SUBTOPIC
NUMBER OF QUESTIONS
2011 2012 2013 2014 2015 2016 2017
3.1 Relative Atomic Mass and Relative
Molecular Mass 1 1 1
3.2 The Mole and the Number of
Particles z 1 1 1
3.3 The Mole and the Mass of
Substances 1 1 1 2
3.4 The Mole and the Volume of Gas 1 1 2 1 1 1
3.5 Chemical Formulae 3 2 z 2 2 1
3.6 Chemical Equations ? 1
PAPER
1A2
Bt2
c24
D48
Cll
Relative
Atomic
Mass
and
Relative
Molecular
Mass
SPM
20ll
Question
6I
The average mass of a magnesium atom is 24 times greater than of the mass of a carbon-12 atom. What is the relative atomic massof
magnesium? Puratajisim
satu atom magnesium adalah 24kali
lebih besar daripada
jisim
satu atomkarbon-\2.
Apakah
jisim
atomrelatif
bagi magnesium?SPM 2014 Question 44
3
What is the mass of oxygen in 88 g of carbon dioxide? [Relative atomic mass: C:
12,O:
16]
Berapakah
jisim
oksigen
dalam
88
g
karbon dioksida?lJisim atom
relatif:
C:
12, O:
161A l6e
C
50g
B 32e
D
64e
Gl
The
Mote and
the Number
of
Particles
SPM 2011 Question 21
4
Diagram 4 showstwo
typesof
gasesfilled
in two
balloons.
Rajah 4 menunjukkan
duajenis
gasyang
diisi
ke dalam duabiii
belon.SPM 2013
Question
132
Why
carbon-I2 was chosen as a reference standard for relative atomic mass and relative molecular mass?Mengapakah
karbon-|2
telah
dipilih
sebagairujukan
piawai
untukjisim
atomrelatif
danjisin
molekulrelatifl
A
Carbon has three isotopes Karbon mempunyaitiga
isotopB
Carbon is non-metal elementKarbon merupakan unsur bukan logam
C
Carbon is a solid and easier to be handleKarbon adalah pepejal
dan
lebih
senangdikendalikan
D
Carbon is locatedin
Group 14in
the Periodic Table of ElementsKarbon terletak dalam Kumpulan
14 dalamJadual
Berkala
Unsur32
gof
oxygen gas 32 g gas oksigen 2gof
hydrogen gas 2ggas hidrogen Diagram 4 Rajah 4 ANALYSIS OF THE 2011_ 2017 SPM PAPERSWhich
statementis
correct aboutthe
numberof
particles in oxygen gas?
[Relative atomic mass:
H:
l,
O:
l6]
Pernyataan manakah yang betul tentang bilangan zarah dalam gas oksigen?
fJisim atom
relatif
H:
1, O:
l6]
A
Same as in hydrogen gasSama seperti dalant gas hidrogen
B
More than in hydrogen gasLebih banyak daripada dalam gas hidrogen
C
Two times more than in hydrogen gasDua
kali
lebih
banyakdaripada
dalam gashidrogen
D
l6
times more thanin
hydrogen gas16
kali
lebih
banyak daripada dalam
gas hidrogenSPM 2011
Question
355
What
is
the
number
of
atoms
in
0.5
mol
of
ammonia gas,
NHrt
[Avogadro constant
:
6.02x
l0:] mol
r]Berapakah
bilangan etom dalam
0.5 mol
gasammonia, NH.?
fPemalar Avogadro
:
6.02X
1023 mol-r]A
6.02x
l0rr
B
0.5X
6.02X
l0rl
c
0.5x2x6.02x102r
D
0.5x4x6.02x1023
SPM 2012 Question 40
6
0.58 gflavouring
substance is used to improve the tasteofa
pineapple cake.What is the number of molecules of the
flavourins
substance?
