Ž .
Aquaculture 183 2000 31–44
www.elsevier.nlrlocateraqua-online
Vaccination of Atlantic halibut Hippoglossus
hippoglossus L., and spotted wolffish Anarhichas
minor L., against atypical Aeromonas salmonicida
Marina Ingilæ, Jan Arne Arnesen, Vera Lund
), Guri Eggset
1Fiskeriforskning, Norwegian Institute of Fisheries and Aquaculture, N-9291 Tromsø, Norway
Abstract
Atypical furunculosis caused by atypical Aeromonas salmonicida is an increasing problem in commercial halibut farming, and a potential problem in farming of spotted wolffish in Norway. Halibut, Hippoglossus hippoglossus L., and spotted wolffish, Anarhichas minor L., vaccinated with oil-emulsified vaccines against atypical furunculosis, demonstrated a relative percent survival ŽRPS of approximately 90 when challenged with homologous isolates. Bath and aqueous injection. vaccines failed to protect against disease when challenged 10 weeks post-vaccination. High antibody titres were produced in both species after vaccination with oil-emulsified vaccines, and
Ž .
the major antibody response were against A-layer, LPS and some minor outer membrane OM proteins.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Atypical Aeromonas salmonicida; Furunculosis; Vaccination; Halibut; Hippoglossus hippoglossus
L.; Spotted wolffish; Anarhichas minor L.
1. Introduction
The species Aeromonas salmonicida is, according to Bergey’s Manual of
Determina-Ž . Ž
tive Bacteriology 1994 , divided into three subspecies: subsp. salmonicida Griffin et
. Ž . Ž .
al., 1953 , subsp. achromogenes Smith, 1963 , and subsp. masoucida Kimura, 1969 .
Ž .
Other subspecies have been suggested as subsp. smithia Austin et al., 1989 in addition
) C o rresp o n d in g au th o r. T el.: q4 7 -7 7 -6 2 -9 0 -0 0 ; fax : q4 7 -7 7 -6 9 -9 1 -0 0 ; e-m ail: [email protected]
1
Present address: Tromsø Science Park, Forskningsparken, N-9291, Tromsø, Norway. 0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
to an increasing number of reported strains that have not been assigned to any of these subspecies. A. salmonicida subsp. salmonicida comprises a homogenous group of strains and are referred to as typical A. salmonicida causing classical furunculosis, while all other A. salmonicida strains are referred to as atypical and comprise a heterogenous group causing diseases referred to as ulcer disease or atypical furunculosis
Žreviewed by Wiklund and Dalsgaard, 1998 ..
The number of published reports of disease outbreaks associated with atypical strains of A. salmonicida has increased significantly during the last decade, and these isolates have been reported from an increasing number of fish species and geographical areas
Žreviewed by Wiklund and Dalsgaard, 1998 . The virulence of atypical A. salmonicida.
varies according to the host species, and variations in susceptibility to different strains of atypical A. salmonicida are observed in both salmonids and non-salmonids. Some of the atypical strains isolated from wild fish adversely affect salmonids and therefore may represent a significant risk to farmed fish. At present, several non-salmonid fish species have been introduced into farming, while other possible species are being tested for that purpose. The susceptibility of these fish species to atypical A. salmonicida has to be examined. Atypical furunculosis is an increasing problem in commercial halibut farm-ing, and a potential problem in farming of spotted wolffish in Norway. Wild wolffish caught and used as breeding stocks may be carriers of the bacterium, and outbreaks frequently occur when the fish are stressed or when water temperature increases above
Ž .
88C–108C Hellberg et al., 1996 . Commercial furunculosis vaccines based on A. salmonicida subsp. salmonicida and produced for the prevention of furunculosis in salmonids, did not give the same degree of protection against disease caused by atypical
Ž .
bacterial strains in salmonids Gudmundsdottir and Gudmundsdottir, 1997 , and proba-bly neither against atypical furunculosis in non-salmonids. The aim of the present study was to examine whether vaccines based on bacterial strains isolated from diseased halibut and wolffish, may yield protective effect against atypical furunculosis in these fish species.
2. Materials and methods
2.1. Fish
Ž .
