Trends affecting the next generation of U.S. agricultural
biotechnology: Politics, policy, and plant-made pharmaceuticals
Patrick A. Stewart
a,*, Andrew J. Knight
ba
Department of Political Science, Masters of Public Administration Program, P.O. Box 1750, Arkansas State University, State University, AR 72467, USA
b
Department of Criminology, Sociology and Geography, Arkansas State University, State University, AR 72467, USA
Received 7 December 2003; received in revised form 3 March 2004; accepted 4 March 2004
Abstract
This paper analyzes the structure and history of regulatory policies in the United States, focusing on recent regulatory changes due to the promise and threat posed by plant-made pharmaceuticals (PMPs). PMPs are the latest advance in the genetic engineering of plants and promise to produce medicines inexpensively and abundantly by using a range of different plants as factories to express active medicinal ingredients; however, PMPs may pose a risk to the public’s health if they enter the food supply. How the benefits and risks of PMPs are addressed by the respective government’s regulation and how this will affect what, if any, products make it to the marketplace and their ultimate success are of great concern to many different parties, ranging from consumers and farmers to health and food production industries. As a result, this paper addresses the history of agricultural biotechnology regulatory policy since 1972, arguing that three distinct periods may be identified: (1) from 1972 to 1986 when the new biotechnology was focused on scientific self-regulation in the laboratory; (2) from 1987 to 2002, as the technology was being developed and widespread release of certain technologies became more common and was not perceived as an environmental threat, regulations became increasingly laxer; and finally, (3) we argue that we are entering a third phase with a series of controversies over regulatory infractions involving genetically engineered (GE) plants and the potential threats posed by PMPs.
D2004 Elsevier Inc. All rights reserved.
Keywords:Genetic engineering; Agricultural biotechnology; Regulation; Field releases; Plant made pharmaceuticals, PMPs; Plant-made industrial products, PMIPs
0040-1625/$ - see front matterD2004 Elsevier Inc. All rights reserved.
doi:10.1016/j.techfore.2004.03.001 * Corresponding author.
1. The next generation in U.S. agricultural biotechnology
While genetically engineered (GE) crops, such as Round-Up Ready soybean and Bacillus thur-ingiensis(Bt) corn and cotton, have become a pervasive part of agricultural production in the United States over the past 7 years, their place in the market is by no means assured. International trade concerns and recent crises played out in front of the public have the potential to not only stifle support for these products, but also lead to their being discarded if they are perceived by producers and retailers as too much of a risk. With many nations following the lead of Europe by not accepting goods derived from GE plants into their markets, or demanding their labeling, consumers will not have an opportunity to purchase these products, as these markets will remain closed. In countries that are willing to embrace genetic engineering plants, like the United States, if consumers are unwilling to buy these products and the public is unwilling to accept the risk of GE plants being grown, it is unlikely that GE crops will survive as part of the agricultural system. Public opinion is often the key driver in regulatory change. As a reaction to public perception of potential threats and not experienced events, the biotechnology regulatory arena has experienced a good deal of change since 1986. Because of the lack of substantive experience with health and/or environmental threats from the release of biotech products, federal government agencies established an amalgam of existing regulations to respond to potential, but not established, threats. These regulations use genetic engineering as the trigger and have undergone a series of alterations, as more knowledge of the risks associated with the release of GE plants has been accumulated, as well as in response to public reactions, or lack thereof, to perceived risks.
Likewise, change in the field testing of plant biotechnology has occurred since the regulatory regime was put in place in 1986 and field releases began in 1987. Three different generations of alterations to plants have been identified as likely taking place. First-generation biotechnologies alter the character-istics of plants so that they require less agricultural inputs such as herbicides, pesticides, and fertilizer as well as other chemicals. Second-generation biotechnologies focus on improving product quality so the plants are more nutritious, tastier, or stay fresh longer. Third-generation GE plants are ones in which cash crops act as ‘‘factories’’, producing industrial goods, pharmaceuticals, and other products more efficiently and cheaper than traditional approaches [1].
First-generation products, such as Round-Up Ready herbicide tolerant plants and Bt insecticidal crops, are used extensively by farmers. While crops exhibiting product quality characteristics have been given regulatory approval, the second-generation crops have yet to catch on in the marketplace. For instance, Calgene’s Flavr Savr tomato, which was designed to have a longer shelf life and a better taste than traditional tomatoes, appeared briefly in grocery stores but was eventually pulled from the shelves due to marketing and transportation problems.
Finally, the third generation of GE crops includes plant-made pharmaceuticals (PMPs) and plant-made industrial products (PMIPs). PMPs are designed to produce vaccines and antibodies for a wide range of diseases like rabies, traveler’s diarrhea, cholera, hepatitis B, antibodies to fight cancer, and tooth decay, and therapeutic proteins for cystic fibrosis, liver disease, and hemorrhages. PMIPs can be used for a variety of industrial purposes, such as to accumulate heavy metals in the plant to clean up soil, perform as biosensors for hazardous materials such as explosives found in landmines, produce enzymes and epoxies for industrial uses and plastics to replace petroleum-based products, and to produce cosmetics
This paper considers the future of new agricultural biotechnology applications, particularly third-generation products such as PMPs and PMIPs, by analyzing the regulations that allow these products to be field-released and marketed. This paper will first examine the regulatory history of new agricultural biotechnology products by analyzing the events that led to the promulgation of regulations and whether the events led to more stringent or relaxed regulations. Next, the paper will consider trends in the new agricultural biotechnology development by analyzing the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection System’s databases. Specifically, we analyze trends in field releases considering the types of plants that are being genetically engineered and the types of interventions being considered. We conclude by considering the future of the new agricultural biotechnology applications in light of trends in regulation, field experimentation, and politics.
