Possible involvement of protein phosphorylation in the
wound-responsive expression of
Arabidopsis
plastid
v
-3 fatty acid
desaturase gene
Hiroaki Kodama
a,* , Takumi Nishiuchi
a,1, Shigemi Seo
b,c, Yuko Ohashi
b,c, Koh Iba
aaDepartment of Biology,Kyushu Uni6ersity,Fukuoka812-8581,Japan
bDepartment of Molecular Genetics,National Institute of Agrobiological Resources,Tsukuba,Ibaraki305-8602,Japan cCore Research of Science and Technology(CREST),Tiyoda-ku,Tokyo101-0062,Japan
Received 3 September 1999; received in revised form 21 January 2000; accepted 21 January 2000
Abstract
The plastidv-3 fatty acid desaturase (FAD7) catalyzes the conversion of linoleic acid to linolenic acid. Wounding enhances the expression of theFAD7 gene in leaves and induces its expression in stems and roots. The wound-induced expression of theFAD7 promoter was investigated in transgenic tobacco plants carrying the −825Arabidopsis FAD7 promoter::b-glucuronidase (GUS) fusion gene. The protein kinase inhibitor, staurosporine, and the protein phosphatase inhibitor, calyculin A, suppressed the wound induction of theFAD7 gene in stems. A tobacco mitogen-activated protein kinase (WIPK) was rapidly activated upon wounding not only in leaves but also in stems and roots, indicating that WIPK probably mediates the wound signals in most vegetative organs. The FAD7 promoter::GUS fusion gene was introduced into the transgenic tobacco plants in which thewipk gene was expressed constitutively at a high level or into the transgenic plants in which thewipk gene was suppressed possibly due to the transgene-induced gene silencing. The wound-induced expression of the FAD7 gene in stems was enhanced in the former transgenic tobacco plants and suppressed in the latter plants. These results suggest that the wound activation of the FAD7 promoter depends on both protein phosphorylation and dephosphorylation events especially in stems, and also that WIPK is involved in such signaling cascades. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Arabidopsisplastidv-3 fatty acid desaturase; calyculin A; jasmonic acid; staurosporine; wounding; wound-induced protein kinase www.elsevier.com/locate/plantsci
1. Introduction
Plants confront herbivore feeding by activating the defense systems that include the transcriptional activation of defensive genes, such as genes for proteinase inhibitor II (PI-II). Jasmonic acid (JA)
is one of the signaling molecules for mechanical wounding (reviewed in [1]). Accumulation of JA in response to wounding causes a de novo induction of defensive genes and, therefore, is essential in plant defenses against insects [2] and pathogen attacks [3]. JA is synthesized from linolenic acid through a lipoxygenase-dependent process called the octadecanoid pathway [4,5]. Thev-3 fatty acid desaturases convert linoleic acid to linolenic acid in lipids and thus play a role in supplying a substrate for JA synthesis [6,7]. They are both localized in the microsome membranes and in plastid envelopes as two distinct isoforms (re-viewed in [8]).
The FAD7 gene encodes the plastid v-3 fatty acid desaturase [9]. Wounding enhances the accu-Abbre6iations: GUS,b-glucuronidase; DMSO, dimethyl sulfoxide;
JA, jasmonic acid; MAP kinase, mitogen-activated protein kinase; PI-II, proteinase inhibitor II; SIPK, salicylic acid-induced protein kinase; WIPK, wound-induced protein kinase.
* Corresponding author. Present address: Department of Biore-sources Chemistry, Faculty of Horticulture, Chiba University, Mat-sudo 648, Chiba 271-8510, Japan; tel./fax: +81-47-308-8857.