Halibut, Hippoglossus hippoglossus L. ;15 g and spotted wolffish, Anarhichas
Ž .
minor L. ;39 g in experiment 2 and ;28 g in experiment 3, see Table 1 produced from wild caught breeding stocks were used in this study. The halibut were obtained from Lofilab, Leknes, Norway, while the wolffish were bred at the Aquaculture Research Station in Tromsø, Norway, where the vaccination and experimental chal-lenges were performed. The halibut were kept at 128C in a 500 l tank, and the wolffish in raceways measuring 40=17=210 cm and 20=20=100 cm in experiments 2 and 3, respectively, both at 108C during the experiments. Prior to all handling such as injection of vaccines or challenge bacteria or individual weighing, the fish were
Ž .
anaesthetized with benzocain 50 mgrl water . The different groups were spotmarked
Ž .
()
M.
Ingilæ
et
al.
r
Aquaculture
183
2000
31
–
44
33
Table 1
Experimental design
Ž . Ž . Ž .
Vaccine type Administration Dose Experiment 1 halibut Experiment 2 wolffish Experiment 3 wolffish
Fish per group Fish per group Fish per group
a 8 b c
Aqueous vaccine Immersion 5=10 bacteriarml 50 50
a 9
Aqueous vaccine i.p. injection 2.5=10 bacteriarfish 50 50
a 9
FIA-emulsified i.p. injection 2.5=10 bacteriarfish 50 50
a 9
Alpharma-emulsified i.p. injection 2.5=10 bacteriarfish 50
Unvaccinated control 50 50 50
Challenge method Bath 60 min i.p. injection i.p. injection
5 7 4
Challenge dose 10 LFI 4050rml 10 LFI 4048rfish 10 LFI 4048rfish
a
The halibut and the spotted wolffish vaccines contained the A. salmonicida isolates LFI 4050 and LFI 4048, respectively.
b
Immersion for 15 min.
c
2.2. Bacteria
Two atypical A. salmonicida strains, LFI 4050 isolated from diseased halibut, and LFI 4048 from diseased spotted wolffish, were used for vaccination and challenge of halibut and spotted wolffish, respectively. They were shown to be different by
biochemi-Ž .
cal tests API 20E and API 50 CH , and by production of extracellular proteases demonstrated in substrate gels. In addition, the strain LFI 4061 isolated from another outbreak of atypical furunculosis in the breeding stock of spotted wolffish was used for challenge. A typical A. salmonicida subsp. salmonicida LFI 4045 was used as a reference strain in Western blots.
The bacteria used in the vaccines and for challenge were grown in Brain heart
Ž .
infusion broth BHI, Difco containing 2% NaCl at 128C for 24 h with shaking. The cells used in vaccines were inactivated by addition of 0.5% vol.rvol. formaldehyde
Ž . Ž .
solution 37% for 48 h, washed in PBS 20 mM phosphate, 150 mM NaCl, pH 7.4 and
Ž .
resuspended in PBS before used to prepare the different vaccines Table 1 . BHI agar
ŽOxoid , supplemented with 0.005% Coomassie brilliant blue, and Oxoid blood agar.
base no. 2 supplemented with 2% human red blood cells and 1.5% NaCl, were used for reisolation of bacteria from dead fish.
2.3. Vaccines andÕaccination
Three vaccination and challenge experiments were performed, one on halibut and two on spotted wolffish. Each experiment was run in separate tanks or raceways. The experimental design is summarised in Table 1. Four different vaccines were tested for protection against atypical furunculosis; one immersion and three injection vaccines. The bacterial stock suspension described above were diluted in order to obtain appropriate cell concentrations in the different vaccines. The immersion vaccines were diluted in sea water, the aqueous injection vaccines in PBS, and in the oil-emulsified vaccines the bacterial suspension was diluted in PBS before emulsification in the oil adjuvant in
Ž .
proportion 1:1 Table 1 . The adjuvant used was either Freunds incomplete adjuvant
ŽFIA-emulsified or the vegetable–animal oil adjuvant Alpharma-emulsified used in. Ž . Ž
the commercial furunculosis vaccines for Atlantic salmon Apoject-Fural vaccines,
.
Alpharma, Norway . In 1998 the Apoject vaccines were replaced with the Alphaject series which instead contains a mineral oil adjuvant. The vaccines used for the halibut and spotted wolffish contained the A. salmonicida strains LFI 4050 and LFI 4048, respectively. Fifty fish of each group were vaccinated either by immersion or by
Ž .
intraperitoneal i.p. injection of 0.1 ml of vaccines, and one group of 50 unvaccinated
Ž .
fish was left as control in each experiment. In experiment 3 Table 1 , each fish was weighed at the time of vaccination and challenge.