2. Regulatory change and prevailing sentiment
The regulation of agricultural biotechnology can be seen as having been sequestered in a fairly well insulated policy subsystem, with little public involvement due most likely to its highly technical nature. As a result, there was little need for institutional intervention by Congress, the Executive Branch, or the Judicial Branch [3]. More recently, forces within the policy subsystem have led to the relaxation of regulations through the promise of new products and dearth of experienced difficulties in the field experiments. However, recent focusing events, such as the GE corn’s effects on the monarch butterfly, GE animal feed entering the United State’s food supply, and a plant pharmaceutical nearly entering the food supply, have publicized and politicized agricultural biotechnology and the regulatory arena, leading to greater scrutiny by more parties and stricter regulations[4].
When looking at the regulatory history of new agricultural biotechnology applications since 1972, a pattern seems to emerge, with three different identifiable periods. The first time period can be considered to start with the impetus to self-regulate by the scientific community, beginning with the Asilomar Conference Center and ending with formal government regulation of field releases, with the promul-gation of the Coordinated Framework by the Executive Branch’s Office of Science and Technology Policy (OSTP). The second time period extended from the Coordinated Framework until recently, with the widespread release of the new products of agricultural biotechnology in the fields, especially Bt corn and cotton, and Round-Up Ready soybean. This period is marked by a deregulatory trend, as regulations concerning the field release of GE plants were progressively relaxed. The third time period, the one into which we are currently entering, marks a return to scientific concern and regulatory restriction, as a series of events have called into question the safety of GE crops. These events have spurred a systematic questioning of the regulation of GE plants in general, and PMPs and PMIPs specifically.
2.1. In the lab: Asilomar (1972) to Coordinated Framework (1986)
over the use of recombinant DNA technology, instead highlighted the uncertainty of elite scientists and their desire to restrict debate to within the scientific community by limiting public involvement and press coverage [6,7]. While the result, scientific self-regulation with restraints only enforced on Federally funded projects [specifically, by the National Institutes of Health (NIH)], was as intended, the Asilomar conference and the events attending it served to set in motion a risk-averse perspective in which the threat of the new biotechnology was assumed before it was proved. This in turn led to it being the first technology to be regulated before risk was shown to exist ([8,9]p.223).
Over the next decade, most research tended to be laboratory-based. However, as the new biotechnology started moving from the lab to the field, concerns over field releases of GE organisms were raised, especially by such interest groups as the Foundation on Economic Trends (FET). One GE organism in particular raised concern—a soil bacterium genetically altered to reduce the likelihood of frost damage by lowering the point at which ice forms on a plant, in turn preventing an estimated US$1 billion in losses annually. Unfortunately dubbed ‘‘ice-minus’’, the perceived threat of the bacterium escaping, proliferating, and altering the environment was used as a focusing event to draw attention to the potential dangers raised by the new biotechnology, especially as the FET brought suit against the Environmental Protection Agency (EPA) for not protecting the environment against this threat.
This, combined with the need to clarify administrative turf who had regulatory primacy over the nascent industry and broader environmental concerns, led to the Reagan Administration’s OSTP proposing the Coordinated Framework for the Regulation of Biotechnology (hereafter the Coordinated Framework) in 1985, and its being promulgated in 1986 ([6]p. 192–197). The resultant Coordinated Framework coordinated the regulatory jurisdictions of the Food and Drug Administration (FDA), NIH, EPA, USDA, and the National Science Foundation (NSF). In all cases, a ‘‘pragmatic’’ approach was used in which preexisting regulations were utilized on a product-by-product basis, but with the use of genetic engineering processes to set off the regulatory trigger ([10]p. 79). The Coordinated Framework put in place dealt with jurisdictional overlap between the USDA, EPA, and FDA1 with GE plant products as they move from the field to consumers.
The first line of regulatory oversight with the field release of GE organisms was and remains the USDA’s Animal and Plant Health Inspection Service (APHIS), primarily through the Plant Quarantine Act (PQA) and the Federal Plant Pest Act (FPPA), although USDA also claims oversight through the Federal Meat Inspection Act (FMIA), the Poultry Products Inspection Act (PPIA), the Virus, Serum, Toxin, and Analogous Products Act (VSTA), and the Federal Seed Act (FSA). The EPA’s regulatory oversight comes into play when products reach the commercial stage of development through the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and the Toxic Substances Control Act (TSCA). Finally, FDA regulates the new biotechnology under the Federal Food, Drug and Cosmetic Act (FFDCA) and the Food Quality Protection Act (FQPA), which also affects EPA to a lesser extent.
