www.elsevier.comrlocateratmos
Voltammetric evidence for the presence of
humic-like substances in fog water
A. Gelencser
´
a,), M. Sallai
b, Z. Krivacsy
´
a, G. Kiss
a, E. Meszaros
´ ´
b aAir Chemistry Group of the Hungarian Academy of Sciences, UniÕersity of Veszprem, P.O. Box 158,´
H-8201 Veszprem, Hungary´
b
Department of Earth and EnÕironmental Science, UniÕersity of Veszprem, P.O. Box 158,´
H-8201 Veszprem, Hungary´
Received 15 December 1999; received in revised form 24 February 2000; accepted 24 February 2000
Abstract
Humic-like substances have recently become candidates for making up an important fraction of bulk organic carbon in various atmospheric samples. Their properties were exploited by various analytical — mostly spectroscopic — techniques. For lack of any decisive analytical evidence, their presence in the samples was proved only by comparison with arbitrarily selected humic or fulvic acid standards. This series of evidences requires as many independent principles of determination as possible to minimise false identification and to better characterise the properties of the bulk organic matter, which may also be important in various atmospheric processes. In this paper, we used anodic stripping voltammetry to characterise the complexing and electrochemical behaviour of organic matter in fog water under conditions prevalent in most surface waters. Although the method provided no quantitative information, important interference effects — formation of electrochemically labile complexes, adsorption on the working electrode, complex dissociation, etc. — were observed. Evaluation of the results revealed all of the major electro-chemical attributes of natural complexants as had been reported for humic or fulvic acids. These may provide indirect evidence that natural complexants similar to humic or fulvic acids are present in fog water in concentrations sufficiently large to produce these effects. This finding, which supplements previous independent measurements, may be useful in assessing the sources and effects of humic-like substances in atmospheric samples.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Humic-like substances; Fog water; Anodic stripping voltammetry; Natural complexants
)Corresponding author. Tel.:q36-88-422-022; fax:q36-88-423-203.
Ž .
E-mail address: [email protected] A. Gelencser .´
0169-8095r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
1. Introduction
The importance of aerosol particles in climate change has been recently recognised. However, what prevents us from better assessing the direct and indirect climate forcing of tropospheric aerosol is the limited knowledge on its chemical composition. In particular, the bulk of organic matter of continental fine aerosol and precipitation seems to have defied identification very effectively. Even by using powerful analytical techniques such as gas chromatography-mass spectrometry, only a fraction of total organic carbon can be attributed to individual organic compounds such as mono- and dicarboxylic acids, n-alkanes, etc. Recently, there has been increasing evidence that a considerable fraction of organic carbon is theoretically impossible to speciate because they are made up of natural humic-like macromolecules. It was speculated that such compounds may be predominant in Amazonian aerosol during the wet season when no
Ž .
biomass burning occurred Artaxo et al., 1990; Andreae and Crutzen, 1997 . In temperate regions, from the residues of rainwater and melted snow, yellow, brown or
Ž .
black aggregations and tar-like droplets were separated Went, 1966 . In terrestrial cloud water some degradation products and an unidentified orange-brown material were found
ŽBank and Castillo, 1987 . Mukai and Ambe 1986 extracted a brown substance having. Ž .
the solubility characteristics of humic acid from airborne particulate matter collected in a rural area in Japan. Using ultrafiltration and 13C-nuclear magnetic resonance
spec-Ž .
troscopy, Havers et al. 1998 demonstrated that humic-like substances separated from particulate matter made up a significant fraction of organic carbon and they can be characterised primarily by polysaccharide and aliphatic substructures. Using various spectroscopic techniques such as infrared, UV–VIS or fluorescence spectrometry, we recently proved that in continental background aerosol, a significant amount of these humic-like macromolecules were present in the fine mode, and they also accounted for a
Ž .
considerable part of water soluble organic carbon Zappoli et al., 1999 . In all the above cases, the occurrence of humic-like substances was established using separation methods and spectroscopic or elemental analytical techniques. However, it is well known that humic substances, especially humic and fulvic acids, show high complexing ability towards transition metal ions such as lead, copper, nickel, etc. It is therefore possible to prove the presence of humic-like substances in atmospheric samples on the basis of a completely different principle, making use of their complexing properties in electro-chemical analysis. To the best of our knowledge, there is only one study that focuses on
Ž
organic metal complexation in rainwater using complexing ligand titration Spokes et
.
al., 1996 .
Transition metal complexation can be readily studied by anodic stripping voltamme-try with two different approaches. In the first method, metal is added to the organic ligands under conditions held constant, and stability constants and coordination numbers
Ž
are determined from the shift in the redox potential of the metal ion Wilson et al.,
.
