shaping foraging strategies.
Ž
Ruminants are known to avoid grazing herbage which is contaminated with faeces e.g.
.
Gruner and Sauve, 1982; Hutchings et al., 1998, 1999 . Foraging in close proximity to faeces carries a risk of parasite infection as such areas are reservoirs of gastrointestinal
Ž .
parasite larvae Williams and Bilkovich, 1973 . Consequently, it has long been thought that avoidance of contaminated swards by ruminants might be a parasite defence strategy.
It is not clear which cues a grazing animal uses when discriminating against faeces-contaminated herbage. There is evidence that animals can distinguish between
Ž .
uninfected and infected individuals Kavaliers and Colwell, 1995b and also between
Ž .
faeces from uninfected and infected animals Evans et al., 1992; Pappas et al., 1995 , presumably by olfactory means. Consequently, in the current study the role of three
Ž
possible cues faeces from parasite-free sheep, faeces from parasite-infected sheep, and
.
parasite larvae alone in the detection of contaminated patches of pasture were investi-gated.
If avoidance of faecally contaminated herbage is a parasite defence strategy, it must
Ž .
only one previous study has experimentally examined whether avoidance of faeces by
Ž .
ruminants results in a reduction in the number of parasites consumed. Michel 1955 compared the number of DictyocaulusÕiÕiparus larvae isolated from herbage
immedi-ately adjacent to where cattle grazed with the number isolated from random samples of pasture herbage. He concluded that cattle grazed in a manner that resulted in the consumption of fewer larvae than if grazing was random and that selective grazing was a parasite avoidance strategy. However, this conclusion was based only on estimates of the numbers of larvae that cattle ingested. In the study reported here we used direct measurements of consumed parasite larvae to lend weight to our findings.
Environmental and internal factors, other than simply the presence of faeces on herbage, may affect the ability of ruminants to distinguish between clean and contami-nated areas. Selection of alternate foods by sheep is known to be influenced by the scale
Ž .
of aggregation of patches Edwards et al., 1994 . Hence, in this study the influence of the scale of aggregation of faeces-contaminated patches had on diet selection was investigated. Factors, internal to the animal, which play a part in diet selection include
Ž .
parasitism. Hutchings et al. 1998, 1999 conducted a series of small-scale diet-choice experiments and observed that parasite-infected sheep avoid faeces contaminated swards more than uninfected sheep. Although demonstrative, these studies were conducted in artificial conditions. The current study approaches more natural conditions, as the effect of parasite infection on faeces avoidance behaviour by sheep on pasture was investi-gated.
This paper presents two experiments, the aims of which were to investigate the cues used by sheep to avoid faecal contaminated patches, how avoidance of faecal-contamina-tion and ingesfaecal-contamina-tion of parasite larvae by sheep on pasture was influenced by the spatial distribution of contaminated patches, and how parasite infection status influences grazing by animals.
2. Materials and methods
This paper reports on two choice experiments: in experiment 1, the presence of faeces as an environmental cue in parasite avoidance was investigated. In experiment 2 we determined the extent to which avoidance of faecal-contaminated patches of pasture was affected by the parasite infection status of grazing sheep and how avoidance behaviour was influenced by the spatial distribution of the patches. Experiment 2 also sought to investigate the relationship between the parasite infection status of the animal, patch distribution pattern, and the numbers of parasite larvae which were actually consumed by the animal whilst grazing on pasture. Experiments were conducted during August
Ž . Ž .
1993 Experiment 1 and September 1995 Experiment 2 at the Macaulay Land Use
Ž .
Research Institute’s Glensaugh Research Station Kincardineshire, Scotland .
2.1. Animals
Ž y1.
All animals were dosed orally with ivermectin Oramec drench; 200 mg kg to
individually in indoor pens and fed a daily ration of 0.75 kg of a standard lamb and ewe
Ž .
dry feed North Eastern Farmers, Aberdeen .
2.1.1. Experiment 1
Ž
Five, Scottish Blackface, castrate male sheep, approximately 1-year-old mean live
.
weight 34.5 kg; range 31.5–38.0 kg were used in the study. To mimic a light, sub-clinical parasite infection, animals were trickle-dosed daily with 1500 Ostertagia circumcincta infective larvae for 15 days. O. circumcincta is an abomasal nematode
Ž
frequently associated with outbreaks of parasitic gastroenteritis in lambs Coop et al.,
.
1982 . Dosing ended 23 days before commencement of the grazing trials to ensure that all parasites had matured. One week before the start of the grazing trials animals were
Ž .
moved to an outdoor paddock and grazed on a perennial ryegrass Lolium perenne pasture. The pasture had not been grazed for a year and a silage cut had been taken prior to the experiment to minimise the number of viable O. circumcincta larvae present. The number of O. circumcincta larvae on the pasture was not determined but was assumed to be low.
2.1.2. Experiment 2
Ž
Twenty, Scottish Blackface, castrate male sheep, approximately 1-year-old mean live
.
weight 35.0 kg; range 30.0–42.5 kg , were weighed and ranked by live weight and then divided into four treatment groups, each of five animals, balanced for weight.