2.2. In the fields: Coordinated Framework (1987) to widespread field release (2002)
As previously stated, the initial point where GE organisms are regulated is by the USDA as field releases, or the movement of GE organisms into or through the United States, under 7 CFR part 340 of the FPPA and the PQA. Under these acts, APHIS asserts broad regulatory authority over organisms, products, and articles that are plant pests or could harbor plant pests, whether they are genetically
1
engineered or not. Although the Coordinated Framework explicitly states federal agencies should focus on characteristics of risk posed by the product, APHIS uses genetic engineering to trigger regulatory oversight. With the permitting process, which was established in 1987 to allow for field testing of GE plants, the process applies to organisms using genetic materials from organisms defined as plant pests, unknown or unclassified organisms, or organisms that the APHIS deputy administrator determines to be or has reason to believe is a plant pest[11].
These regulations, however, are not encompassing of all GE plants. While the use of recombinant DNA inserted through Agrobacterium is a trigger for regulation, recombinant DNA inserted through a gene gun, genes inserted that do not come from a listed plant pest, or a plant whose pest status is undetermined do not trigger the same regulations. Although creators of such plants have, to date, sent courtesy notifications or permit applications ([12,13]p. 107), there is no certainty that this practice will continue.
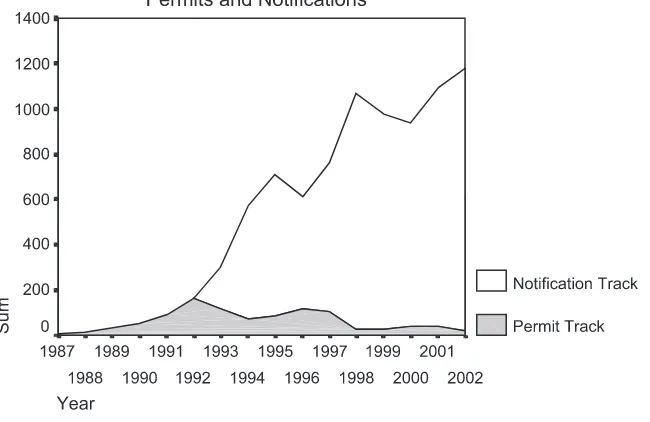
In March 1993, the permitting process was changed by APHIS to include a notification track in order to simplify the process. Six plant species, corn, cotton, potato, soybean, tobacco, and tomato, which were considered genetically well characterized, and in which the transmission of GE characteristics were seen as limited due to the lack of wild relatives in North America, were given notification status. The reduction of paperwork through the use of the notification track, instead of the permitting procedure, led to a decrease in the average waiting period from 120 to 30 days, and costs from US$5000 to US$250 dollars. As can be expected, there was a sudden upturn in field release activity, especially regarding these crops (seeFig. 1).
In May 1997, further changes to the field release regulations were put in place by APHIS to allow the introduction of the great majority of GE plants under the notification procedure. With this approach, a plant is eligible for the notification process if it meets the following requirements: the plant species is not listed as a noxious weed in the area where it is to be released; the inserted DNA is stably integrated into the host genome; the inserted DNA’s function is known and does not cause production of an infectious
entity; encoded substances are not toxic to nontarget species likely to feed on the plant or encode products for pharmaceutical use; virus derived sequences must be unlikely to facilitate virulence and spread in plants; and, finally, the new genes must not be derived from human- or animal-disease-causing agents ([13](p. 109–109);[11]). In other words, unless there is seen to be an environmental threat from the new plants, the less rigorous data collection standards of the notification approach is applied. As can be seen inFig. 1, this led to an increase in use of the notification track as well as a decrease in utilization of the permitting track.
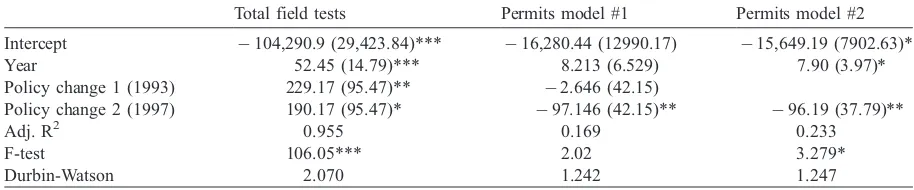
Statistical analysis supports the contention that policy changes put in place since 1987 have led to greater field release activity. Regression analysis of the effect of policy change on total field release activity over 15 years, measured as permits plus notifications, suggests this, as the model is highly significant and explains a good proportion of the variance (adjusted R2=0.955), while not showing autocorrelation (Durbin – Watson = 2.070). Analysis of the parameter coefficients suggests that all variables are significant at the 0.10 level and have a positive effect. Specifically, the year variable is highly significant and shows an increase in, on average, 52 field releases a year since the APHIS program was put in place. The two regulatory changes also had a significant, though lesser, effect on the amount of field releases with the 1993 policy change accounting for an additional 229 field releases per year and the 1997 policy change leading to an added 190 field releases a year(Table 1).