2.4. Prechallenge and challenge of fish
Challenge was performed 10 weeks post-vaccination, unless otherwise stated, with
Ž .
experi-( )
M. Ingilæ et al.rAquaculture 183 2000 31–44 35
Ž
ment, where groups of 10 fish were challenged with different bacterial doses by bath 60
.
min or i.p. injection of 0.1 ml bacterial suspension. The bath and i.p. infected groups were kept in the same raceway as an uninfected group to be infected through the water by cohabitation.
Bath challenge, using the same bacterial concentration as had successfully been used in previous experiments, was performed on halibut. The water level in the tank was lowered to 50 l and the flow stopped before addition of the bacterial suspension. The water was oxygenated during challenge.
Mortality in each group was recorded daily. The cause of death was verified by reisolation of bacteria from kidney samples of dead fish. Cumulative mortality was
Ž .
registered and relative percent survival RPS on the last day of the experiment was
w
calculated according to the formula: RPSs 1y% mortality in vaccinated
x .
groupr%mortality in control group =100% .
The fish in experiment 3 surviving challenge with homologous bacterial strain, together with a group of 50 unvaccinated, previously unchallenged fish of approximately the same size and from the same batch, were challenged 15 weeks after the first challenge with a heterologous A. salmonicida strain LFI 4061.
2.5. Humoral immune responses
Ten additional fish of each group in experiments 1 and 2 were vaccinated to study specific antibody responses. The fish were sampled at the time of challenge and blood was drained. Specific antibody responses were quantified in the sera collected, using homologous formalin killed whole A. salmonicida cells in an enzyme-linked
immuno-Ž . Ž .
sorbent assay ELISA , as described by Lund et al. 1991 . Bound antibodies were detected with alkaline phosphatase conjugated rabbit anti-wolffish and anti-halibut IgM produced in the author’s laboratory.
The antibody responses were evaluated qualitatively by Western blotting, using outer
Ž . Ž .
membrane OM preparations Filip et al., 1973 of the two atypical A. salmonicida strains LFI 4050 and 4048, and one typical A. salmonicida subsp. salmonicida LFI 4045 strain. The OM preparations were boiled for 5 min in 5% sodium dodecyl sulphate
ŽSDS, Sigma with 0.2 M dithiothreitol DTT, Sigma for denaturation and reduction,. Ž .
Ž .
prior to separation in a 12% SDS–polyacrylamide gel Laemmli, 1970 . The
elec-Ž .
trophoretic separation of the OM proteins and the transfer of the proteins Western blot
Ž w .
onto a 0.45mm nitrocellulose membrane Trans-Blot , Bio-Rad was performed on a
Ž .
Mini-PROTEAN II system Bio-Rad according to the Bio-Rad Instruction Manual.
Ž .
Three parallel gels were run, one was silver stained Morrissey, 1981 and two were
Ž .
used for Western blotting. Low Molecular Weight markers Pharmacia or Kaleidoscope
Ž .
markers Bio-Rad were used when gels were silver stained or electroblotted, respec-tively.
In order to identify OM proteins reacting with the fish antibodies, the membranes
Ž .
membrane was incubated with the different antibodies for 60 min at room temperature with gentle shaking, and washed 3=10 min with PBS–Tween between each incubation step. Finally, the membranes were stained by adding the substrate nitroblue tetrazolium
Ž .
chloride and 5-bromo-4-chloro-3-indolylphosphate NBT and BCIP; Gibco BRL in
Ž .
substrate buffer 0.1 M Tris pH 9.5, 0.1 M NaCl and 50 mM MgCl2 as recommended by the producer. The staining was stopped by washing the membrane in distilled water. The specificity of the primary antisera had previously been verified by comparing the reactivity of normal sera and immune sera from both halibut and wolffish to A. salmonicida OM preparations on Western blot.
3. Results
3.1. Vaccination and challenge
The halibut were challenged by bath, as more than 50% mortality had been obtained
Ž 4 5 .
by such challenge 10 –10 cfurml in previous trials. Prechallenge experiments performed on wolffish showed that more that 50% of the fish died after injection challenge with doses corresponding to 104–107 cfu
rml, dependent of fish size, while bath challenge resulted in mortalities of approximately 20% even at doses of 108cfu
rml
Ždata not shown . Challenge by cohabitation was not successful as the fish were not.