[Relative molecular mass
of
flavouring
substance:
116 gmol
r;Avogadro constant
:6.02
x
1023mol
r]0.58 g bahan
perisa
digunakan untuk memperbaik rasa sebiji kek nanas. Berapakah bilangan molekul bahanperisa
itu?lJisin
molekulrelatif
bahanperisa
:
I I 6 gmol
r;Pemalar Avogadro
:
6.02
x
l02rmol
r]A
8.31x
10 27B
3.32
x
l0
22c
3.01x
l02rD
1.20x
1026SPM 2013 Question 40
7
Which of thefollowing
contains the same number of molecules asin
8.8 g carbon dioxide gas?fRelative atomic mass
:
H
:
l;
C
:
12;O
:
16, S-
32;l:
l27l
Antara
yang
berikut,yang
manakah mempunyaibilangan molekul yang sama seperti yang terdapat dalam 8.8 g gas karbon diol<sida?
lJisim
atomrelatif
:H: t;C:
12;O:
l6;
S:32;
t:
l27l
A
3.6 g of water 3.6 gair
B
25.4 gof
iodine 25.4 g iodinC
3.2 gofoxygen
gas 3.2 g gas oksigenD
9.6 g of sulphur dioxide 9.6 gsulfur
dioksida SPM 2013 Question 498
What is the numberof
nitrogen atoms in 40.0 gof
NHINOr?
[Avogadro's constant
:
6.02X
1023 mol-r;Molar
mass of NH.,NO.
:
80 gmol
r]Berapakah bilangan atom nitrogen dalam 40.0 g NHINO.?
fPemalar
Avogadro
:
6.02
X
1023 mola1, JisimrnolarNH.NO,:80
gmol
r]A
6.02X
l0,r
B
3.01x
l02lc
2.41x
102rD
1.51x
10r' SPM 2015 Question 29
What is the meaningofAvogadro
constant?Apakah
yang
dimalcsudkan
dengan
pemalar
Avogadro?
A
Mass of one moleof
a substance Jisim bagi satu mol bahanB
Pressure of one mole of a substance Tekanan bagi satu mol bahanC
Volume occupied by one mole of gas Isi padu yang dipenuhi oleh satu mol gasD
Numberof
particles in one mole of a substanceBilangan zarah dalam satu mol bahan
SPM 2016 Question 48
10
What is the numberof
nitrate ions,NO,- in
2 molof
iron(IlI)
nitrate, Fe(NOr)r?[Avogadro constant
:6.02
x
1023mol
r]Berapakah bilangan ion
nitrat,
NOr- dalam2 mol
.fe ru
m(lll)
n i t r at, F e(N O r) r?lPemalar Avogadro
:
6.02X
1023mol
r]A
1.204x
t0r4B
1.806x
t0r4c
3.010x
1014@
The Mole and the Mass of
Substances
SPM 2012
Question
611
Which of thefollowing
particles equalto
I
mole?Antara zarah
yang
berikut,
yang
manakah bersamaqn denganI
mol?A
The numberofatomin I
g ofhydrogen gas Bilangan atomdalam
I
g gas hidrogenB
The number of moleculein
I
g of hydrogen gas Bilangan molekul dalamI
g gas hidrogenC
6.02x
1023 ofhydrogen atoms in hydrogen gas 6.02x
1023 atom hidrogen dalam gas hidrogenD
6.02X
lG3ofhydrogenmoleculeinhydrogengas6.A
x
lG3 molelail hidrcgen dalam gas hidrogen SPM 2013 Question 4512
14.9g of
potassium chloride,KCl
is
dissolved indistilled
water to produce 0.4mol
dm 3 potassiumchloride solution.
What is
thevolume
of
distilled water needed to dissolve potassium chloride?[Molar
mass:KCI
= 74.5 g mol-r]14.9
g kalium
klorida, KCI
dilarutkqn dalam
air
suling
untuk menghasilkan0.4 mol dm}
larutankalium klorida.
Berapakah
isi padu
air
suling
diperlukan untuk melarutkan kalium
klorida?
LJisim
molar: KCI
:
74.5gmolal
A
50 cm3B
200 cm3C
500 cmlD
2 000 cm3SPM 2014
Quesfion
3913
Which
substance has a different massfrom
I
molof glucose, C6H1206?