Further analysis of trends in field release through consideration of the utilization of the permitting track bolsters the contention of the notification track replacing the permitting approach. While the model, which incorporates both regulatory changes, does not meet model statistics, removing the effect of the first regulatory policy change in 1993 leads to the model reaching statistical significance at the 0.10 level, although it only explains a fraction of what can be expected of a time series regression analysis and there is suggestion of autocorrelation, meaning the model does not have the correct variables for specification. However, what can be gleaned from the model is an increase of about eight permits a year. The change to regulatory policy in 1997 led to a decrease in permitting activity by an order of about 96 a year, suggesting that other factors are at work.
Finally, the 1993 APHIS policy change put in place a petition process that allowed for the determination that certain plants are no longer regulated articles. Furthermore, an extension process, whereby closely related plants are ascribed a nonregulated status, was put in place ([13]p. 104). Once it has been decided by APHIS that a transgenic plant has nonregulated status, APHIS cannot exercise
Table 1
Field releases of genetically engineered plants
Total field tests Permits model #1 Permits model #2
Intercept 104,290.9 (29,423.84)*** 16,280.44 (12990.17) 15,649.19 (7902.63)*
Year 52.45 (14.79)*** 8.213 (6.529) 7.90 (3.97)*
Policy change 1 (1993) 229.17 (95.47)** 2.646 (42.15)
Policy change 2 (1997) 190.17 (95.47)* 97.146 (42.15)** 96.19 (37.79)**
Adj. R2 0.955 0.169 0.233
F-test 106.05*** 2.02 3.279*
Durbin-Watson 2.070 1.242 1.247
additional oversight over the plant and its descendants, even if separate deregulated lines are crossbred conventionally. This might lead to wild species with potential weediness problems ([13]p. 111–112). Change during the period from 1986 to 2002 can be effectively seen as occurring within the agricultural biotechnology subsystem with minimal public input. Specifically, changes in 1992 and 1997 to USDA-APHIS field release regulations, while spurred by OSTP directives, did not incorporate public input to any great extent. The lack of negative events, along with increased knowledge and experience with rapidly advancing and diversifying GE plant field testing, precipitated the easing of regulations. Furthermore, the lack of public input and likely recalcitrance to allow deregulated field experimentation of an uncertain technology certainly accelerated this trend towards relaxed regulations.
2.3. In the public eye: on the precipice of changes to the regulatory framework 2002—??
The most recent changes to the regulatory structure concerning agricultural biotechnology are coming about, due in great part to concerns ‘‘that the expansion in agricultural biotechnology increasingly will put pressure on seed production and commodity handling systems’’ ([14] p. 50578) to segregate and control its products. Further, the concomitant diversification of GE plants with agronomic properties, consumption traits, and industrial production qualities that may enter into the environment have stirred doubts as to their safety. Specifically, concerns over the current regulatory scheme, with its relatively insulated policy-making approach, have been raised by three separate events at the turn of the century that have called into question the scientific basis for regulation, the effectiveness of regulatory enforcement, and the integrity of the food system.2
The first of these focusing events occurred in 1999 when a laboratory study published by Losey et al. (1999)[15]in the eminent peer-reviewed scientific journalNaturecalled into question the environmental safety of Bt, which was engineered to express a protein that kills targeted insects that attack economically important crops such as corn, cotton, and potato by eating through their guts, leading to sepsis and the inability to digest food. This article suggested that the monarch butterfly, a highly visible symbol of the environment, as well as other beneficial insects, would be harmed by Bt corn pollen while in their larva stage. While technically correct and seen by those in the industry as acceptable collateral damage due to its having a negligible effect on these butterflies, this study led to a debate and follow-up studies that lasted for over 2 years and drew a good deal of media coverage ([16]p. 189–192). Additionally, it pointed out potential flaws in the Coordinated Framework, as the effect of pollen that expressed Bt was not considered until after Bt corn was in the field. Specifically, the Bt corn in question moved through the APHIS field release regulatory process, which only considers the likelihood that a plant will become a plant pest and only indirectly considered potential harms to nontarget species, without consideration of potential harms to such species as monarch butterflies. EPA regulations, inasmuch as they deal with plant-incorporated protectants (PIPs)3 such as Bt crops
2
It is not the case that other compliance infractions have not occurred. USDA states that of the 7402 field tests carried out between 1990 and 2001 and regulated by APHIS, 115 resulted in compliance infractions[22]. For the most part, however, these were relatively minor infractions and did not raise public concern. Of potentially greater long-term concern were infractions concerning EPA regulations over the management of Bt corn, in which large numbers of farmers have not been following standards[24]. The nature of the technology and the form of EPA’s regulatory authority, however, make it difficult to observe and punish individual infractions[26], and thus, these infractions have likewise not been of public concern.
3The term PIPs (plant incorporated protectants) reflects a desire by industry to avoid the more accurate, yet more
under FIFRA, does have regulatory authority if the pesticidal substance (the crops with PIPs) harms nontarget species. While regulatory action was not taken, the result was that by the 2001 field season, Ciba Seeds (Novartis), the company producing the type of Bt corn most toxic to the monarch butterfly, removed that particular Bt corn from the market in spite of it being ‘‘a significant market force during 1996–1999’’ ([13] p. 72–75).