To enable the collection of ingested herbage, animals in Groups 1 and 3 were
Ž .
fistulated at the oesophagus Pfister et al., 1989 and allowed to recover for a period of 3 weeks. Animals were monitored twice daily following recovery. One animal in Group 1 became dehydrated and lost condition due to loss of saliva during the recovery period and was euthanased. To mimic a light, sub-clinical parasite infection, animals in Groups 1 and 2 were trickle-dosed daily with 1500 O. circumcincta infective larvae. Dosing began after the fistulated animals’ recovery period and lasted for 15 days. Dosing ended 23 days before commencement of the grazing trials. One week before the start of the grazing trials infected and uninfected animals were moved to seperate paddocks and grazed on perennial ryegrass pasture. The pasture had not been grazed for a year and a silage cut had since been taken to minimise the number of viable O. circumcincta larvae present. The number of O. circumcincta larvae on the pasture was not determined but was assumed to be low.
2.2. Plot layout and contamination
composition of the herbage. At the same time, plots were cut to ensure a uniform sward height of 6–8 cm at the start of the experiment. Plots were divided into a grid of patches that were marked with wooden pegs, painted white to enhance their detection on video tape.
2.2.1. Experiment 1
Plots were divided into four patches, each patch measuring 5 m=5 m. Six replicates of three different types of contamination were created on the 18 plots in the experimen-tal area. Plots were contaminated with either faeces from animals infected with O.
Ž . Ž .
circumcinta FP , faeces from worm-free animals F , or infective O. circumcincta
Ž . Ž .
larvae only P . Six kg of faeces wet weight was spread on each of two of the four patches within a plot to give a contamination rate of 240 g my2. Contaminated plots were diagonally opposite each other within the plot. Plots treated with faeces were contaminated 3 weeks before they were to be grazed. To minimise larval losses due to desiccation or heavy rain, plots treated only with larvae were contaminated the day before they were to be grazed. Larvae were applied using a watering can at a rate of 46,000 larvae per contaminated patch in a volume of 500 ml of water. This was equivalent to the number of larvae isolated from 6 kg of faeces from a sheep whose faeces had also been used to contaminate the FP plots.
2.2.2. Experiment 2
Plots were divided into a grid of 16 patches, each patch measuring 2.5 m=2.5 m. Quantities of faeces were obtained from animals which had patent O. circumcincta infections. Three weeks before the plots were to be grazed, quantities of these faeces were distributed across the patches within plots to create three distribution patterns of
Ž . Ž . Ž .
faecal contamination Fig. 1 : i Dispersed D , faecal pellets evenly spread over all
Ž . Ž . Ž .
squares; ii Small patch S , faecal pellets spread on every other square; iii Large
Ž .
patch L , faecal pellets spread on half the squares in such a way so as to create four
Ž .
patches. Faeces were spread at a level of 1.5 kg wet weight per 2.5 m=2.5 m contaminated square to give a contamination rate of 240 g my2. Six replicates of each of the three patterns were created within the 18 plots.
2.3. Grazing trials
Animals were without food for 1 h prior to each trial to encourage grazing. Throughout each trial, animals were fitted with faeces collection bags to avoid unwanted faecal contamination of plots.
2.3.1. Experiment 1
Two trials were conducted each day for 9 days. Each day, one trail was run in the morning and the second trial was run in the afternoon. Animals were randomly rotated around plots from trial to trial. In each trial, sheep grazed an experimental plot for 30 min.
2.3.2. Experiment 2
Ž .
Four trials one for each treatment group were run daily for a period of nine consecutive days. Two trials were run each morning and two were run each afternoon.
Ž . Ž .
Each day, infected Groups 1 and 2 and uninfected Groups 3 and 4 groups of animals were randomly allocated to graze one of the three different distribution treatments. Allocation of infected animals to morning or afternoon trials was alternated daily to eliminate any influence that time of day might have on collected data. This whole allocation procedure was replicated until each treatment group had grazed each of the
Ž .
three larva distribution plots three times nine trials per treatment group . Fistulated
Ž .
animals Group 1 or 3 were introduced to a plot and allowed to graze the pasture for 20 min. During this time, ingested herbage samples were collected in polythene bags. Occasionally samples were contaminated with regurgitated material and these were discarded. Fistulated animals on each plot were then replaced by unfistulated animals of the same infection status, i.e. Group 1 replaced by Group 2, Group 3 replaced by Group 4. Unfistulated animals grazed for 90 min.
2.4. Herbage measurements
In both experiments herbage samples were taken by cutting all grass from within a 1.25 m=0.20 m quadrant immediately before each trial. In experiment 1, one sample
Ž .
was taken from each patch of the plot four samples per plot . O. circumcincta larvae were isolated from each sample and counted using the technique described by Thomas
Ž1959 to give the mean larval count per patch type. In experiment 2, three samples were. Ž .
taken from plots composed of only contaminated squares D . Where plots were
Ž . Ž .
composed of contaminated and uncontaminated squares, S and L , two samples were
Ž .
taken from each patch type contaminated and uncontaminated . Mean larval counts were then calculated for each plot.
In each experiment, subsamples of pasture herbage were freeze-dried and analysed to determine their nitrogen content by a Carlo Erba NA1500 elemental analyser, using an
Ž .
automated Dumas combustion system. In vitro organic matter digestibility OMD of the
Ž
herbage was determined using a standard technique Alexander and McGowan, 1961;
.
2.5. Animal measurements
Faecal egg counts were conducted on each animal using a modification of the method
Ž .
of Jackson 1974 on the first day of each experiment to ensure that infections had been established in all challenged animals and that unchallenged animals were uninfected.