[Relative atomic mass:
H:
l,
C=
12,O:
16]Bahan
manakah mempunyaijisim yang
berbezadaripada
I
mol glukosa, C6H1206?LJisim atom
relatif.H =
1, C:
12, O:
16)A
I
mol
of naphthalene, C,oH,I
mol naftalena,C,oH,
B
3mol ofpropanol, C.H,OH
3 mol
propanol, C,H,OH
C
6 molof
ethane, CrHu6 mol of etana, CrHu
D
l0
mol
of water, HrOl0
molair; HrO
SPM 2015
Quesfion
3514
Eight
atomsof
elementX
hasthe
same mass astwo
atoms of Tellurium, Te.What is the relative atomic mass of X? [Relative atomic mass:
Te:
128]Lapan atom unsur
X
mempunyaijisim
yang sama dengan dua atomklurium,
Te.Berapakah
jisim
atomrelatifX?
lJisim
atomrelatif.
Te= l28l
A8
B16
c32
D64
SPM 2015
Question
3615
What
is
the
percentage compositionby
massof
water
in
hydrated iron(II)
sulphate, FeSOo.THrO? [Relative atomic mass: H= l,O =
16, S = 32,Fe =561
Berapakah peratus komposisijisim bagi
air
dalamferum(ll
) sulfat
terhidrat, FeSOo. TH2O?LJisim atom
relatif.
H:
1,O:
16, S:32,Fe=
561A
9.00%B
14.12'Yoc
45.32%D
7t.20%
Gl
The
Mole and
the Volume
of
Gas
SPM 2011
Quesfion
3616
Whencopper(Il)
carbonate, CuCO,is
heated, the gas released turns the lime water chalky.What is the volume of gas released when 0.62
gof
copper(ll)
carbonate is heated at room conditions?[Relative atomic mass: C
:
12,O:
16, Cu = 64; Molarvolume ofgas:
24 dm3 mol I at room conditions]Apabila
kuprum(ll)
karbonat, CuCO, dipanaskon,gas
yang
terbebas menukarkanair
kapur menjadikeruh.
Berapakah isi
padu
gos
yang
terbebasapabila
0.62
g
kuprum(Il) karbonat
dipanaskan pada
keadaan
bilik?
lJisim atom
relatif:
C:
12, O:
16,Cu:
64;Isi
padu
molargas
=
24
dm3 mol-tpada
keadaanbilikl
A
5cm3B
120 cm3C
240 cm3D
360 cm3 SPM 2012 Question 44l7
Thefollowing
equation represents the combustionofpropane
in excess oxygen.Persamaan berikut mewakil i pembakaran propana
dalam ol<sigen berleb ihan.
CrHr+50,
t
3CO2+4H2OWhat is the volume of carbon dioxide gas produced when 48 cm3 of propane is completely bumt?
[Molar
volume
of
gas:
24
dm3mol-r at
roomtemperature]
Apakah isi padu gas karbon dioksida yang
terhasil
apabila 48 cm3 propana terbakar dengan lengkap?
llsi padu
molar
gas
:
24
dm3 mol-tpada
suhubitikl
A
28 cmlB
48 cm3C
96 cm3SPM 2013 Question 41
18
A
compoundof
magnesiumnitrite
contains 72o/oof magnesium and 28o/o of nitrogen.
What
is
the
empirical formula
of
magnesiumnitrite?
[Relative atomic mass:N
:
l4;Mg:241
Suatu sebatian
magnesiumnitrit
mengandungi72o/o magnesium dan 28Yo nitrogen.