The second controversy garnering national attention and concern likewise dealt with Bt corn. A variant of Bt, which is expressed in Starlink corn and not deemed fit for human consumption due to potential human allergic reactions but seen as safe for use as animal feed, found its way into the human food supply. The public interest group ‘‘Genetically Engineered Foods Alert’’ performed tests on taco shells and other corn-based products being sold in grocery stores, like Safeway, and in fast food restaurants, such as Taco Bell, and found that these products contained Starlink Bt corn [16]. Indeed, within a single year, of 110,000 grain tests by Federal inspectors, Starlink corn showed up in one tenth[1].
The resulting uproar led to actions by EPA to cancel the registration of this corn in spite of Starlink’s parent company Aventis attempting to win approval based on its safety as Generally Recognizable As Safe (GRAS) from FDA. However, when this was discarded as an option, Aventis and USDA bought back existing grain supplies and recalled food with Starlink corn in it. Further, EPA no longer allows ‘‘split’’ registrations in which PIPs may be registered for animal feed but not for human consumption. As a result of this, public attention was drawn to flaws in the regulatory system, especially the ease in which food security may be breached, and Congressional hearings were held to discuss this and other concerns with agricultural biotechnology[16].
The final focusing event, that of Prodigene’s PMP corn, has likewise led to public concern over the safety of the food supply. In September and October of 2002, in Iowa and Nebraska, respectively, APHIS found ‘‘volunteer’’ corn plants genetically engineered to produce a pharmaceutical to prevent ‘‘traveler’s diarrhea’’ growing in soybean fields in violation of permit conditions. Specifically, Prodigene did not abide by the conditions of their field release of PMPs from the previous year as small quantities of this corn ended up in soybean that was to be processed and sold for human consumption. As a result of this, Prodigene had to pay a civil penalty of US$250,000, destroy 500,000 bushels or $2.7 million dollars worth of soybean in Nebraska, and incinerate 155 acres of corn in Iowa due to concern that cross-pollination occurred, as well as post a US$1-million-dollar bond and accede to higher compliance standards for future field tests[17]. Further, and perhaps more important in terms of long-term political implications, the Grocery Manufacturers of America (GMA) and other food processing interest groups expressed concern over plant made pharmaceutical field test regulations, with John R. Cady, CEO of the National Food Processors Association commenting, ‘‘nothing short of alarming to know that at the earliest stages of development of crops for PMPs, the most basic preventative measures were not faithfully observed. This apparent violation of rules. . .very nearly placed the integrity of the food supply in jeopardy.’’[18].
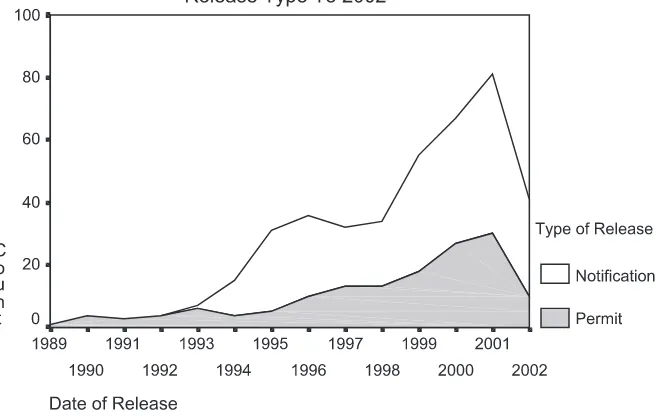
As a result of these focusing events, especially the Prodigene fiasco, a certain degree of uncertainty over the shape of the federal regulatory system was experienced,4 with a concomitant drop in permit
4
activity (seeFig. 1) and experimentation with PMPs (see Fig. 2). To address the decreasing trust in the regulatory structure, OSTP published ‘‘Proposed Federal Actions To Update Field Test Requirements for Biotechnology Derived Plants and To Establish Early Food Safety Assessments for New Proteins Produced by Such Plants’’ in August 2002. Specifically, the notice was published to provide guidance to USDA, EPA, and FDA to update field-testing requirements for food and feed crop plants and establish early food safety assessments for new plant proteins, most specifically PMPs and PMIPs, in line with the 1986 Coordinated Framework.
According to the document, three principles are relied upon in updating the Coordinated Framework. First, the level of field test confinement should be consistent with the level of environmental, human, and animal health risk associated with the introduced proteins and trait(s). Second, if a trait or protein presents an unacceptable or undetermined risk, field test confinement requirements would be rigorous to restrict outcrossing or commingling of seed. Further, the occurrence of these genes or gene products from these field tests would be prohibited in commercial seed, commodities, and processed food and feed. Finally, even if these traits or proteins do not present a health or environmental risk, field test requirements should still minimize the occurrence of outcrossing and commingling of seed, although low levels of genes and gene products could be found acceptable based upon meeting applicable regulatory standards ([14] p. 50 579).