All grazing trials were recorded using a GYYR time lapse video cassette recorder
Žtape speed y1. Ž
s37 frames min , with the recorder mounted on a vertical tower 11 m
.
tall located at the centre of the plot system. The video tapes were used to estimate the total proportion of time each sheep spent grazing in uncontaminated patches of a plot and the duration of each discrete foraging bout in an uncontaminated patch. This was achieved by determining the grazing location of each animal at 60-s intervals. For the first experiment, the proportion of time spent in each patch type was calculated for the
Ž . Ž .
first grazing period 1 and last grazing period 2 30 min of the 90-min grazing trial involving unfistulated animals. Data from these two grazing periods were compared to see whether the behaviour of the experimental animals changed during the time course of the trial. Where animals were seen to be obviously resting, data were excluded from the analysis.
Larvae from extrusa samples collected from fistulated animals were recovered using
Ž .
the technique used by Thomas 1959 for pasture herbage.
2.6. Statistical analysis
Ž
Unless stated otherwise, statistical analyses were performed using Unistat 4 Unistat,
.
1997 . Sheep graze in a synchronised manner and so individual animals should not be
Ž .
treated as replicates in grazing experiments Rook and Penning, 1991 . Consequently, although data was available for all individuals, mean values were calculated for each grazing trial and these mean values were used in the analysis. Hence, the grazing trial
Ž .
was the unit of replication and not the individual animals . Each experiment involved a total of 18 grazing trials. Hence, results from experiment 1, which involved infected animals grazing plots with three different sources of contamination, are based on six replicates. The results from experiment 2, which involved both uninfected and infected animals grazing plots with three different patch distributions, are based on three replicates.
Unless otherwise stated, data was analysed using standard one-way analysis of
Ž .
variance ANOVA or t-tests. Analysis of data obtained from video tapes was restricted to trials involving unfistulated animals grazing plots with both contaminated and uncontaminated patches. The mean proportion of time sheep spent in uncontaminated plots was calculated for each trial replicate as was the duration of each grazing bout in an uncontaminated patch. Proportional data were subjected to an angular transformation prior to analysis to stabilise variance. In the second experiment, data obtained from the
Ž .
video was analysed using an analysis of variance ANOVA procedure for a split unit design with trial as the main experimental unit and grazing period as the split unit. The effects of parasite infection and pattern of contamination together with appropriate interactions was investigated. The structure of the ANOVA table is given in Table 1.
Ž
These analyses were carried out using Genstat 5, Release 3.1 Lawes Agricultural Trust,
.
Table 1
Structure of the ANOVA table used for analysis of data obtained from video tapes. Data were analysed using an ANOVA procedure for a split unit design with trial as the main experimental unit and grazing period as the split unit. The effects of infection status, contamination pattern and appropriate interactions were investigated
Ž .
Source of variation Degrees of freedom missing values
Main residual stratum
Contamination pattern 1
Grazing period 1
Contamination pattern=grazing period 1
Residual 8
Main residual units stratum
Infection status 1
Infection status=contamination pattern 1
Infection status=grazing period 1
Ž .
Residual 8 1
Ž .
Total 22 1
Log transformations were taken of larval count data prior to analysis. The difference between larval counts from pasture and ingested herbage was determined and this difference was subjected to a two-way ANOVA to investigate the effects of infection status and contamination pattern.
3. Results
3.1. Herbage parameters
In experiment 1 contamination of patches with faeces had no significant effect on the
Ž
in vitro OMD uncontaminated: 0.69"0.01; contaminated: 0.69"0.01; F1,34s0.02,
. Ž y1
P)0.8 or the concentration of nitrogen uncontaminated: 26.0"0.5 g kg DM ;
y1 .
contaminated: 25.2"0.4 g kg DM ; F1,34s1.30, P)0.2 . In experiment 2,
contami-Ž
nation of patches with faeces had no significant effect on the in vitro OMD
uncon-.
taminated: 0.81"0.01; contaminated: 0.81"0.01; F1,28s0.13, P)0.7 or the
con-Ž y1
centration of nitrogen uncontaminated: 24.7"0.7 g kg DM ; uncontaminated: 24.5"
y1 .
0.5 g kg DM ; F1,28s0.06, P)0.8 . This confirmed that faecal contamination had no effect on the sward chemical characteristics measured.
3.1.1. Experiment 1
Larvae were recovered from both contaminated and uncontaminated patches within
Ž .
plots regardless of the source of contamination treatment Table 2 , although counts were negligible on uncontaminated patches and patches contaminated with faeces from uninfected animals. The source of contamination on plots had no significant effect on
Ž .
Table 2
Pasture larva counts expressed as number of larvae kg DMy1 herbage for plots with three different types of
Ž .
contamination mean values"s.e.; ns6
Type of contamination Patch type
Contaminated Uncontaminated
Ž .
Faeces from parasite-free animal F 25"11 6"4
Ž .
Faeces from parasite infected animal FP 3644"1636 29"16
Ž .
Parasites P 4766"1942 12"6
no significant difference between larva counts from contaminated and uncontaminated
Ž .
plots from plot type F ts1.38, dfs0.10, P)0.1 Larval counts differed significantly
Ž
between contaminated and uncontaminated patches on plot type FP F1,10s27.1,
. Ž .
P-0.001 and on plot type P F1,10s47.5, P-0.001 . There was no significant difference between larval counts obtained from contaminated patches on plot type FP
Ž .
and those from plot type P F1,10s0.25, P)0.6 .