Apakahformula
empirik bagi magnesiumnitrit?
fJisim atom
relatif
:N
:
14;Mg--
2al
A
MgN,
B
MgrN
SPM 2013 Question 42
19
3.2
g of
gasX
occupies
ll20
cm3at
standard temperature and pressure (STP).What is the relative molecular mass of X?
fMolar
volume of gas at STP:
22.4 dm3mol-I]
3.2 g gas
X
menempatlll20
cm3pada
suhu dan tekanan pia,uat (STP).Berapakah
jisim
molekulrelatif
bagiX?
llsi
padu molar gos pada STP:22.4
dm3mofr]
C
MgrN,
D
MgrN,
c64
D70
A16
832
SPM 2015 Question 3820
Diagram9
showsa
graphof
the volume
of
gas released against time for a reaction between calcium carbonate, CaCO, and hydrochloric acid,HCl.
Rajah
9
menunjukknngraf
isi padu gas
yangterbebas melawan masa
bagi tindak
balqs qntarakalsium karbonat, CaCO,
dan asid
hidroklorik,HCI..
Volume of gas released (cm3)
Isi padu gas yang terbebas (cm3)
Time (s) Masa (s) Diagram 9
Raiah 9
What is the mass of calcium carbonate used in the reaction?
[Molar
volume
of
gas:
24
dm3mofr at
roomconditions;
Relative atomic mass: C
:
12,O:16,Ca--401
Berapakahj isim kalsium karbonat yang digunakan dalam tindak bolas
in?
llsi
padu molargas:24
dm3 mol't pada keadaanbilik;
Jisim atom
relatif:
C--
12, O:
16, Ca:
401A
0.14g
C
0.40 gB
0.20g
D
2.00 gSPM 2016
Question
502l
Diagram
16
shows volcanic eruptions
whichrelease gases such as
CO,,
SO2,H,,
steam, H"S, CO andHCl.
Rajah 16
menunjukkanletusan
gunung
berapiyang membebaskan gas seperti CO,, SO",
H,,
waPair,HrS,CO
danHCl.
Diagram 16
Rajah 16
To simulate the eruptiori
in
thelaboratory
apupil
added 12.6
g of
ammonium
dichromate(Vl),
(NH)rCrrO,
in a mortar and immediately ignited.The
decomposition
reaction
of
ammoniumdichromate(Vl)
produces
three
substances,chromium(lll)
oxide, nitrogen gas and steam.What
is
the volume
of
steam producedat
roomcondition?
[Relative atomic
mass:H: l;N:
14;O:
16;Cr
:
52;Molar
volumeof
gas atroom
condition:24
dm3mol-']
(Jntuk mensimulasikan letusan
di
dalam makmal,seorang
murid
memasukkan12.6
g
ammoniumdikromat(Yl),
(NHo)rCrrO,
dalam mortar
dandiny alakan dengan s erta-mer ta.
Tindak balas penguraian ammonium
dikromat(YI)
menghasilkan
tiga
bahan
iaitu
kromium(llI)
oksida, gas nitrogen dan wap air.
Berapakah
isipadu
wap
air yang
terhasil padakeadaan
bilik?
lJisim atom
relatif:
H:
I ; N:
14; O:
16: Cr:
52:'Isi padu gas pada keadaan
bilik:24
dm3mol ']
A
4.80 dm3B
1.20 dm3C
Q.30 dmlSPM 2017 Question 21
22
Diagram 4 shows a cooking gas cylinder. Rajah 4 menunjukkan sebuah silinder gas memasak.I
mol of butane gasI mol gos butana
Diagram 4 Rajah 4
How many hydrogen atoms are there in the cylinder? [Avogadro constant: 6.02
x
1023)Berapakah bilangan
atom
hidrogenyang
terdapat di dalam silinder itu?fPemalar Avogadro:6.02
X
l0r3]A tx6.02x1023 c
9x6.02x1023
B 2x6.02x1023 D
10x6.02x1023
@!
Chemical Formulae
SPM 2011
Question
723
DiagramI
shows the apparatus set-up to determinethe empirical formula of
copper(Il)
oxide. Raj ah I menunjukkan susunan radas bagi menentukan formula empirik bagi kuprum(ll) ol<sidaCopper(Il)
oxide
Combustion tube Kuprum(Il)oksida
Tiub pembakaran Dry hydrogengas
Gas hidrogen kering
./
Porcelain
dish
lHeat Piringporselin
PanaskanDiagram I
Rajah I
The dry hydrogen gas must be flowed through the apparatus
for
several minutes before heating thecopper(Il)
oxide.What is the reason for this action to be taken?