‘‘labile.’’ Complexes that undergo dissociation during the measurement period are defined as kinetically ‘‘labile.’’ In voltammetry, it is essential that the presence of ligands should not affect the diffusion coefficient of the aquo ion in solution nor should the ligand adsorb onto the working electrode where it may complex metal ion or may change the rates of analytical redox reactions being monitored. Natural complexing agents such as humic or fulvic acids were shown to exhibit properties that render such
Ž
complexation studies difficult or often impossible to interpret quantitatively Filella et
.
al., 1990 . These complexants tend to form electroactive metal–ligand complexes, cause complex dissociation during the voltammetric measurements andror adsorb on the electrode surface. These effects are evident from the shifts in peak current potentials andror half-width relative to those observed in their absence. The same interferences, however, can be used to prove the presence of these natural complexants in atmospheric samples, thus supplementing evidences obtained from spectroscopic measurements. In this paper, we attempt to make use of these interference effects in anodic stripping voltammetry to substantiate the presence of humic-like substances in fog water. To enable comparisons with results available for aquatic humic matter, the pH of fog water is adjusted to that of most aquatic environment.
2. Methods
Fog sampling was carried out at the FISBAT field station of S. Pietro Capofiume, in the Po Valley, Italy. The area is characterised by high fog occurrence during the fall–winter period and also by a high aerosol loaded air due to the high levels of pollution. In addition, the fog system in the Po Valley is characterised by high atmospheric stability and long residence time of the air masses.
An automated, computer-driven, sampling system was set up to concurrently sample fog droplets and interstitial particles. The sampling system was essentially composed of:
Ž .a Particulate Volume Monitor PVM-100 Gerber, 1991 for detecting the presenceŽ .
of fog;
Ž .b temperature sensor to detect subfreezing conditions; and Ž .c stainless steel fog droplets string collector.
The sampling of fog droplets was performed using an active string collector designed
Ž .
by Fuzzi et al. 1997 . This instrument collects fog droplets suspended in an air stream created by a fan, by impacting them on cylindrical strings. Sampling flow rate is 17 m3
miny1, and the collection efficiency is 43% of the actual fog liquid water content
ŽLWC , with calculated 50% cut-off for each individual string, with an aerodynamic.
diameter of ca. 6mm. All parts that come in contact with the fog droplets, including the
were obtained, 11 of which were used for the experiments. Sampling duration ranged from 2 to 19 h.
Fog samples were first weighed and pH was then determined in a small aliquot of the sample. The sample was then filtered through a 47-mm quartz filter and the water
Ž .
soluble organic carbon in the filtrate WSOCF was determined with a total carbon
Ž .
analyser TOC Astro 2100 liquid module using potassium hydrogen phthalate solutions for calibration. TOC liquid measurements had an analytical error of 5%. The
concentra-Ž q y 2y.
tion of inorganic ions NH , NO , SO4 3 4 was determined by ion chromatography. The
Ž .
total concentration of Cu II in filtered fog samples was determined by atomic absorp-tion spectrometry. All vials, sampling bottles and Petri dishes used for sampling, collection and storage of the aerosol and liquid samples were of Pyrex glass and were pre-cleaned and conditioned. The pH of the samples and model solution was adjusted using Britton–Robinson buffer solution. The stock buffer solution was composed of acetic acid, phosphoric acid and boric acid at a concentration of 0.04 M each.
Ž . y5
The copper II solution of 1.00=10 M was prepared from analytical grade
Ž .
copper-nitrate Merck with sequential dilution in calibrated flasks using MilliQ water
Ž . Ž .
containing 0.10 M potassium-nitrate Reanal . The humic acid standard Aldrich solution of 100 mg dmy3 was prepared gravimetrically using 100 ml of a solution made of 10.0 ml Britton–Robinson buffer and 90.0 ml of 0.10 M potassium nitrate and pH adjusted to 9.00. While we are aware that the pH of fog water is mainly acidic, our objective was not to study copper complexation in fog water but to compare the electrochemical behaviour of dissolved organic carbon present in fog water to that of
Ž .
natural humic matter in aquatic environments e.g. in seawater . Throughout the experiments, 3-h equilibrium time was allowed for copper complexation before analysis. One milliliter of the buffer solution was added to 9.00 ml of fog samples and model solution and the pH was then adjusted to 9.00 with 1.00 M sodium-hydroxide solution. The ionic strength of all solutions was adjusted to 0.10 M potassium-nitrate concentra-tion. The instrument was an AUTOLAB electrochemical instrument with a dropping mercury electrode, the deposition potential was y1.000 V, deposition time of 60.0 s. The volume of the sample cell was 10.0 ml. The potential was scanned fromy1.000 to 0.000 V, purge time was 120 s with nitrogen, equilibrium time was set to 30.0 s.