3.2. Parasitic status of animals
In experiment 1, all animals excreted nematode eggs on the first day of grazing trials
Žmean faecal egg count 458; range 126–905 eggs per gram of faeces . In both.
experiments, infected animals were free of clinical signs of infection throughout the study. In experiment 2, nematode eggs were not excreted by any of the non-parasitized animals. Parasitized animals all excreted nematode eggs on the first day of grazing trials
Žmean faecal egg count 543; range 99–1517 eggs per gram of faeces ..
3.3. Patch selection
3.3.1. Experiment 1
Single-sample t-tests revealed that sheep spent significantly more time grazing in
Ž
uncontaminated patches than in faecal contaminated patches expected value with no discriminations0.500; Plot F: proportion of observations in uncontaminated patchess
0.759, ts8.56, dfs5, P-0.001; Plot FP: proportion of observations in
uncontami-.
nated patchess0.805, ts10.57, dfs6, P-0.001 . However, where plots were
Ž .
contaminated only with O. circumcincta larvae P , sheep showed no preference for
Ž
either contaminated or uncontaminated patches expected value with no discrimination
s0.500; proportion of observations in uncontaminated patchess0.529; ts0.56, dfs5,
. Ž .
P)0.5 Fig. 2a .
The proportion of time sheep spent grazing uncontaminated patches on plots
contami-Ž .
nated with faeces from infected animals FP did not differ from the proportion of time spent grazing uncontaminated patches on plots contaminated with faeces from infected
Ž . Ž .
animals F F1,10s1.2, P)0.2 . The proportion of time spent grazing uncontaminated
Ž .
Ž .
Fig. 2. a Proportion of total grazing time spent in uncontaminated patches by infected sheep grazing plots
Ž . Ž .
with three different sources of contamination mean values"s.e. ; ns6. b Duration of grazing bouts in
Ž
uncontaminated patches by infected sheep grazing plots with varying sources of contamination mean
.
values"s.e. ; ns6.
Ž . Ž .
when they grazed plots contaminated with faeces from infected FP or uninfected F
Ž .
animals F2,15s14.7, P-0.001 .
Ž .
they grazed plots contaminated with only parasites F2,15s12.39, P-0.001 . The average length of a single encounter with an uncontaminated patches was not influenced
Ž . Ž .
by whether the faeces came from a worm-free F or a worm-infected sheep FP
ŽF1,10s0.612, P)0.4. ŽFig. 2b ..
3.3.2. Experiment 2
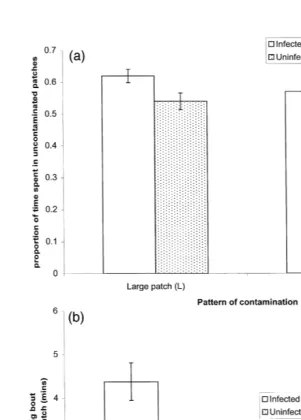
The total proportion of each grazing period that was spent in uncontaminated patches is summarised in Table 3. A single-sample t-test showed that overall sheep spent more
Ž
time grazing in uncontaminated patches than in contaminated patches total mean proportion of observations in uncontaminated patches, 0.575; s.e.s0.011; expected
.
value with no discriminations0.500; ts6.87, dfs22, P-0.001 . Neither the pattern
Ž . Ž .
of contamination F1,8s0.23, P)0.6 nor the grazing period F1,8s1.60, P)0.2 had a significant effect on proportion of time spent grazing in uncontaminated patches.
Ž .
However, infected animals Group 2 spent a significantly higher proportion of their
Ž .
time F1,8s8.03, Ps0.022 grazing in uncontaminated areas than did uninfected
Ž . Ž .
animals Group 4 Table 2 . A significant infection status=patch distribution pattern
Ž .
interaction F1,8s6.51, Ps0.034 indicated that discrimination between uncontami-nated and contamiuncontami-nated patches was considerably greater by infected animals in the
Ž .
plots with the Large patch L contamination pattern whilst there was no significant
Ž . Ž .
difference in plots with the Small patch S contamination pattern Fig. 3a .
The duration of discrete foraging bouts in uncontaminated patches is summarised in Table 4. Grazing period had no effect on the amount of time which was spent in each
Ž .
uncontaminated patch during a foraging bout F1,8s0.01, P)0.9 . The average amount of time spent in an uncontaminated patch during any particular encounter was
Ž .
greater for infected animals than for uninfected animals F1,8s14.77, Ps0.005 . Animals grazing the Large patch distribution spent longer in uncontaminated patches on
Ž
each individual encounter than when grazing the small patch distribution F1,8s12.29,
.
Ps0.008 . There was a significant interaction between infection status and patch
Ž .
distribution pattern F1,8s8.65, Ps0.019 on the amount of time spent in an uncon-taminated patch on each foraging bout. This was due to the difference between infected
Table 3
Ž .
Mean proportion of total time unfistulated animals Groups 2 and 4 spent grazing uncontaminated patches
Ž"s.e. . Observations were recorded over two grazing periods period 1. Ž s0–30 min; period 2s60–90 min.
Period Contamination pattern Infection status and group Mean Infected — Group 2 Uninfected — Group 4
Ž .
Fig. 3. a Proportion of total grazing time spent in uncontaminated patches by infected and uninfected sheep
Ž .
grazing plots with two different contamination patterns mean values"s.e. . Sheep rested during the second
Ž .
period of one grazing trial and data was not collected. Hence, for infected animals Group 2 , grazing Large
Ž . Ž .
patch L contamination pattern, ns5. In all other cases ns6. b Duration of grazing bouts in
uncontami-Ž .
nated patches for infected and uninfected sheep on plots of varying contamination pattern mean values"s.e. . Sheep rested during the second period of one grazing trial and data was not collected. Hence, for infected
Ž . Ž .