Gas hidrogen kering mesti dialirkan
melaluiradas untuk
beberapaminit
sebelum pemanasankuprum(ll)
oksida.Apakah sebab tindakqn
ini
diambil?A
To ensure all the copper(Il) oxide has changed into copperUntuk memastikan semua
kuprum(Il)
oksida telah bertukar kepada kuprumB
To
ensureall
air
has been removedso
that explosion can be preventedUntuk memastikan semua
udarq
dikeluarkan supqya letupan dapat dielakkanC
To
prevent copperfrom
reactingwith air
to formcopper(Il)
oxideUntuk
mengelakkan
kuprum
daripadabertindak
balas
dengan
udqra
bagi mem b e nt ukkuprum(ll)
o l<s i d aD
To prevent the water fromflowing
towards the hot porcelain dish and cracks the combustion tubeUntuk
mengelakkanair
daripada
mengalir
ke
arah
piring
porselin yang panas
danmeretakkan tiub pembakaran SPM 2011 Question 30
24
Table 2 shows the proton number and the nucleon numberof
atoms of elementsX
and Y.X
and Y are not the actual symbols of the elements. Jadual 2 menunjukkan nombor proton dan nombor nukleon bagi atom-atom unstrrX
dan unsur y .X
danY
bukan simbol sebenar unsur-unsur ilu.Element Unsur Proton
number
Nomborproton
Nucleonnumber
Nombor nukleonX
IJ 27Y
8 16Table2
Jadual2Element
X
reacts
with
element
y
to
form
acompound.
What is the molar mass of the compound?
Unsur
X
bertindak
balas dengan unsury
untukmemb entu k s uatu s ebatian.
Berapakah
jisim
molar bagi sebatian itu?A
43gmol'
B
50 gmol-'
SPM 2011 Question 33
25
ElementM
isin
the same group as magnesium in the Periodic Table. It reacts with oxygen gas to form a compoundwith
the formula MO.What is the formula of the fluoride of element
M?
[Proton number:
F:9, Mg:
12]Unsur M berada dalam lotmpulan yang sama dengan magnesium dalam Jadual Berkala. Ia boteh bertindak balas dengan
gas
ol<sigen untuk membentuk satu sebatian yang mempunyaiformula MO.
Apakah
formula
bagifluorida
bagi unsur M2 lNomborproton:
F =9,Mg
:
12]AMF
B
MF,
C
l02gmol'
D
113 gmol-'
C
M,F
D
M,F,
ffi
f
\---l
)
SPM 2012
Question
1826
Which
chemical
formula
is
correctly
named according to the IUPAC nomenclature system?Formula kimia
manakah yang dinamakan denganbetul berdasarkan sistem penamaan IUPAC? Chemical
formula
Formala
kimis
Name Nqma
Mgo
magnesium oxide magnesium oksida SO, sulphur oxidesulfur oksida
CO carbon oxide
karbon oksida FerO, iron oxide
ferum oksida SPM 2012 Question 37
27
The formulafor
potassium hexacyanoferrate(Il) isgiven
as K"Fe(CN)u.Its
relative formula
mass is368. What i's the value of Y?
[Relative atomic mass:
C:
12,N:
14,K:39,
Fe = 561Formula bagi kalium
heksasianoferat(ll) diberi
sebagai KrFe(CN)u. Jisim
formula relatif
sebotianini
ialah368. APakahnilaiY?
fJisim
atom
relatif'
C:12, N:
14,
K:39,
Fe:
561A2C4
B 3
D5
SPM 2014
Question
728
The
molecular
formula
of
calcium nitrate
andpotassium
phosphateare Ca(NOr), and
K,PO.
respectively.
What is the molecular formula ofcalcium phosphate?