E-mail address:[email protected] (H. Kodama)
1Present address: Plant Molecular Biology Laboratory, Molecular
Biology Department, National Institute of Bioscience and Human Technology, AIST, MITI, 1-1 Higashi, Tsukuba, Ibaraki 305-8566, Japan.
mulation of FAD7 mRNA in leaf tissues of to-bacco [10] and Arabidopsis thaliana [11]. In un-wounded plants, the FAD7 gene is preferentially expressed in chloroplast-containing tissues. How-ever, induction of the FAD7 gene by wounding was evident not only in leaf tissues but also in stem and root tissues. These changes in the spatial expression pattern of theFAD7 gene by wounding is thought to be mediated through different signal transduction pathways [11]. In leaf and stem tis-sues, the transcriptional activation of the FAD7 gene by wounding appears to occur independently of the wound-triggered JA accumulation. On the other hand, the wound activation of the FAD7 gene in root tissues is considered to be mediated by a JA-dependent signaling pathway (reviewed in [12]). In potato leaves, accumulation of transcripts of the plastid v-3 desaturase gene preceded the wound-responsive expression of thePI-IIgene. JA treatment had no effect on the level of potato plastid v-3 desaturase mRNA, suggesting that wound-induced expression of this gene is also regulated by a JA-independent signaling pathway in leaves [7].
Protein phosphorylation cascades often consti-tute the intracellular signaling pathways that are activated in response to external signals. Several reports suggest that mitogen-activated protein (MAP) kinases are involved in the wound-acti-vated signal transduction. In tobacco plants, sev-eral protein kinases with properties of MAP kinases have been identified [13 – 18]. Wound-in-duced protein kinase (WIPK) and salicylic acid-in-duced protein kinase (SIPK) were demonstrated to be both wound-activated MAP kinases using anti-bodies specific for these protein kinases [19,20]. The role of WIPK in the wound-signaling cascade has been suggested by the analysis of transgenic tobacco plants in which the WIPK activity was modulated. When the wipk cDNA was introduced into tobacco plants under the control of a consti-tutively expressed promoter, two independent plant lines with distinct properties were isolated. In one transgenic line (TMP1S-13), the endoge-nouswipk gene was inactivated possibly by trans-gene-induced gene silencing and the accumulation of both JA and mRNAs for JA-inducible genes were suppressed after wounding [13]. Another line (35S::wipk-8) conferred a constitutive activation of WIPK without wounding and showed 3 – 4-fold higher levels of JA than did nontransformed
to-bacco plants. In the latter transgenic plant, the PI-II mRNA accumulated in unwounded leaves [20]. These results suggest that the level of acti-vated WIPK is closely related to the JA level.
To delineate the signal transduction pathways that ultimately lead to the wound-responsive ex-pression of the FAD7 gene, we investigated here the possible involvement of protein phosphoryla-tion in the wound activaphosphoryla-tion of the FAD7 pro-moter in presence of inhibitors for both protein kinases and protein phosphatases. We also investi-gated the wound responsiveness of theFAD7 pro-moter in the transgenic tobacco plants in which WIPK activity is modulated.
2. Materials and methods
2.1. Plasmid construction
The Ti-plasmid, pfD82, contains the −825
Arabidopsis FAD7 promoter::b-glucuronidase
(GUS) reporter fusion gene (FAD7 pro::GUS, [21]). The 2-kb EcoR I fragment of the pYL3 [22] contains promoter sequence from the nopaline synthase gene, the hygromycin phosphotransferase gene and the terminator sequence from the nopa-line synthase gene. This fragment was cloned into the EcoR I site of the pfD82 to generate plasmid, pfD82h.
2.2. Plant materials
Tobacco (Nicotiana tabacum cv W38) plants transformed with pfD82 were generated as previ-ously described [21].
Mu-rashige – Skoog medium supplemented with 20 mg/ ml hygromycin B. The parental plants, that were transformed with the 35S::wipk, are resistant to kanamycin but sensitive to hygromycin. Therefore, the R1 seedlings that are hygromycin-resistant must carry the T-DNA of the pfD82h. The hy-gromycin-resistant R1 plants were transferred to soil, grown at 26°C under continuous light illumi-nation (2000 lux) and subjected to analyses of the segregation of 35S::wipk transgene and of their wound responsiveness.
All experiments used 2 – 3-month-old tobacco plants.