In light of concerns raised by increased experimentation with PMPs and plants expressing industrial compounds and addressed by OSTP in their notice[14], USDA-APHIS changed rules concerning their field testing of PMPs in March 2003[19]. The amount of comments in response to this Federal Register notice reflects the changing salience concerning the field release of GE plants. While the changes to the APHIS regulations in 1993 garnered 84 comments and the even more wide-ranging changes in 1997 attracted only 50 comments ([13]p. 104 –105), the Federal Register notice concerning PMP field-testing
requirements attracted at least 847 comments (of which 77 were late), many of them from concerned citizens. A high percentage of comments were sent by individuals not commonly associated with the agricultural biotechnology debate, when compared with comments to the previous two Federal Register notices.
While critiques were raised in many comments by those who appeared to have ties with the organic movement or with environmental groups such as Greenpeace, as evidenced by the large number of comments received via email, concerns were raised by other politically powerful groups. GMA and affiliated groups expressed concern over uncontained field releases of PMPs and PMIPs, especially in food and feed plants, which account for 75% of all field releases under APHIS notification and permit regulations. Interestingly enough, while support for a total ban on PMPs was expressed by a small number of individuals, concern by consumer groups and traditional biotechnology opponents was tempered, likely mitigated by the potential for medical benefits from this new technology.
While the resulting regulations are expected to be modified further over the coming years, they currently incorporate significant changes in how PMPs and PMIPs are regulated[20]. Specifically, for all plants genetically engineered to produce pharmaceutical and/or industrial compounds and field-tested under permit, APHIS established seven conditions that can be grouped into three categories. The first considers field test siting, the second considers the dedication of equipment and facilities to their production, and the third considers procedural matters.
Field test siting regulations proposed by APHIS provide two conditions to be met, with special consideration for pharmaceutical corn. First, the perimeter fallow zone will be increased from 25 to 50 ft to prevent inadvertent commingling with plants to be used for food or feed. Second, production of food and feed plants at the field test site and perimeter fallow zone will be restricted for the following season to prevent inadvertent harvesting. Furthermore, specific permit conditions for pharmaceutical corn have been instituted, likely due to corn being the organism of choice, accounting for three quarters of PMP field releases [1]. The large percentage of experiments with corn derives from a variety of reasons, including farmer experience and expertise with raising it, the ideal storage nature of its seeds, the large amount of scientific knowledge concerning its genetics, and the ease in which its genetics are transferred
[1]. The first permit condition requires no corn grown within 1 mile of the test site during any field tests involving open pollinated corn—an eightfold increase from standards for foundation seed. When pollen flow is controlled by bagging, the spatial buffer is reduced to 1/2 mile, and a temporal buffer is established with pharmaceutical corn not to be planted less than 28 days before or 28 days after corn grown in the zone from the 1/2- to 1-mile boundary. With the establishment of these buffers, whether they are 1/2 or 1 mile out, border rows will not be allowed to reduce the isolation distance.
A key factor in any regulatory arrangement is the ability to ensure that those regulated are complying with the requirements set forth. As a result of the potentially contentious nature of PMPs and PMIPs, APHIS plans to increase the number of field site inspections ‘‘to correspond with critical times relevant to the confinement measures.’’ ([19]p. 11338) Therefore, in addition to maintaining records of activities related to meeting permitting conditions and increasing the likelihood of auditing them to verify that required permit conditions were met, APHIS might inspect permitted field tests up to five times during the growing season—once at preplanting to evaluate the site location, once at the planting stage to verify site coordinates and adequate cleaning of planting equipment, at midseason to verify reproduction isolation protocols and distances, at harvest to verify cleaning of equipment and their appropriate storage, and again at postharvest to verify cleanup of the field site. In addition, two postharvest inspections may occur to verify that the regulated articles do not persist in the environment. Finally, APHIS may inspect more frequently if deemed necessary. ([19]p. 11338–11339).
Possibly due to the number of responses received as a result of the Federal Register request for comments concerning APHIS changing their PMP field release regulations and/or the vehemence of concern voiced by those participating in the process, the potential for both PMPs and PMIPs entering into the food supply were cited as points of concern. As a result, and using the PMP regulatory changes as a starting point, APHIS took immediate action to remove the notification track option, requiring complete permit track review in their recent (August 6, 2003) interim rule and request for comments. As stated in the Federal Register notice, ‘‘. . .we believe it is prudent and necessary to remove the notification option for all industrials pending the completion of our ongoing review of part 340.’’ ([21] p. 46435).
The rationale given in the interim rule and request for comments was that while 14 field releases (nine notifications, five permits) have been carried out to date, the type of genetic engineering being carried out was to enhance such nutritional components as oil content. However, recent genetic modifications have been for ‘‘nonfood traits with which APHIS has little regulatory experience or scientific familiarity.’’ ([21]p. 46434) As such, the definition of PMIPs has three criteria: (1) the plants produce compounds new to the plant; (2) this compound has not normally been used in food or feed; and (3) the compound is being expressed for nonfood/feed purposes ([20] p. 46435).