Formula
molekulbagi
kalsiumnitrat
dan knliumfosfat adal ah Ca(NOr), dan K rP O o m as ing- mas i n g'
Apakah
formula
mo lekul bagi kals iumfosfat?
A
CaPOoB
CarPO,, SPM 2014 Question 48C
Ca,(POr).,D
Ca,(PO,,),29
The following
equation
representsthe
reactionbetween sodium and oxYgen.
Persamaan
berikut mewakili tindak
balas antara natrium dan oksigen.4Na+Oz
'2NazO
What
is
the
maximum mass
of
sodium
oxideformed when I I .5 g of sodium is heated completely in oxygen?
[Relative atomic mass:
Na:
23'O:
l6]
CD
Berapakah
jisim
maksimumnatrium
oksidayang
terbentuk
apabila
I1.5
g
natrium
dipanaskandengan lengkap dalam oksigen? lJisim atom
relatif:
Na:23,
O:
l6]
A
15.5 gB
19.5 g SPM 2015Question
730
DiagramI
shows the apparatus set-up to determine the empirical formula of magnesium oxide.Rajah
I
menunjukkan
susunan
radas
untuk menentukanformula
empirik magnesium oksida.Lid Penutup
C
31.0 gD
62.0 g Crucible Mangkukpijar
Magnesium ribbon Pito magnesium"l*
Panaskan"';:::[,'
Which
stepis
correct
to
ensurethe
magnesiumribbon burnt comPletelY?
Langkah
manakahyang
betul untuk
memastikanpita
magnesiumitu
terbqkar dengan lengkap?A
RaisethecruciblelidonceinawhileduringheatingBuka penutup
mangkukpijar
sekali-sekalasemasa pemanasan
B
Heat the
magnesiumribbon strongly
in
thecrucible
without
itslid
Panaskan
pita
magnesiumitu
dengan kuat dalam mangkukptjar
tanpa penutupnyaC
Cover the cruciblewith
its
lid
as soon as the magnesium ribbon starts burningTutup mangkuk pij ar dengan penutupnya sebaik sahaja
pita
magnesium itu mula terbakarD
Repeatthe
processof
heating,
cooling
and weighinguntil
a constant mass is obtained(Jlang proses
pemanasan,penyeiukan
danpenimbangan sehingga
jisim
tetap diperoleh SPM 2015 Question 373l
The
decomposition
of
lead(Il) nitrate
produceslead(Il)
oxide, nitrogen dioxide and oxygen.Which
of
thefollowing
is
the balanced chemicalequation for the reaction?
Penguraian plumbum(Il)
nitrat
menghasilkanplumbum(ll)
oksida, nitrogen dioksida dan oksigen'Antara
yang
berikut,
yang
manakah persamaankimia seimbang bagi tindak balas tersebut?
A
Pb(NO3),-
PbO2 + 2NO + OzB
Pb(NO3),*
PbO + 2NO + OrC
2Pb(NO3),+
2PbO + 4NO2 + 02SPM 2016 Question 30
32
Diagram 9 shows the apparatus set-up to determine the empirical formulaof lead(Il)
oxide.Rajah
9
menunjukkan
susunan
radas
untuk menentukanformula
empirikplumbum(ll)
oks ida.Lead(II) oxide Plumbum(ll\ oksida
Dry hydrogen gas
+
Gas hidrogen kering
Diaprram 9
niion
sWhich
statement explainswhy
the method isnot
suitable
to
determine
the empirical formula
of
magnesium oxide?
P ernyataan manakah yang menerangkan mengapa kaedah
ini tidak
sesuqi untuk menentukanformula
empir ik magnes ium o ks i da?A
Magnesium burnsvigorously
in oxygen Magnesiumterbakar
dengansqngat
cergas dalam oksigenB
Magnesium explodes whenit
is heated Magnesium meletup apabila dipanaskanC
Magnesium is more electropositive than leadMagnesium
lebih
elektropositif
daripadaplumbum
D
Magnesium is more reactive than hydrogen Magnesium lebih reaktif daripada hidrogen SPM 2016Quesfion
3933
0.40 gX
metal reactswith
fluorine to produce 0.78 gofX
fluoride.What is the empirical formula of the
X
fluoride? [Relative atomic mass: F=
19;X:40]
0.40
g
logam
X
bertindak balas
denganfiuorin
untuk menghasilkan 0.7 8 g X
fiuorida.