Ž .
diseased or died within a time scale of 4 weeks. Thus, injection challenge i.p. was chosen for spotted wolffish.
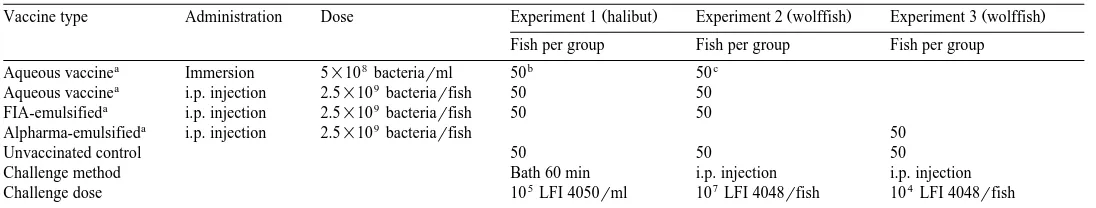
In experiments 1 and 2, respectively, groups of halibut and spotted wolffish were vaccinated with the different vaccine formulations as described in Table 1, and chal-lenged with homologous A. salmonicida strains 10 weeks later. The results are presented as cumulative mortalities in Fig. 1. The onset of death in the bath challenged
Ž .
halibut groups started 11–12 days post-challenge for all groups Fig. 1a . The mortality of the group vaccinated by immersion was similar to that of the unvaccinated group. Compared to these, the group that obtained aqueous injection vaccine showed a lower mortality throughout the challenge experiment. However, at the end of the experimental
Ž .
period day 39 post-challenge , all three groups reached similar cumulative mortalities
Ž43%–47% . On the other hand, the protection obtained in the group vaccinated with.
Ž .
oil-emulsified vaccine FIA-emulsified was convincing. In this group the mortality
Ž5% was significantly lower P. Ž -0.05 than those of the other groups at the end of the.
experiment, yielding RPSs89.
The onset of death in the i.p. challenged wolffish groups occurred on day 3
Ž .
post-challenge Fig. 1b . All vaccinated groups and the unvaccinated control group reached 100% mortality on day 17 post-challenge, indicating that a too high bacterial
Fig. 1. Experimental challenge of differently vaccinated and unvaccinated groups of halibut and spotted
Ž . Ž .
wolffish 10 weeks post-vaccination Table 1 . A The atypical A. salmonicida strain LFI 4050, from halibut,
Ž 5 . Ž .
was used in the vaccines and for bath challenge 10rml, 60 min . B The atypical A. salmonicida strain LFI
Ž 7 .
( )
Fig. 2. Experimental challenge of spotted wolffish 10 weeks after i.p. vaccination with A. salmonicida LFI
Ž 4
4048 emulsified in Alpharma’s oil adjuvant. The homologous bacterial strain was used for i.p. challenge 10
.
cellsrfish .
dose had been used for infection. However, on day 7, the groups vaccinated with oil-emulsified vaccine, aqueous injection vaccine, or immersion vaccine showed cumula-tive mortalities of 38%, 60% and 82%, respeccumula-tively, indicating the FIA-emulsified vaccine to be the most promising vaccine candidate.
Ž .
A second vaccination and challenge experiment experiment 3, Table 1 was
per-Ž .
formed on wolffish, using Alpharma’s commercial oil adjuvant Alpharma-emulsified instead of FIA. Also, a lower bacterial dose was used for this challenge. The results of the challenge with the homologous A. salmonicida strain are shown in Fig. 2. The unvaccinated control group started to die 5 days post-challenge, and 90% mortality was reached on day 12. In the vaccinated group, however, only 14% cumulative mortality was obtained 26 days post-challenge, corresponding to RPSs90. Thus, a significant protection against A. salmonicida was obtained in spotted wolffish when using a vaccine containing a commercial oil adjuvant. Fifteen weeks later, 43 vaccinated fish surviving this experiment were subjected to challenge by i.p. infection with a
heterolo-Fig. 3. Specific antibody responses against atypical A. salmonicida in the different vaccinated groups analysed
Ž . Ž .