Table 4
Ž . Ž
Duration of discrete sampling events min in uncontaminated patches by unfistulated animals Groups 2 and
. Ž . Ž
4 . Observations were recorded over two grazing periods Period 1s0–30 min; Period 2s60–90 min mean
.
values"s.e.
Period Contamination pattern Infection status and group Mean Infected — Group 2 Uninfected — Group 4
Ž . Ž . Ž . Ž .
and uninfected animals being greater when animals grazed the Large patch distribution
ŽFig. 3b ..
3.4. LarÕa counts from pasture and ingested herbage samples
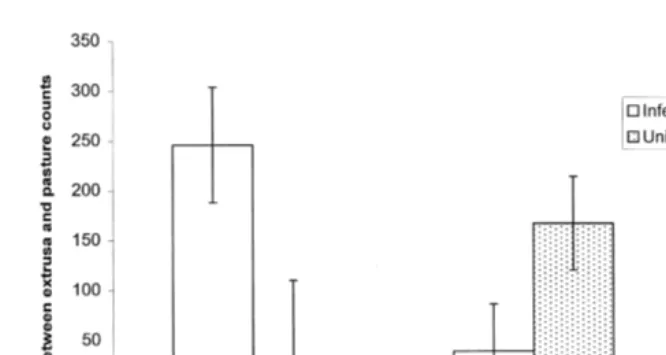
Larval counts from pasture and ingested herbage obtained in experiment 2 are summarised in Table 5. Overall, the number of larvae recovered from ingested herbage
Ž155"19 kg DMy1. were lower than the number that were obtained from pasture
Ž y1.
herbage 286"63 kg DM . Paired t-test results showed that this difference verged on
Ž .
significance ts2.09, dfs17, P-0.052 . ANOVA results showed that overall, the difference between larval counts from ingested and pasture herbage was not influenced
Ž .
by the infection status of sheep F1,12s0.047, P)0.8 or by the distribution pattern of
Ž .
contamination F2,12s0.668, P)0.5 . However, there was a significant status=pattern
Ž . Ž .
interaction F2,12s6.215, Ps0.014 Fig. 4 . The largest difference between the number of larvae isolated from pasture and the number isolated from ingested samples occurred when infected animals grazed plots with a dispersed pattern of contamination
Ž .D . When plots with a dispersed contamination pattern D were grazed by uninfectedŽ .
sheep the difference between the number of larvae from pasture and ingested herbage
Table 5
Larval counts from herbage ingested by fistulated animals, expressed as number of larvae per kg dry matter, for infected and uninfected animals grazing plots with three patterns of contamination. Counts from
Ž .
corresponding pasture herbage samples are also given mean values"s.e.
Infection status Herbage Contamination pattern Mean
Fig. 4. The difference between ingested and pasture herbage counts for infected and uninfected sheep grazing
Ž .
plots with different patterns of contamination mean values"s.e. ; ns3.
Ž
samples was lower than when aggregated patterns of contamination were grazed S and
. Ž . Ž .
L . When sheep grazed plots with either a Small S or Large L patch contamination pattern, the difference between the number of larvae on herbage and the number that were ingested was greatest for uninfected animals.
4. Discussion and conclusions
In both experiments, sheep preferentially grazed from patches free from contamina-tion with faeces. While such avoidance of contaminated pasture has been reported by
Ž .
other workers e.g. Gruner and Sauve, 1982; Hutchings et al., 1998, 1999 , it was not clear how sheep detected contaminated areas. Hence, one objective of this study was to investigate which environmental cues were used by sheep when discriminating against patches of pasture which carried a risk of parasite infection. It was not known whether sheep avoided the faeces, the parasites, or a combination of the two. It has been shown that cattle can visually detect ticks and hence avoid eating in areas of high infestations
ŽSutherst et al., 1986 . However, parasite-infected sheep did not avoid patches contami-.
nated solely by O. circumcincta larvae. This suggests that either the larvae cannot be detected, or if they can be, they are not associated with any risk and consequently not avoided. Instead, the presence of faeces acts as an environmental cue for sheep enabling them to detect and avoid contaminated patches. Sheep are known to associate cues with
Ž .
food rewards and use them to locate preferred food patches Edwards et al., 1997 . The use of faeces as a cue for discrimination shows that sheep also associate cues with unfavourable food items.
parasite infected mice have reduced aversive responses to odours associated with
Ž .
parasite infection Kavaliers et al., 1998 and in this experiment parasite infected animals were used. A more complete picture of the mechanisms involved in discrimina-tion would be available if parasite-free sheep had also been used in experiment 1. However, in a natural environment, most animals are infected with parasites or pathogens of some kind. Grazing animals are unlikely to encounter faeces that did not present some risk. Hence, it would be surprising if sheep had evolved a mechanism that enabled them to distinguish between parasite-free and parasite-associated faeces. Futher studies are required to demonstrate this experimentally.