2.3. Segregation of the 35S::wipk transgene
To investigate the inheritance of the 35S::wipk, total DNA was prepared from leaves of each hygromycin-resistant R1 plant according to the method of Edwards et al. [23]. The 35S::wipk transgene in the genome was PCR-amplified using the CaMV 35S promoter-specific primer, 35Sp1 (5%-ACTATCCTTCGCAAGACCCT-3%), and the wipk gene-specific primer, WIPK-N1 (5%
-GGT-GAAGATCAGTATCCATG-3%). The
474-bp-fragment with a portion of wipk was amplified from total DNA prepared from the hygromycin-resistant R1 plants harboring the 35S::wipk trans-gene but the trans-gene was not amplified from hygromycin-resistant R1 plants in which the 35S::wipk transgene was segregated out.
2.4. Wound treatments with or without inhibitors
Tobacco leaves were cut into discs 7.5 mm in diameter with a cork borer. Stems were cut into about 1-mm-thick slices with a razor blade and roots were cut into about 1-cm-long sections as previously described [11]. Soluble proteins of an aliquot from each sample were prepared immedi-ately after the preparation of tissue segments and subjected to the measurement of their GUS activ-ity as unwounded tissues. Other tissue fragments were floated on 50 mM sodium phosphate buffer, pH 7.0, with or without dimethyl sulfoxide (DMSO), staurosporine, or calyculin A at indi-cated concentrations. Inhibitors were dissolved in DMSO, and final concentration of DMSO was 1% (v/v) or less in these wound treatments. The above solutions were vacuum-infiltrated into intracellular spaces of each organ tissue as described by
Con-rath et al. [24]. Leaf and stem fragments were incubated at 26°C under light, and root segments were placed in the dark for 6 h. After wounding, soluble proteins were prepared from leaves, stems, and roots and the GUS activities in soluble proteins were fluorometrically determined as pre-viously described [21].
2.5. Assay of myelin basic protein kinase acti6ity
of immune-complex proteins
The wild-type tobacco plants (N. tabacum cv Samsun NN) grown in a greenhouse were sub-jected to an immune-complex kinase assay. Wounding were performed by cutting each organ with a pair of scissors. The soluble proteins are prepared from each organ as previously described [20]. Immunoprecipitation was performed with anti-WIPK antibody and myelin basic protein ki-nase activities of resultant immune-complexes were determined by the kinase assay as described [20].
3. Results
3.1. Effects of inhibitors of protein
phosphorylation and dephosphorylation on the
wound acti6ation of the FAD7 promoter
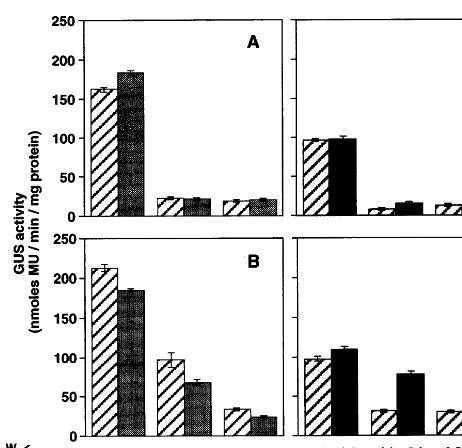
stau-Fig. 1. Effects of staurosporine and calyculin A on the wound induction ofGUSexpression driven by theFAD7 promoter. GUS activities in each organ of healthy plants transformed with theFAD7 pro::GUS gene were assayed in unwounded tissues (open boxes). Aliquot samples of each organ were incubated in sodium phosphate buffer for 6 h (hatched boxes). Alternatively samples were incubated for 6 h in sodium phosphate buffer supplemented with a solvent only (+DMSO), staurosporine (+Sta) or calyculin A (+Cal) at the concentrations indicated in this figure (closed boxes). GUS activities were then assayed in wounded tissues. GUS activity was expressed as nmole of 4-methylumbelliferone (MU) generated per min per mg protein. (A) Promoter activity in leaves. (B) Promoter activity in stems. (C) Promoter activity in roots. Values are means 9S.E.M. (n=5).