3. Results and discussion
The pH and the concentrations of total organic carbon, ammonium, nitrate and sulphate in the filtered fog samples are given in Table 1. Details of the analysis are
Ž .
reported elsewhere Facchini et al., 1999 . As can be seen in the table, the concentra-tions of all species and the pH vary considerably in the samples. In all cases, however, the concentrations of total carbon expressed in units of mg CPdmy3 are comparable to
those of the major inorganic ions. Furthermore, if we consider that the mass concentra-tion of organic compounds may be higher by a factor of 1.4 than that of organic carbon
ŽZappoli et al., 1999 , it can be concluded that organic compounds are important.
Table 1
Concentrations of ammonium, nitrate, sulphate and total organic carbon in mg dmy3 and pH in the fog samples used in voltammetric studies
q y 2y
Sample code pH NH4 NO3 SO4 Total organic carbon
y3 y3 y3 y3
ŽFrmonthrday. Žmg dm . Žmg dm . Žmg dm . Žmg C dm .
F1217 3.73 12.4 45.5 5.70 19.1
F1223 4.13 82.6 180 94.2 65.2
F0107 4.64 66.2 163 87.0 96.1
F0120 3.53 95.3 257 46.5 84.5
F0122 3.95 125 294 153 108
F0130 4.95 85.4 150 60.9 76.8
F0201 4.04 36.9 78.0 22.0 30.0
F0212 6.05 18.4 32.6 15.1 14.0
F0213 6.16 54.3 158 38.9 42.0
F0224 4.30 75.3 125 88.8 38.8
F0226 6.44 28.4 30.7 24.6 15.1
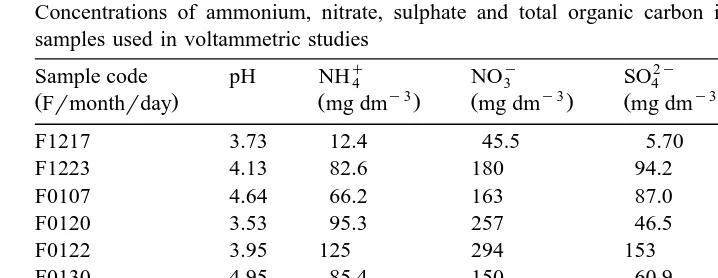
The voltammograms of a copper solution at a concentration of 1.00=10y5 M at pHs9.00 are shown before and after the addition of 2.00mg of humic acid standard in
Fig. 1. It can be seen from the figure that the addition of humic acid caused a cathodic
Ž .
shift y0.113 V in the peak potential, which suggests reversible complex formation as
Ž .
was also reported for copper–fulvic acids complexes Piotrowicz et al., 1982 . Secondly, a cathodic shift in the Hg-wave can be observed, which affects the base line quite prominently, caused by adsorption of macromolecules on the Hg-electrode, and were
Ž .
also described for humic and fulvic acids Wilson et al., 1980 . Thirdly, peak broadening occurred, which may be due to the presence of electroactive copper–humate complexes or complex dissociation on the electrode surface. This phenomenon is well established
Ž
for natural complexants in anodic stripping voltammetry Wilson et al., 1980; Filella et
.
al., 1990 .
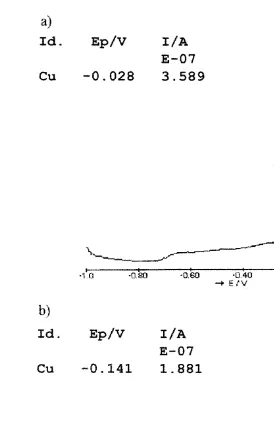
For comparison, the voltammogram of fog sample F1223 originally containing 0.53
Ž .
mM copper II is shown in Fig. 2. The voltammogram of the fog sample closely Ž .
resembles that of b in Fig. 1, i.e. the copper solution with the addition of standard humic acid. The peak is shifted cathodically, the Hg-wave is also affected and peak broadening is also evident, though all of them to a lesser extent than in the case of the
Ž .
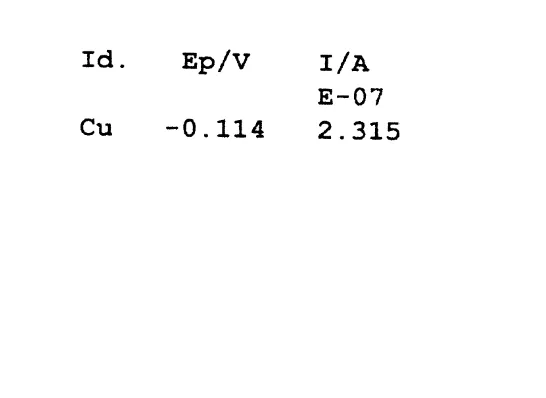
model solution. The effects of further addition of 2.00 then 4.00 nmol copper II in the form of buffered copper solution of 1.00=10y5 M are indicated in Fig. 3.