Sight and smell are likely to be the principal senses involved in the recognition of
Ž .
faeces. Arnold 1966 observed that blinkered sheep would eat from pasture with a characteristic ‘dung-patch’ appearance, whereas unblinkered sheep would not. Unfortu-nately, it is not clear whether faeces were actually present in the patches or whether the patch simply consisted of herbage with different sward characteristics. The herbage surrounding such a patch is often a brighter shade of green than the rest of the pasture due to the nutrient influx from the faeces. Sheep can distinguish between different
Ž .
degrees of brightness Bazely and Ensor, 1989 , and may have been using either herbage characteristics or the presence of faeces to discriminate against the dung-patches in Arnold’s experiment. In the current experiment, contamination had no effect on sward characteristics; hence, sheep were not discriminating between patches using brightness of herbage as a visual cue.
Although the visual detection of faeces may be important, the sense of smell is almost certainly involved as well. Specific feeding deterrents have been found to cattle
ŽDohi et al., 1991 and goats Aoyama et al., 1994 in the odour of cattle faeces. The. Ž .
presence of specific feeding deterrents in sheep faeces has yet to be demonstrated but their existence is likely.
Another major objective of this study was to investigate how avoidance of faecal-con-tamination by sheep on pasture was influenced by their parasite infection status and by the spatial distribution of contaminated patches. In experiment 2, infected animals spent more of their total grazing time in uncontaminated patches than uninfected animals. The average duration of each visit to an uncontaminated patch was also longer for infected animals than it was for uninfected animals. These differences were most pronounced when animals grazed plots with a large patch distribution pattern. Hence, both spatial aggregation of patches and parasite infection status play a role in determining how effectively sheep avoid areas of contaminated pasture.
Sheep used in these experiments were fasted prior to each grazing trial. Hunger has
Ž .
been shown to affect diet selection Newman et al., 1994 and hence might be expected to influence the results obtained in these experiments. However, the analysis showed
Ž .
that grazing period either the first 30 min of each trial or the last 30 min did not affect the amount of time spent in uncontaminated patches, suggesting that the sheep did not become more selective as they became less hungry. Hence, it seems that in this instance, fasting had little effect on diet selection.
This is the first study to investigate the effect of spatial aggregation of faecal
Ž .
study, more of a preferred diet was consumed when patches were aggregated at a large scale even though there was the same total availability of patches as at lower scales of
Ž . Ž .
aggregation 50% favourable and 50% unfavourable . Edwards et al. 1994 suggested that the different diet selected at the largest aggregation size did not imply that foraging behaviour differed with different scales of aggregation. They hypothesised that searching behaviour remained the same but the way in which patches were encountered was altered and subsequently so was the diet selected.
Overall, sheep appeared to consume fewer larvae than the mean number that were present on the sward. This may initially appear to be a result of sheep grazing discriminately. However, such a result would be expected if grazing was random when we consider that sheep graze the upper section of the sward whereas parasite larvae tend
Ž .
to concentrate in the lower sections Sykes, 1987 . Mastication should also be consid-ered when comparing larval counts from ingested and pasture herbage. Mastication
Ž
enhances the release of infective larvae from herbage Heath et al., 1970; Gettinby et al.,
.
1985 , resulting in higher rates of larval recovery from ingested herbage than from pasture herbage. Such considerations make direct comparisons of larval counts from pasture and ingested herbage inappropriate as their exact relationship to each other is uncertain. Hence, in this study the difference between the two counts was analysed rather than the actual counts.
On plots composed entirely of contaminated patches, the difference between larval counts from ingested and pasture herbage was far greater for infected than uninfected
Ž .
animals. This supports the observations of Hutchings et al. 1998, 1999 who found that sub-clinical parasitism affected grazing behaviour by lowering bite rates and reducing
Ž .
bite depths. As larvae are concentrated in the bottom of the sward Sykes, 1987 , a reduction in bite depth should result in a reduction in the number of larvae consumed. However, on plots where contamination was patchily distributed, differences between larval counts from pasture and ingested herbage were greatest for uninfected animals. This presents us with what appears to be a paradoxical situation in that infected animals spend more time in uncontaminated patches but yet consume more parasite larvae than uninfected animals. This suggests that parasitism influences grazing strategy and its effectiveness, with respect to parasite avoidance, in more complex ways than have previously been considered.
The importance of sight and smell in the detection of contaminated patches has been previously discussed. Parasite infection affects the ability of a host to locate resources
ŽKavaliers and Colwell, 1995a and to choose the most profitable food items Milinski,. Ž .
1984 . Parasite-infected animals also show reduced aversive responses to odours
associ-Ž .
ated with other infected animals Kavaliers et al., 1998 . Consequently, parasitism may compromise the olfactory system making it more difficult for animals to distinguish between contaminated areas through the sense of smell. If olfactory senses are impaired, a parasite-infected animal might be more likely to rely on visual cues to detect contaminated patches. Another consideration to be taken into account is that anorexia
Ž .
frequently accompanies parasite infection Symons, 1985; Kyriazakis et al., 1998 , and hence infected animals have a lower appetite drive than uninfected animals.
in the general location of profitable food items, using visual cues for orientation, whilst keeping bite depth consistently low. On the other hand, uninfected animals have a greater appetite than uninfected animals, which will encourage them to graze deeper and to explore contaminated patches more than infected animals. However, uninfected animals may be better able to detect faecal contamination on a finer spatial scale using the olfactory senses. Within contaminated patches, there will be pockets of uncontami-nated herbage due to the pelletted, and hence aggregated, nature of the faeces. Uninfected animals may be able to detect these uncontaminated pockets and graze the sward more deeply within them. This would allow uninfected animals to exploit the patchy aggregation of preferred food patches more effectively than infected animals. This hypothesis would explain why uninfected animals spend more time grazing in contaminated patches than infected animals and yet consume fewer larvae.