An administrative reorganization of how USDA-APHIS regulates biotechnology recently created the Biotechnology Regulatory Services (BRS). This reorganization can be seen as another move to address concerns raised by PMPs and PMIPs specifically and GE organisms generally. According to USDA-APHIS, ‘‘Given the growing scope and complexity of biotechnology, now more than ever, APHIS recognizes the need for more safeguards and greater transparency of the regulatory process to ensure that all those involved in the field testing of GE crops understand and adhere to the regulations set forth by BRS.’’ Changes instituted by BRS include new training for APHIS inspectors in auditing and inspections of field trials, the use of new technologies such as global positioning systems, and analysis of historical trends to inform monitoring and inspection.
resolution in which criteria will be established to determine the extent of an infraction and the response, whether this be further investigation, the issuance of a guidance letter, the issuance of a written warning, or referral to APHIS’ Investigative and Enforcement Services (IES) unit for further action; (5) documentation, in which a database will be set up to track field test inspections and resulting compliance infractions; transparency to keep stakeholders and the public informed on the regulatory decision-making process; (6) continuous process improvements, where as the science of biotechnology advances, regulations and permit conditions to allow safe field testing will also do so; (7) an emergency response protocol, being developed with input from EPA and FDA, in which a quick response plan will be put in place ‘‘to counteract potential impacts on agriculture, the food supply, and the environment’’; (8) training for field test inspectors in their dealings with PMP and PMIP field test sites, as well as the latest in auditing; and (9) certification concerning compliance with the highest level of auditing standards[22]. Although the reorganization can be seen as streamlining and focusing enforcement efforts, the potential for unduly high levels of workload stresses placed on this 26-member unit can be foreseen. First, BRS draws on APHIS inspectors to inspect field tests; however, more than 2600 of these agriculture quarantine inspectors have been transferred to the Department of Homeland Security (DHS)
[20]. The current agreement between USDA-APHIS and DHS allows for continued access by APHIS and BRS, although it can be expected that problems might occur as a result of split responsibilities and duties.
3. Conclusions
The awareness of the potential for agricultural biotechnology to transform the landscape of American farming through the development of economically important new products, including PMPs and PMIPs, has long been recognized. Just less than 10 years ago, this journal devoted a special issue to ‘‘Biotechnology and the Future of Agriculture and Natural Resources’’ [23]. Then, uncertainty over the future of agricultural biotechnology was based upon the lack of financial support for research and development as well as vague and unfocused regulations[24]. These same concerns exist now in spite of better characterized biotechnology-based science and technology and a better understanding of economic and ecological risks and benefits.
The concerns over the new agricultural biotechnology are often termed as one in which the issue is less about the science of GE crops and more about the social issues in which this technology is nested. This ‘‘surrogate for safety’’ is a reflection on the idea that ‘‘in many areas of life there is less and less control. For some segments food offers some control.’’[25]. The threat of drugs and medicines, as well as a variety of industrial compounds, entering the food supply through normal production channels can be seen as particularly dreaded by the American public, which, while largely unaware of the extent of genetically modified products in their food supply, have been attenuated to threats to their security since 9-11. In spite of the lack of evidence of human disability through consumption of GE foods, concern has increasingly been raised in the European Union, which is establishing labeling standards, and Africa, where GE corn destined for famine relief was turned down due to health and ecological concerns.
system have alerted the American public to potential threats, rupturing the previously insular policy subsystem. While these events provide evidence that the regulatory system is being successfully implemented, their occurrence has drawn attention to gaps in the Coordinated Framework.
At least two recent events have the potential to further expand the scope of concern and thus conflict. A report by the Center for Science in the Public Interest (CSPI) called into question the enforcement of guidelines set by EPA requiring growers using Bt corn to set aside land as refuge for pest management purposes[26]. Here, corporations have been called upon to regulate farmers directly due to the use of preexisting pesticide regulations under the Coordinated Framework—a task for which they are not well suited [27]. And most recently, on November 12, 2003, a coalition of environmental groups and consumer advocates sued USDA in federal court to stop the field testing of PMPs due to lack of risk assessment concerning other crops, wildlife, and humans[28].
In light of these concerns and reflected in the rapidly changing field release regulations of PMPs and PMIPs put forward for comment in the Federal Register in March and August of 2003, there is a high likelihood that the Coordinated Framework for the Regulation of Biotechnology will continue to change. Whether this change will occur in the form of marginal alterations in the regulatory approach by EPA, FDA, and USDA, especially in the case of the latter with the newly constituted APHIS-BRS, while retaining the Coordinated Framework, or a major change in the regulations through the creation of a new agency or approach, remains to be seen. As more becomes known about this still young technology and its potential for health, ecotoxicological and ecological effects, as well as the complex and nonlinear environment it operates in, the more likely negative side effects will be discovered and dealt with. Already, both USDA-APHIS and EPA are strengthening their ties with each other with monthly coordinated phone calls and are enhancing transparency and ties with stakeholders through public workshops and meetings. Additionally, greater attention is being given to different means of approaching ecological control of these products, in light of a newly released National Academy of Sciences report on the biological confinement of GE organisms [29].
Regardless, new agricultural– environmental biotechnologies stand on a precipice of change. Over the next 15 years, they may continue to change how food, drugs, and industrial products are produced, or they may be yet another failed technology along the lines of nuclear power with its plants withdrawn from farmers’ fields, depending on how issues dealing with public trust in regulations are addressed. In either case, it is social support for the technology and trust in regulatory institutions that matter most.
Acknowledgements
This report was funded by the Arkansas Biosciences Institute, Arkansas State University.