rosporine did not affect the induction of GUS expression significantly. However, the wound-in-duced elevation of GUS activity rewound-in-duced to about 18% of controls by incubation of stems in 5 mM staurosporine. Incubation of stem tissues in caly-culin A resulted in remarkable inhibitory effects on the induction ofGUS expression by wounding. In the presence of 0.2 and 1 mM calyculin A, the wound-induced elevation of GUS activity de-creased to about 55 and 6% of controls, respec-tively. In roots, 1 mM staurosporine or 0.2 mM calyculin A did not affect an increase in GUS activity in response to wounding. However, wound induction of GUS expression was partly inhibited when root tissues were treated with 5 mM stau-rosporine or 1mM calyculin A. These results sug-gest that the wound-induced expression of the GUS gene under control of the FAD7 promoter is mediated by a signal transduction pathway involv-ing the phosphorylation and dephosphorylation of unidentified proteins. These phosphorylation and dephosphorylation events seem to be relevant for FAD7 expression mainly in stems, partially in roots, and not in leaves.
3.2. Wound-induced WIPK acti6ation in stem and
root tissues
Possible candidates for wound-signal mediating protein kinases are MAP kinases [19,20]. In these
studies, leaf tissues alone had been subjected to the kinase assays. Therefore, we assessed the activa-tion of WIPK in other vegetative organs of N.
tabacum cv Samsun NN plants. Total soluble
proteins from leaves, stems and roots were im-munoprecipitated with the WIPK-specific anti-body, and the resultant immune-complexes were subjected to the kinase assay. Myelin basic protein kinase activity of WIPK drastically increased within 5 min after wounding in stems and roots, similar to leaf tissues (Fig. 2). This result suggests that protein kinases such as the WIPK participate in wound-induced signal transduction not only in leaves, but also in the stems and roots.
Fig. 3. Wound induction of GUS activity driven by theFAD7 promoter in tobacco plants in which the activity of WIPK would be modulated. GUS activities of aliquot samples were determined in each organ of healthy plants immediately after preparation of samples. Other aliquot samples were then incubated in sodium phosphate buffer for 6 h, then the GUS activities were determined. (A, B) Promoter activity of the TMP1S-13 progeny plants in unwounded condition (A) and after wounding (B). The shaded boxes indicate the GUS activity in theFAD7 pro::GUS/35S::wipk plants and hatched boxes indicate the GUS activity in the corresponding FAD7 pro::GUS/null plants. (C, D) Promoter activity of the 35S::wipk-8 progeny plants in unwounded condition (C) and after wounding (D). The closed boxes indicate the GUS activity in theFAD7 pro::GUS/35S::wipk plants and hatched boxes indicate the GUS activity in the corresponding FAD7 pro::GUS/null plants. Values are means 9S.E.M. (n=5). The ratios of average GUS activity in wounded tissues (W) to that in unwounded tissues (U) are indicated below each box. Similar results were obtained in another independent experi-ment.
Several hygromycin-resistant R0 plants were se-lected in which significant GUS activities in chlorophyllous tissues were observed as previously reported [21]. Among them, the transgenic lines that showed a segregation of T-DNA as single functional T-DNA insertional plants were sub-jected to the cross with the TMP1S-13 or 35S::wipk-8 plants. The resultant R1 seeds were aseptically germinated on hygromycin-containing medium. Then, hygromycin-resistant R1 plants were classified into two groups based on the PCR-amplification of a portion of the wipk gene (Sec-tion 2); one group had the 35S::wipk transgene (FAD7 pro::GUS/35S::wipk), and in another group the 35S::wipk transgene was crossed out (FAD7 pro::GUS/null). These two groups were provided from a common seed population that had been produced by crossing a R0 FAD7 pro::GUS transformant with the 35S::wipk plants, and thus shared the same genetic backbone except for the inheritance of the 35S::wipk transgene. It has not been confirmed whether the 35S::wipk transgene confers the same phenotype to the FAD7 pro::GUS plants when compared to the previously published phenotypes for the 35S::wipk plants [13,20]. However the expression of thePI-II gene in the progeny plants produced by self-polli-nation of the TMP1S-13 and 35S::wipk-8 plants was nearly the same as those of the parental plants (Seo et al., unpublished results).
The GUS activities of these FAD7 pro::GUS transgenic plants in unwounded and wounded conditions are shown in Fig. 3. When the FAD7 pro::GUSplants were crossed with the 35S::wipk-8 plants or TMP1S-13, the resulting progenies showed markedly higher GUS activity in un-wounded leaves than those in unun-wounded stems and roots (Fig. 3A and C). There was no signifi-cant difference in the GUS activity between plants with or without the 35S::wipk transgene in un-wounded condition.