Apakahformula
empirik bagi Xfluorida
itu?fJisim atom
relatif:
F:
19;X:401
AXF
B
XF,
C
X,F
D
XFoSPM 2017
Quesfion
3634
The following
chemical equation
represents thephotosynthesis process.
Persamaan kimia berikut mewakili proses folosintesi.s.
6Co,
+
r2H2o#ffic6H,2o6
+ 602 +6H,o
What is the mass of glucose produced
if
1.2 dmrof
carbon dioxide gas is used? +
I I
Heat
Panaskan
[Relative atomic mass: H
:
l;
C:
12;Q:
16;Molar
volume of gas at room
conditions:24
dm3 mol-r] Apakahjisim
glukosa yang terhasiljika
1.2 dm3 ga.skar bon d io ks ida digunakan?
fJisim atom relatif.
H:
I ; C:
12; O:
161' Isi padu molargas pada keadaan
bilik:24
dm3 mol-r]A
0.81 gB
1.50 gc
4.e!
eD
9.00 gf[!
Chemical Equations
SPM2011Question
335
The following
equation
representsa
chemicalreaction.
NqCO,(aq) +
Cacl(aQ
*
CaCO:(s) + 2NaC(aq)Persamaan
berikut mewakili satu tindak
balaskimia.
NqCQ(ak)
+Cacl(ak)
---+CaCQ(d
+ 2NaC(ak)Which statement is correct? Pernyataan manakah yang betul?
A
Two
moles
of
sodium
carbonate reactwith
one mole of calcium chloride
Dua mol natrium
karbonat bertindok
balasdengan satu mol kalsium
klorida
B
The products are calcium carbonate precipitate and sodium chloride solutionHasil
tindak balas
ialah
mendakan kalsiumkarbonat dan larutan natrium
klorida
C
The reactants aresolid
sodium carbonate andcalcium chloride solution
Bahan tindak balas ialah pepejal
natriumkarbonat dan larutan kalsium
klorida
D
Two
moles
of
calcium
carbonateand
onemole
of
sodium chloride are formedDua mol
kalsium karbonat dan satu
mol natriumklorida
terbentukSPM 2014 Question 41
36
What
is
the
massof
sodium chloride
obtainedwhen 50.0 cm3
of
1.0mol
dm-3 sodium hydroxide reactswith
50.0 cm3of
1.0 mol dm-3 hydrochloric acid?[Relative atomic mass: Na
:
23,Cl:
35.5]
Berapakahjisim
natrium klorida yang
diperoleh apabila 50.0 cm3 natrium hidrol<sida 1.0 mol dm 3 bertindak balas dengan 50.0 cm3 asidhidroklorik
1.0
mol
dmr?
lJisim atom
relatif:
Na:
23, Cl:
35.51A
0.025 gB
0.050 gC
1.463 gSPM 2014 Question 43
37
The reaction between barium chloride solution andlead(Il)
nitrate solution produceslead(Il)
chlorideand barium nitrate.
Which ionic equation represents the reaction?
Tindak
balas
antara larutan
barium
klorida
dan
larutan plumbum(Il)
nitrat
menghasilkonplumbum(Il)
klorida dan barium nitrat.Persamaan
ion yang
manakahmewakili
tindak balas itu?A
pb2* +NO3-
---
pb(NO3),B
Pb2*+
2Cl-
---+ PbCl,C
Ba2*+
2Cl-
___+BaCl,
D
Ba2* +2NO3-
-
Ba(NOr)2 SPM 2016 Question 2238
The
reaction between
aluminium and iron(III)
oxide produce iron and substances
X.
What is the chemical formula of X?