( )
gous A. salmonicida strain, isolated from moribund wild caught spotted wolffish. Also, 50 non-vaccinated and hitherto unchallenged fish were challenged as controls. All the
Ž .
fish in the latter group died, but none in the vaccinated group data not shown . This indicates that vaccines based on a single atypical A. salmonicida strain may be protective against heterologous bacterial strains, at least those pathogenic to the same fish species.
3.2. Humoral immune responses
The quantitative antibody responses in halibut and spotted wolffish against whole cells of the A. salmonicida strains used in the vaccines, were determined by ELISA. By defining the antibody titre as serum dilution yielding an optical density at 405 nm of
;1, the oil-emulsified and aqueous injection vaccines induced titres of )1280 and
;160, respectively, in both halibut and wolffish, while no specific responses were
Ž .
observed in sera of immersion vaccinated fish Fig. 3 .
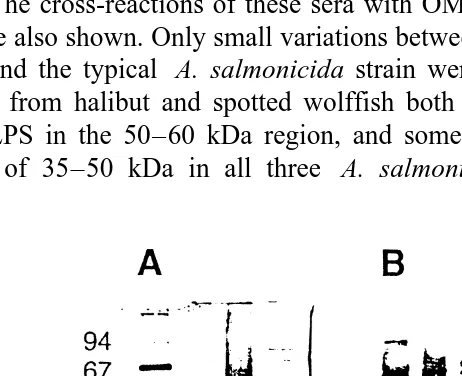
Qualitative antibody responses of halibut and spotted wolffish to OM antigens of the two atypical A. salmonicida strains used in the vaccines are shown on Western blots in Fig. 4. The cross-reactions of these sera with OM antigens of a typical A. salmonicida strain are also shown. Only small variations between the OM patterns of the two atypical strains and the typical A. salmonicida strain were observed on the silver stained gel. Antisera from halibut and spotted wolffish both reacted with the A-layer protein and HMW LPS in the 50–60 kDa region, and some unidentified proteins with molecular weights of 35–50 kDa in all three A. salmonicida strains. In addition the halibut
Ž .
Fig. 4. Qualitative antibody responses in halibut and spotted wolffish. A Silver stained gel with OM proteins; lane 1: low molecular weight marker, lane 2: atypical A. salmonicida LFI 4050 isolated from halibut, lane 3: atypical A. salmonicida LFI 4048 isolated from spotted wolffish, lane 4: typical A. salmonicida subsp.
Ž .
salmonicida LFI 4045. B Western blot of parallel gel as in A immunostained with halibut antiserum to A. Ž .
salmonicida LFI 4050. C Western blot of parallel gel as in A immunostained with spotted wolffish antiserum
( )
M. Ingilæ et al.rAquaculture 183 2000 31–44 41
Ž .
antiserum seems to cross-react with some low molecular weight components ;10 kDa in all three isolates.
4. Discussion
Vaccines formulated with oil adjuvants yielded better protection than aqueous injection or bath vaccines. A protection of approximately 90% RPS could be obtained on challenge with homologous A. salmonicida strains in both halibut and spotted wolffish
ŽFig. 1a and Fig. 2 . The efficacy of the vaccines correlated with specific antibody.
responses, as the highest antibody titres were demonstrated in fish vaccinated with the oil-emulsified vaccines. Since atypical A. salmonicida strains comprise a heterogeneous group of bacteria, vaccinated fish should be challenged with several isolates of different species and geographical locations, in order to determine if more than one isolate is needed in the vaccines. In this experiment, fish shortage did not allow for challenge with several bacterial strains. However, in experiment 3, vaccinated wolffish surviving challenge with homologous bacterial strain, were subjected to a second challenge with a heterologous strain. The vaccine also protected effectively against this particular strain. The first challenge might, however, have functioned like a boost and increased the protection of the vaccinated fish compared to the unvaccinated and previously unchal-lenged control fish.
The high final mortalities in all wolffish groups in experiment 2 were probably due to
Ž 7 . Ž 4
the high challenge dose 10 cells per fish . Therefore, a reduced dose 10 cells per
.
fish was used in experiment 3. Even though the mortality of the unvaccinated control group in the latter experiment also reached nearly 100%, a RPS as high as 89 was obtained in the vaccinated group.