In a tobacco cultivar, W38, we repeatedly ob-served the wound-responsive elevation of GUS activity in leaves as seen in the control data in Fig. 1 [11]. However in the FAD7 pro::GUS/null plants, activation of the FAD7 promoter upon wounding was not obvious (Fig. 3B and D), and similar results were observed in another FAD7 pro::GUS transgenic line that had been generated by using N. tabacum cv Samsun NN plants (data not shown). The reason for such different wound
3.3.Wound responsi6eness of the FAD7promoter in
the transgenic tobacco plants with a modulated
WIPK acti6ity
The wound induction mediated by the FAD7 promoter was investigated in the transgenic to-bacco plants in which the activity of WIPK is modulated [13,20]. Since these 35S::wipk plants were generated by using a different tobacco
culti-var,N. tabacumcv Samsun NN, we here provided
responses of the FAD7 promoter between W38 and Samsun NN is not clear at present. The progenies of TMP1S-13 plants containing the 35S::wipk transgene showed no apparent wound induction of GUS activity in leaves, and those of 35S::wipk-8 plants showed only a barely signifi-cant elevation of GUS activity (Fig. 3B and D). In stems, wounding induced an increase in the GUS activity (about 4-fold) in the FAD7 pro::GUS/null plants. In the progenies of 35S::wipk-8 plants, the FAD7 pro::GUS/35S::wipk plants exhibited higher GUS activity in wounded stems than those of corresponding null plants. By contrast, the wound induction of the FAD7 pro-moter was weaker in the presence of the 35S::wipk transgene in the progenies of TMP1S-13 plants compared to the null plants (Fig. 3B and D).
There were only slight differences in the GUS activity of wounded roots between 35S::wipk and the corresponding null plants. However, the ratio of GUS activity of wounded roots to that of unwounded roots decreased by the 35S::wipk transgene in the TMP1S-13 progenies and in-creased significantly in the 35S::wipk-8 progenies (Fig. 3B and D).
4. Discussion
In this study, we showed that the staurosporine-sensitive protein kinase(s) might play a role in the signal transduction cascade that leads to wound-induced activation of the Arabidopsis FAD7 pro-moter (Fig. 1). Staurosporine exhibited the most pronounced effects in stem tissues. In tobacco plants, several MAP kinases have been found to be activated upon wounding [13,15,19,20]. Al-though in most previous studies, the wound acti-vation of MAP kinases had been demonstrated only in leaf tissues, we found that WIPK was also activated in stem and root tissues by wounding (Fig. 2). Therefore, WIPK is one of the possible candidates for the protein kinases responsible for the wound induction of the FAD7 gene in stems. Analysis of the phenotypes of FAD7 pro::GUS/ 35S::wipk plants also suggested that WIPK medi-ates the wound signal to induce the expression of theFAD7 gene (Fig. 3). In unwounded tissues, the expression levels ofGUSgene driven by the FAD7 promoter were not much different between the progenies of the TMP1S-13 and 35S::wipk-8.
Al-though the responsiveness of transcriptional activ-ity of the FAD7 gene to wounding was lower and
higher in all tissues examined of the
FAD7pro::GUS/35S::wipk progenies of TMP1S-13 and 35S::wipk-8, respectively, when compared with the corresponding FAD7 pro::GUS/null plants (see the ratios shown under the Fig. 3B and D), the only significant difference of the FAD7 promoter activity between the two transgenic lines was observed when stem tissues were wounded (Fig. 3B and D). These results suggest that WIPK might phosphorylate certain proteins that function as positive regulators for transcriptional activation of the FAD7 promoter in stem tissues. In stems, a nuclear protein was detected that interacted with the wound-responsive region (−242 to −223) of theFAD7 promoter, and formation of this nuclear protein – DNA complex was stimulated signifi-cantly by wounding [27]. Interestingly, nuclear extracts treated with alkaline phosphatase lost the ability to form this complex, suggesting that a phosphorylated nuclear protein binds to this wound-responsive region (Nishiuchi et al., unpub-lished data). Therefore, this nuclear protein is a possible candidate for the target protein of the WIPK or its downstream protein kinases, al-though further study is required to clarify the identity and role of this phosphorylated nuclear factor in the wound-inducible expression of the FAD7 gene. In this context, it is implied that WIPK is involved not only in the regulation of the wound-induced responses via JA biosynthesis [13,20] but also in JA-independent wound signal-ing cascade at least in stem tissues.