Ž .
This study supports the work of others Hutchings et al., 1998, 1999 who have found that infected and uninfected sheep use different foraging strategies. It is not known at what level of infection one strategy switches to the other. The change in grazing behaviour may be linked to anorexia and there is a threshold parasite dose below which
Ž .
anorexia is not observed Steel et al., 1980; Kyriazakis et al., 1998 . However, the switching of strategies appears to be based on an interaction between the occurrence of anorexia and the diminished ability of an animal to exploit its environment effectively. Further studies are required to investigate this interaction.
The results of these experiments throw doubt on the hypothesis considered previously
Ž . Ž .
by Cooper 1997 and Kyriazakis et al. 1998 that the occurrence of anorexia in parasitic infections allows an infected animal to forage more selectively. The grazing strategy adopted by infected animals was only effective in terms of minimising the number of parasite larvae ingested when sheep grazed plots were all patches were contaminated. This was a somewhat artificial situation as faeces tend to have an
Ž .
aggregated distribution on pasture Morton and Baird, 1990 , leaving areas free of contamination.
In conclusion, sheep do not discriminate against parasites per se while foraging. Rather, they discriminated against faeces regardless of the risk of infection. Parasite infection status and faecal distribution in the environment influenced grazing behaviour and rate of infective larvae consumption. Sheep avoided areas of faecal contamination when faeces were aggregated at a large spatial scale. Parasite infection may alter the grazing strategy of sheep, increasing the uptake of infective parasite larvae. This may be due to the interaction between infection induced anorexia and the detrimental influence that infection has on resource location and the olfactory senses. The relationship between parasite infection, faecal distribution and foraging behaviour is a complex one with many issues that still require clarification. Further investigation of these relation-ships will improve our understanding of how animals exploit their environment whilst foraging and would also add to our knowledge regarding the transmission of parasites.
Acknowledgements
Robertson, Iain Thomson and all the staff at Glensaugh who assisted in running the experiment. Statistical advice was provided by staff at the University of Reading’s Central Statistical Service. This work was funded by the Aberdeen Research Consortium and The Scottish Executive Rural Affairs Department and was conducted under a Home Office licence.
References
Alexander, R.N., McGowan, M., 1961. A filtration procedure for the in vitro determination of herbage digestibility. J. Br. Grassl. Soc. 16, 275–277.
Aoyama, M., Dohi, H., Shioya, S., Takeuchi, Y.M., Okubo, T., 1994. Feeding deterrent substance in cattle feces: its effects on ingestive behaviour in goats. Appl. Anim. Behav. Sci. 40, 253–262.
Arnold, G.W., 1966. The special senses in grazing animals. I. Sight and dietary habits in sheep. Aust. J. Agric. Res. 17, 531–542.
Bazely, D.R., Ensor, C.V., 1989. Discrimination learning in sheep with cues varying in brightness and hue. Appl. Anim. Behav. Sci. 23, 293–299.
Coop, R.L., Sykes, A.R., Angus, K.W., 1982. The effects of three levels of intake of Ostertagia circumcincta larvae on growth rate, food intake and body composition of growing lambs. J. Agric. Sci. 98, 247–255. Cooper, J. 1997. The behavioural control of helminth infection by sheep. PhD thesis, University of Aberdeen. Dohi, H., Yamada, A., Entsu, S., 1991. Cattle feeding deterrents emitted from cattle feces. J. Chem. Ecol. 6,
1197–1203.
Edwards, G.R., Newman, J.A., Parsons, A.J., Krebs, J.R., 1994. Effects of the scale and spatial distribution of the food resource and animal state on diet selection: an example with sheep. J. Anim. Ecol. 63, 816–826. Edwards, G.R., Newman, J.A., Parsons, A.J., Krebs, J.R., 1997. Use of cues by grazing animals to locate food
patches: an example with sheep. Appl. Anim. Behav. Sci. 71, 59–68.
Edwards, J., 1983. Diet shifts in moose due to predator avoidance. Oecologia 60, 185–189.
Evans, W.S., Hardy, M.C., Singh, R., Moodie, G.E., Cote, J.J., 1992. Effect of the rat tapeworm, Hymenolepis diminuta, on the coprophagic activity of its intermediate host, Tribolium confusum. Can. J. Zool. 70, 2311–2314.
Folstad, I., Nilssen, A.C., Halvorsen, O., Andersen, J., 1991. Parasite avoidance: the cause of post-calving migrations in Rangifer? Can. J. Zool. 69, 2423–2429.
Gettinby, G., McKellar, Q.A., Bairden, K., Theodoridis, Y., Whitelaw, A., 1985. Comparison of two techniques used for the recovery of nematode infective larvae from pasture. Res. Vet. Sci. 39, 99–102. Godin, J.G.J., Sproul, C.D., 1988. Risk taking in parasitized sticklebacks under threat of predation: effects of
energetic need and food availability. Can. J. Zool. 66, 2360–2367.
Gruner, L., Sauve, C., 1982. The distribution of Trichostrongyle larvae on pasture and grazing behaviour in calves. Vet. Parasitol. 11, 203–213.
Ž .
Gulland, F.M.D., 1992. The role of nematode parasites in Soay sheep OÕis aries L. mortality during a
population crash. Parasitology 105, 493–503.
Hart, B.L., 1990. Behavioural adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 14, 273–294.