Vaccination with oil-emulsified vaccines seemed to reduce the growth of both halibut and spotted wolffish. In experiments 1 and 2, an inhibition of growth was only visually observed, since the mean weights of each vaccination group were not measured. Therefore, in experiment 3, the mean weights of the groups were measured both at the time of vaccination and challenge. The growth of the fish was seriously repressed as the mean weight of the fish was 28 g when vaccinated, while at challenge 10 weeks later, the vaccinated and unvaccinated groups were 33 and 76 g, respectively. Also, the intestines were pale and shrunken compared to the control group, but adherances between internal organs were rare. However, 5 months post-vaccination, the intestines of
Ž .
the surviving vaccinated fish were normal and the growth rate % was the same as for unvaccinated backup fish of the same batch.
The Alpharma-emulsified vaccine seemed to have a more serious effect on the growth of the spotted wolffish compared to that observed in Atlantic salmon vaccinated with Alpharma’s Apoject vaccines containing the same vegetable–animal oil adjuvant
Žinformation from Alpharma . However, recently a vaccine containing the mineral oil.
used in Alpharma’s Alphaject vaccines, was shown to have less effect on the growth of
Ž .
spotted wolffish data not shown , indicating that the period of growth inhibition can be reduced.
Atypical and typical A. salmonicida strains share major cell-surface antigens such as
Ž . Ž
. Ž . Ž
1986 , iron-regulated OM proteins Hirst and Ellis, 1994 and porins Lutwyche et al.,
. Ž .
1995 , while the production of exotoxins differs Gudmundsdottir, 1996 . In this study, western blots using OM preparations as antigens showed that both halibut and spotted wolffish produced antibodies to the A-layer protein, LPS and some unidentified proteins of homologous isolate. The antisera also cross-reacted with corresponding proteins of typical A. salmonicida subsp. salmonicida. Little information is available about im-munological protection of fish against infection with atypical A. salmonicida. Vaccina-tion against typical furunculosis has been shown to protect against atypical furunculosis
Ž .
in both salmonids and cyprinid fish Jones et al., 1996 . The authors of the present study have experienced that A-layer protein on bacterial cells is an important protective antigen in vaccines against typical furunculosis in salmonids, since no protection was
Ž
obtained with vaccines containing a bacterial strain lacking the A-layer unpublished
. Ž .
data . Hirst and Ellis 1994 found that the iron-regulated membrane proteins of A. salmonicida represented protective antigens important for successful vaccines against both typical and atypical strains of A. salmonicida in salmon. Extracellular products
ŽECP appeared to be important for protection against atypical A. salmonicida in carp. ŽEvenberg et al., 1988 and Atlantic salmon Gudmundsdottir and Magnadottir, 1997 .. Ž .
ECP of the subsp. achromogenes elicited better protection than whole bacteria in Atlantic salmon, and the protection strongly correlated with the detection of antibodies directed against the 20 kDa extracellular metallocaseinase AsaP1 in fish sera
ŽGudmundsdottir and Magnadottir, 1997 ..
The only commercial currently available vaccine against atypical furunculosis is an autogenous emulsion vaccine against A. salmonicida subsp. achromogenes in salmonids
Ž .
for use in Iceland where the strain is endemic Iceland Biojec OO, Alpharma N.W. . Both the autogenous vaccine and a commercial vaccine against classical furunculosis
ŽBiojec1500, Alpharma N.W. evoked protection against atypical furunculosis in At-.
lantic salmon, the former yielding an apparently better protection than the latter
ŽGudmundsdottir and Gudmundsdottir, 1997 . On the other hand, no protection against.
classical furunculosis was achieved with the autogenous vaccine. This indicate that the two vaccines contain common antigens capable of yielding some protection against atypical furunculosis, but insufficient to protect against classical furunculosis. However, it remains to be tested whether the salmonid vaccine against atypical furunculosis may protect against atypical A. salmonicida in marine fish species.
Challenge by bath or cohabitation is preferable since they mimic the infection mechanisms in the environment. In this study, bath challenge was used on halibut, while
Ž .
injection challenge i.p. was used on spotted wolffish. Bath or cohabitation infection was not successfully established in prechallenge experiments on the latter species. Both bath and cohabitation models are commonly used, both in fresh and seawater, when challenging commercial vaccines against typical furunculosis in Atlantic salmon. How-ever, using A. salmonicida subsp. achromogenes, Gudmundsdottir and Gudmundsdottir
Ž1997 were only able to establish an intra muscular infection in Atlantic salmon in fresh.