References
[1] N.C. Ellstrand, Going to ‘great lengths’ to prevent the escape of genes that produce specialty chemicals, Plant Physiol. 132 (2003 August) 1770 – 1774.
[2] Pew, Harvest on the horizon: future uses of agricultural biotechnology, The Pew Initiative on Food and Biotech-nology (2001 September).
[4] J.W. Kingdon, Agendas, Alternatives, and Public Policies, Harper Collins, New York, 1984. [5] P. Berg, et al., Potential biohazards of recombinant DNA molecules, Science 185 (1974) 303. [6] S. Krimsky, Biotechnics and Society: The Rise of Industrial Genetics, Praeger, New York, 1991.
[7] S. Wright, Molecular Politics: Developing American and British Regulatory Policy for Genetic Engineering, University of Chicago Press, Chicago, IL, 1994, pp. 1972 – 1982.
[8] R.W.F. Hardy, D.J. Glass, Our investment: what is at stake, Issues Sci. Technol. (1985 Spring) 69 – 82.
[9] S. Krimsky, R. Wrubel, Agricultural Biotechnology and the Environment: Science, Policy and Social Issues, University of Illinois Press, Chicago, IL, 1996.
[10] P.A. Stewart, A.A. Sorensen, Federal uncertainty or inconsistency? Releasing the new agricultural – environmental bio-technology into the fields, Polit. Life Sci. 19 (1) (2002) 77 – 88.
[11] USDA (U.S. Department of Agriculture), Introduction of organisms and products altered or produced through genetic engineering which are plant pests or which there is reason to believe are plant pests, 52 Fed. Regist. 115 (1997 June 16). [12] G. Jaffe, Planting Trouble: Are Farmers Squandering Bt. Corn Technology? An Analysis of USDA Data Showing Significant Non-Compliance with EPA’s Refuge Requirements, Center for Science in the Public Interest, 2003 (www.cspinet.org).
[13] NRC (National Research Council), Environmental Effects of Transgenic Plants: The Scope and Adequacy of Regulation, National Academy Press, Washington, DC, 2002.
[14] OSTP (Office of Science and Technology Policy), Proposed federal actions to update field test requirements for biotech-nology derived plants and to establish early food safety assessments for new proteins produced by such plants; notice, 67 Fed. Regist. 149 (2002) 50577 – 50580.
[15] J.E. Losey, L.S. Rayor, M.E. Carter, Transgenic pollen harms monarch butterflies, Nature 399 (214) (1999).
[16] M. Nestle, Safe Food: Bacteria, Biotechnology, and Bioterrorism, University of California Press, Berkeley, CA, 2003. [17] USDA (U.S. Department of Agriculture), USDA announces actions regarding Plant Protection Act violations involving
Prodigene, Press Release, (http://www.usda.gov/news/releases/2002/12/0498.htm) (2003 December 6). [18] J.L. Fox, Puzzling industry response to Prodigene fiasco, Nat. Biotechnol. 21 (1) (2003 January) 3 – 4.
[19] USDA (U.S. Department of Agriculture), Field testing of plants engineered to produce pharmaceutical and industrial compounds, 68 Fed. Regist. 46 (2003) 11337 – 11340.
[20] USDA (U.S. Department of Agriculture), United States Department of Agriculture pre-briefing for reporters on USDA’s federal register notice on field testing of pharmaceutical-producing plants, www.usda.gov/news/releases/2003/03/084.htm
(2003 (March 6).
[21] USDA (U.S. Department of Agriculture), Introductions of plants genetically engineered to produce industrial compounds, 68 Fed. Regist. 68 (2003) 46434 – 46436.
[22] USDA (U.S. Department of Agriculture), Biotechnology regulatory services: compliance and enforcement. agricultural biotechnology website,www.aphis.usda.gov/brs/compliance(2003).
[23] Biotechnology and the future of agriculture and natural resources, Technol. Forecast. Soc. Change 50 (1) (1995). [24] S.L. Huttner, H.I. Miller, P. Lemaux, U.S. agricultural biotechnology: Status and prospects, Technol. Forecast. Soc.
Change 50 (1) (1995) 25 – 39.
[25] J. McCluskey, Presentation and comments, National Agricultural Biotechnology Council (NABC) Conference (2003 June 2).
[26] G. Jaffe, How to approach the regulatory conundrum, Integrating Agriculture, Medicine and Food for Future Health: NABC Report 14 on Foods for Health, National Agricultural Biotechnology Council, Ithaca, NY, 2003, pp. 51 – 60. [27] P.A. Stewart, D. Harding, E. Day, Regulating the new agricultural biotechnology by managing innovation diffusion, Am.
Rev. Public Adm. 32 (1) (2002) 78 – 99.
[28] R. Fabi, Green groups sue USDA to stop bio-pharm planting, http://www.usatoday.com/tech/news/techpolicy/2003-11-12-biopharm-suit_x.htm (2003).
[29] Proceedings, Plant-Incorporated Protectant (PIP) Experimental Use Permits (EUP): Process and Compliance, Center for Agriculture in the Environment – American Farmland Trust, (2004 February 10 – 11).