In root tissues, it was suggested that the FAD7 promoter activity is modulated by JA accumula-tion in response to wounding [11]. Although the 35S::wipk-8 plants can be expected to contain increased JA levels not only in unwounded leaves [20] but also in roots, the activity of the FAD7 promoter was almost the same in both TMP1S-13 and 35S::wipk-8 progenies (Fig. 3A and C). The elucidation of involvement of JA in the regulation of transcriptional activity of FAD7 gene in the root tissue of 35S::wipk plants requires more de-tailed analyses since JA levels in root tissues of these parental transformants has not been determined.
Arabidopsis, it has been suggested that protein kinases positively regulate the wound-responsive expression of several genes, such as WR3 and choline kinase genes, and that the activity of protein phosphatases is also required for the acti-vation of several other wound-responsive genes [28]. In this case, it was shown that protein kinases and phosphatases may play opposite roles in the activation of wound-responsive genes. By contrast, our results suggest a unique mode of regulation of wound-responsive gene expression, that is, both protein kinases and phosphatases may function in wound signal transduction pathways leading to expression of the FAD7 gene.
Acknowledgements
This research was supported in part by Grant RFTF96L00602 from the Japan Society for Pro-motion of Science to KI.
References
[1] E.E. Farmer, Fatty acid signalling in plants and their associated microorganisms, Plant Mol. Biol. 26 (1994) 1423 – 1437.
[2] M. McConn, R.A. Creelman, E. Bell, J.E. Mullet, J. Browse, Jasmonate is essential for insect defense in Ara-bidopsis, Proc. Natl. Acad. Sci. U.S.A. 94 (1997) 5473 – 5477.
[3] P. Vijayan, J. Shockey, C.A. Le´vesque, R.J. Cook, J. Browse, A role for jasmonate in pathogen defense of
Arabidopsis, Proc. Natl. Acad. Sci. U.S.A. 95 (1998) 7209 – 7214.
[4] B.A. Vick, D.C. Zimmerman, Biosynthesis of jasmonic acid by several plant species, Plant Physiol. 75 (1984) 458 – 461.
[5] E.E. Farmer, C.A. Ryan, Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors, Plant Cell 4 (1992) 129 – 134. [6] M. McConn, J. Browse, The critical requirement for
linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant, Plant Cell 8 (1996) 403 – 416. [7] M. Martı´n, J. Leo´n, C. Dammann, J.-P. Albar, G. Griffiths, J.J. Sa´nchez-Serrano, Antisense-mediated de-pletion of potato leaf v3 fatty acid desaturase lowers linolenic acid content and reduces gene activation in response to wounding, Eur. J. Biochem. 262 (1999) 283 – 290.
[8] P. Mazliak, Desaturation processes in fatty acid and acyl lipid biosynthesis, J. Plant Physiol. 143 (1994) 399 – 406. [9] K. Iba, S. Gibson, T. Nishiuchi, T. Fuse, M. Nishimura, V. Arondel, S. Hugly, C. Somerville, A gene encoding a chloroplast v-3 fatty acid desaturase complements
alter-ations in fatty acid desaturation and chloroplast copy number of the fad7 mutant of Arabidopsis thaliana, J. Biol. Chem. 268 (1993) 24099 – 24105.
[10] T. Hamada, T. Nishiuchi, H. Kodama, M. Nishimura, K. Iba, cDNA cloning of a wounding-inducible gene encoding a plastid v-3 fatty acid desaturase from to-bacco, Plant Cell Physiol. 37 (1996) 606 – 611.