( )
M. Ingilæ et al.rAquaculture 183 2000 31–44 43
bath challenge. Also, halibut skin may be more easily penetrable by the bacterium than the wolffish skin. The virulence of the bacteria used for challenge should also be taken into consideration since autogenous, and not readily comparable strains, were used for challenge of each fish species.
In summary, halibut and spotted wolffish vaccinated with oil-emulsified vaccines against atypical furunculosis, demonstrated a RPS of approximately 90% when chal-lenged with homologous strains. Bath or aqueous injection vaccines failed to protect against the disease. High antibody titres were produced in both species after vaccination with emulsion vaccines, and antibodies were produced against A-layer, LPS and some minor OM proteins. However, it remains to identify which antigens of the halibut and wolffish isolates are important for protection. The heterogeneity of the marine isolates of atypical A. salmonicida suggest that it may be necessary to include several strains in the vaccines, and that separate vaccines may be needed for the different fish species.
Acknowledgements
This work was supported by The Norwegian Research Council and Troms Steinbit. The authors are grateful to Ingrid Ugelstad for participating in the development of challenge methods for halibut and wolffish, and to Alpharma, for formulating the wolffish vaccine.
References
Austin, D.A., McIntosh, D., Austin, B., 1989. Taxonomy of fish associated Aeromonas spp., with the description of Aeromonas salmonicida subsp. smithia subsp. nov. Syst. Appl. Microbiol. 11, 277–290. Evenberg, D., Versluis, R., Lugtenberg, B., 1985. Biochemical and immunological characterization of the cell
surface of the fish pathogenic bacterium Aeromonas salmonicida. Biochim. Biophys. Acta 815, 233–244. Evenberg, D., De Graaff, P., Lugtenberg, B., Van Muiswinkel, W.B., 1988. Vaccine-induced protective immunity against Aeromonas salmonicida tested in experimental carp erythrodermatitis. J. Fish Dis. 11, 337–350.
Filip, C., Fletcher, G., Wulff, J.L., Earhart, C.F., 1973. Solubilization of the cytoplasmic membrane of
Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 115, 717–722.
Griffin, P.J., Snieszko, S.F., Friddle, S.B., 1953. A more comprehensive description of Bacterium
salmoni-cida. Trans. Am. Fish Soc. 82, 129–138.
Gudmundsdottir, B.K., 1996. Comparison of extracellular proteases produced by Aeromonas salmonicida strains, isolated from various fish species. J. Appl. Bacteriol. 80, 105–113.
Gudmundsdottir, B.K., Gudmundsdottir, S., 1997. Evaluation of cross protection by vaccines against atypical and typical furunculosis in Atlantic salmon, Salmo salar L. J. Fish Dis. 20, 343–350.
Ž .
Gudmundsdottir, B.K., Magnadottir, B., 1997. Protection of Atlantic salmon Salmo salar L. against an experimental infection of Aeromonas salmonicida ssp. achromogenes. Fish Shellfish Immunol. 7, 55–69. Hellberg, H., Moksness, E., Hoie, S., 1996. Infection with atypical Aeromonas salmonicida in farmed
common wolffish, Anarhichas lupus L. J. Fish Dis. 19, 329–332.
Kimura, T., 1969. A new subspecies of Aeromonas salmonicida as an etiological agent of furunculosis on
Ž . Ž .
‘sakuramasu’ Oncorhynchus masou and pink salmon O. gorbuscha rearing for maturity: Part 1. On the morphological and physiological properties. Fish Pathol. 3, 34–44.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Lund, V., Jørgensen, T., Holm, K.O., Eggset, G., 1991. Humoral immune response in Atlantic salmon, Salmo
salar L., to cellular and extracellular antigens of Aeromonas salmonicida. J. Fish Dis. 14, 443–452.
Lutwyche, P., Exner, M.M., Hancock, R.E.W., Trust, T.J., 1995. A conserved Aeromonas salmonicida porin provides protective immunity to rainbow trout. Infect. Immun. 63, 3137–3142.
Morrissey, J.H., 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117, 307–310.
Pyle, S.W., Cipriano, R.C., 1986. Specificity of lipopolysaccharide antigens of Aeromonas salmonicida. Microbios Letters 31, 149–155.
Smith, I.W., 1963. The classification of Bacterium salmonicida. J. Gen. Microbiol. 33, 263–274.