Heath, D.D., Southcott, W.H., May, P.F., 1970. The use of sheep fistulated at the oesophagus for the recovery of strongyloid larvae from pasture. Parasitology 60, 281–289.
Hutchings, M.R., Kyriazakis, I., Anderson, D.H., Gordon, I.J., Coop, R.L., 1998. Behavioural strategies used by parasitized and non-parasitized sheep to avoid ingestion of gastro-intestinal nematodes associated with faeces. Anim. Sci. 67, 97–106.
Hutchings, M.R., Kyriazakis, I., Gordon, I.J., Jackson, F., 1999. Trade-offs between nutrient intake and faecal avoidance in herbivore decisions: the effect of animal parasitic status, level of feeding motivation and sward nitrogen content. J. Anim. Ecol. 68, 310–323.
Jackson, F., 1974. A new technique for obtaining nematode ova from sheep faeces. Lab. Pract. 23, 65–66. Kavaliers, M., Colwell, D.D., 1995a. Reduced spatial learning in mice infected with the nematode,
Kavaliers, M., Colwell, D.D., 1995b. Odours of parasitized males induce aversive responses in female mice. Anim. Behav. 50, 1161–1169.
Kavaliers, M., Colwell, D.D., Choleris, E., 1998. Parasitized female mice display reduced aversive responses to the odours of infected males. Proc. R. Soc. Lond. B: Biol. Sci. 265, 1111–1118.
Kyriazakis, I., Tolkamp, B.J., Hutchings, M.R., 1998. Towards a functional explanation for the occurrence of anorexia during parastic infections. Anim. Behav. 56, 265–274.
Loehle, C., 1995. Social barrieres to parasite transmission in wild animal populations. Ecology 76, 326–335. Lozano, G.A., 1991. Optimal foraging theory: a possible role for parasites. Oikos 60, 391–395.
Michel, J.F., 1955. Parasitological significance of bovine grazing behaviour. Nature 75, 1088–1089.
Ž .
Milinski, M., 1990. Parasites and host decision making. In: Bernard, C.J., Behnke, M. Eds. , Parasitism and Host Behaviour. Taylor and Francis, London, pp. 95–116.
Millikan, G.C., Gaddis, P., Pulliam, H.R., 1985. Interspecific dominance and the foraging behaviour of juncos. Anim. Behav. 33, 428–435.
Moller, A.P., Dufva, R., Allander, K., 1993. Parasites and the evolution of host social behaviour. Adv. Study Behav. 23, 65–102.
Moore, J., Gotelli, N.J., 1990. A phylogenetic perspective on the evolution of altered host behaviours: a
Ž .
critical look at the manipulation hypothesis. In: Bernard, C.J., Behnke, M. Eds. , Parasitism and Host Behaviour. Taylor and Francis, London, pp. 193–223.
Mooring, M.S., 1995. The effect of tick challenge on grooming rate by impala. Anim. Behav. 50, 377–392. Morton, J.D., Baird, D.B., 1990. Spatial distribution of dung patches under sheep grazing. N.Z. J. Agric. Res.
33, 285–294.
Newman, J.A., Penning, P.D., Parsons, A.J., Harvey, A., Orr, R.J., 1994. Fasting affects intake behaviour and diet preference of grazing sheep. Anim. Behav. 47, 185–193.
Pappas, P.W., Marchall, E.A., Morrison, S.E., Durka, G.M., Daniel, C.S., 1995. Increased coprophagic activity of the beetle, Tenebrio molitor, on feces containing eggs of the tapeworm, Hymenolepis diminuta. Int. J. Parasitol. 25, 1179–1184.
Pfister, J.A., Hansen, D., Malechek, J.C., 1989. Surgical establishment and maintenance of esophageal fistulae in small ruminants. Small Rumin. Res. 3, 47–54.
Provenza, F.D., Villalba, J.J., Cheney, C.D., Werner, S.J., 1998. Self-organisation of foraging behaviour: From simplicity to complexity without goals. Nutr. Res. Rev. 11, 199–222.
Rook, A.J., Penning, P.D., 1991. Synchronisation of eating, ruminating and idling activity by grazing sheep. Appl. Anim. Behav. Sci. 32, 157–166.
Steel, J.W., Symons, L.E.A., Jones, W.O., 1980. Effects of larval intake on the productivity and physiological and metabolic responses of lambs infected with Trichostrongylus colubriformis. Aust. J. Agric. Res. 31, 821–838.
Sutherst, R.W., Floyd, R.B., Bourne, A.S., Dallwitz, M.J., 1986. Cattle grazing behaviour regulates tick populations. Experientia 42, 194–196.
Ž .
Sykes, A.R., 1987. Endoparasites and herbivore nutrition. In: Hacker, J.B., Ternouth, J.H. Eds. , Nutrition of Herbivores. Academic Press, Harickvale, Australia, pp. 221–232.
Symons, L.E.A., 1985. Anorexia; occurance, pathophysiology and possible causes in parasitic infection. Adv. Parasitol. 24, 103–133.
Thomas, R.J., 1959. Field studies on the seasonal incidence of Nematodirus battus and N.filicollis in sheep. Parasitology 49, 387–410.
Tilly, J.M.A., Terry, R.A., 1963. A two-stage technique for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 18, 104–111.
Williams, J.C., Bilkovich, F.R., 1973. Distribution of Ostertagia ostertagia on pasture herbage. Am. J. Vet. Res. 34, 1337–1344.