[11] T. Nishiuchi, T. Hamada, H. Kodama, K. Iba, Wound-ing changes the spatial expression pattern of the Ara-bidopsis plastid v-3 fatty acid desaturase gene (FAD7) through different signal transduction pathways, Plant Cell 9 (1997) 1701 – 1712.
[12] T. Nishiuchi, K. Iba, Roles of plastid v-3 fatty acid desaturases in defense response of higher plants, J. Plant Res. 111 (1998) 481 – 486.
[13] S. Seo, M. Okamoto, H. Seto, K. Ishizuka, H. Sano, Y. Ohashi, Tobacco MAP kinase: a possible mediator in wound signal transduction pathways, Science 270 (1995) 1988 – 1992.
[14] K. Suzuki, H. Shinshi, Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with fungal elicitor, Plant Cell 7 (1995) 639 – 647. [15] S. Usami, H. Banno, Y. Ito, R. Nishihama, Y. Machida, Cutting activates a 46-kilodalton protein kinase in plants, Proc. Natl. Acad. Sci. U.S.A. 92 (1995) 8660 – 8664.
[16] C. Wilson, R. Anglmayer, O. Vicente, E. Heberle-Bors, Molecular cloning, functional expression in Escherichia coli, and characterization of multiple mitogen-activated-protein kinases from tobacco, Eur. J. Biochem. 233 (1995) 249 – 257.
[17] A.L. A´ da´m, S. Pike, M.E. Hoyos, J.M. Stone, J.C. Walker, A. Novacky, Rapid and transient activation of a myelin basic protein kinase in tobacco leaves treated with harpin fromErwinia amylo6ora, Plant Physiol. 115 (1997) 853 – 861.
[18] S. Zhang, D.F. Klessig, Salicylic acid activates a 48-kD MAP kinase in tobacco, Plant Cell 9 (1997) 809 – 824. [19] S. Zhang, D.F. Klessig, The tobacco wounding-activated
mitogen-activated protein kinase is encoded by SIPK, Proc. Natl. Acad. Sci. U.S.A. 95 (1998) 7225 – 7230. [20] S. Seo, H. Sano, Y. Ohashi, Jasmonate-based wound
signal transduction requires activation of WIPK, a to-bacco mitogen-activated protein kinase, Plant Cell 11 (1999) 289 – 298.
[21] T. Nishiuchi, T. Nakamura, T. Abe, H. Kodama, M. Nishimura, K. Iba, Tissue-specific and light-responsive regulation of the promoter region of the Arabidopsis thaliana chloroplast v-3 fatty acid desaturase gene (FAD7), Plant Mol. Biol. 29 (1995) 599 – 609.
[22] T. Fuse, H. Kodama, N. Hayashida, K. Shinozaki, K. Iba, A Ti-plasmid-convertible l phage vector system harboring the hygromycin B phosphotransferase gene, Plant Biotechnol. 15 (1998) 227 – 230.
[23] K. Edwards, C. Johnstone, C. Thompson, A simple and rapid method for the preparation of plant genomic DNA for PCR analysis, Nucl. Acids Res. 19 (1991) 1349. [24] U. Conrath, H. Silva, D.F. Klessig, Protein
dephospho-rylation mediates salicylic acid-induced expression of
[25] P. Cohen, C.F.B. Holmes, Y. Tsukitani, Okadaic acid: a new probe for the study of cellular regulation, Trends Biochem. Sci. 15 (1990) 98 – 102.
[26] U.T. Ru¨egg, G.M. Burgess, Staurosporine, K-252a and UCN-01: potent but nonspecific inhibitors of protein kinases, Trends Pharmacol. Sci. 10 (1989) 218 – 220. [27] T. Nishiuchi, H. Kodama, S. Yanagisawa, K. Iba,
Wound-induced expression of the FAD7 gene is
medi-ated by different regulatory domains of its promoter in leaves/stems and roots, Plant Physiol. 121 (1999) 1239 – 1246.
[28] E. Rojo, E. Titarenko, J. Leo´n, S. Berger, G. Vancan-neyt, J.J. Sa´nchez-Serrano, Reversible protein phospho-rylation regulates jasmonic acid-dependent and -independent wound signal transduction pathways in
Arabidopsis thaliana, Plant J. 13 (1998) 153 – 165.