Tindak balas di antara aluminium
denganferum(lll)
olrs ida menghos ilkan
ferum
dan bahan X. Apakahformula kimia
b agi X?A
AIO
B
AlO2SPM 2017
Question
38c
Al2o3D
Al3O239
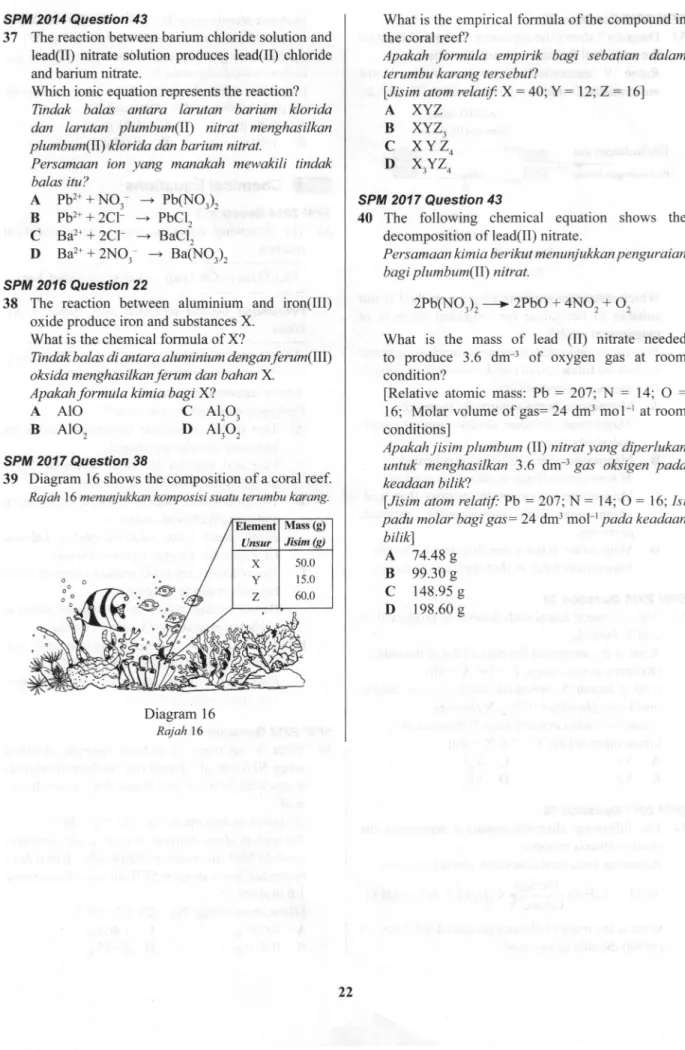
Diagram 16 shows the compositionof
a coral reef. Rajah 16 menunjukkan komposisi suatu terumbu karang.Diagram 16 Rajah 16
What is the empirical formula
of
the compound in the coralreefl
Apakah
formula
empirik bagi
sebatian
dalamterumbu karang tersebut?
lJisim atom
relatif.
X
:
40;Y
:
12;Z:
16)A
XYZ
B
XYZ3c
xYZ4
D
X3YZ4SPM 2017 Question 43
40
The
following
chemical equation shows
the decompositionof
lead(II) nitrate.P ers amaan kimi a b eri kut me nu nj ukkan p e ngu ra i a n
b agi p lum
bum(ll)
n it rat.2Pb(NO3), ----+ 2PbO + 4NO2
+
02What
is
the
mass
of
lead
(II)
nitrate
neededto
produce
3.6
dmr
of
oxygen gas
at
roomcondition?
[Relative atomic
mass:Pb
-
207:
N
:
14;
O
:
16;
Molar
volumeof
gas: 24
dm3mol-r
at roomconditionsl
Apakah
jisim
plumbum(ll)
nitrat
yang diperlukanuntuk
menghasilkan3.6
dm-3gas
oksigen padakeadaan
bilik?
fJisim atom
relatif:
Pb:207:
N
:
14;O:
16;'Isi
padu molar bagi