Ž .
The addition of 2.00 nmol copper II has little effect on the voltammogram. The peak is slightly shifted anodically, implying the formation of an electroactive complex that can be reduced more readily and reversibly than the aquo ions. Such shifts were also
Ž .
reported for copper–fulvic acid complexes Wilson et al., 1980 . The area of the peak remained virtually unaffected, though in the absence of ligands the addition would have increased the peak area considerably, which is the basis for the common standard addition method. The fact that standard addition does not work here is an indication that added copper is incorporated into non-labile complexes in anodic stripping voltammetry,
Ž
which have also been evidenced for fulvic acids Piotrowicz et al., 1982; Filella et al.,
.
Ž . y5 Ž . Ž .
Fig. 1. a Voltammogram of 1.00=10 M of copper II solution at pHs9.00. b Voltammogram of
y5 Ž . y3
1.00=10 M of copper II solution at pHs9.00 after addition of 20.0ml of 0.10 g dm standard humic acid solution.
Ž .
Ž .
Fig. 2. Voltammogram of fog sample F1223 containing 0.53mM copper II at pHs9.00.
ŽBresnahan et al., 1978 . That is, when the ratio of copper. rligand is low, four or threeŽ .
strong donor atoms chelate the Cu2q, which are likely to originate from different
molecules, in agreement with the observation that di- and trivalent metal ions increase the average molecular weight of humic acids. When the copperrligand ratio is high, the more numerous weaker sites predominate. Under these conditions water is more competitive as a ligand and possibly only two donor atoms are bonded to each Cu2q.
The excess Cu2q would therefore tend to cause ligand dissociation of quadridentate
chelation into weaker, bidentate sites. This explanation may be supported by the fact that the area of the new peak formed largely at the expense of the first one, which is shifted slightly anodically. The broad new peak indicates that, probably, a range of electroactive copper–organic complexes were formed, in agreement with what follows from the inherent properties of natural organic complexants. We did not measure individual organic compounds such as formic or acetic acid in the fog samples. We believe that since they are simple organic ligands, they, if present in significant concentrations, do not produce the observed interferences reported for natural complexants. All the phenomena described above were observed in all the fog samples studied. The magni-tude of the effects varied to some extent between samples, but since quantification was not part of this study these variations were not taken into account.
Since a variety of interferences in anodic stripping voltammetry were observed during the measurements, no attempt was made to translate the results into quantitative terms. Nevertheless, it can be estimated that the molar concentration of natural organic complexants should be at least at a level comparable to that of copper to produce the
Ž .
observed phenomena. It was about 11 nmol copper II for 10.0 ml of fog sample,
Ž .
corresponding to a copper II concentration of about 1.1 mM. This assumption is in
Ž . Ž . Ž .
Fig. 3. a Voltammogram of fog sample F1223 after the addition of 2.00 nmol copper II at pHs9.00. b
Ž .
Voltammogram of fog sample F1223 after the addition of 6.00 nmol copper II at pHs9.00.
ŽSpokes et al., 1996 . Furthermore, such macromolecules were found in the same set of.
Ž .
complexants similar to humic or fulvic acids are present in fog water in significant amounts. This statement, corroborating implications of previous measurements, may be of importance for assessing the sources and effects of humic-like substances in atmo-spheric samples.
Acknowledgements
The authors are indebted to Antonella Andracchio, Sergio Zappoli and Maria-Cristina Facchini for providing the samples and carrying out measurements of inorganic ions. The help provided by Erika Schmidt and Klara Polyak in voltammetric and atomic
´
´
absorption measurements, respectively, is also gratefully acknowledged. This work was
Ž
financed by the European Commission Projects ENV4-CT95-0009 and
IC20-CT96-. Ž .
0014 and by the Hungarian Research Fund OTKA T 030186 .
References
Andreae, M.O., Crutzen, P.J., 1997. Atmospheric aerosols: biogeochemical sources and role in atmospheric chemistry. Science 276, 1052–1058.
Artaxo, P., Maenhaut, W., Storms, H., van Grieken, R., 1990. Aerosol characteristics and sources for the Amazon Basin during the wet season. J. Geophys. Res. 95, 16971–16985.
Ž .
Bank, S., Castillo, R., 1987. Analysis of organic matter from cloud particles. Geophys. Res. Lett. 14 3 , 210–212.
Ž .
Bresnahan, W.T., Grant, C.L., Weber, J.H., 1978. Stability constants for the complexation of copper II ions
Ž .
with water and soil fulvic acids measured by an ion selective electrode. Anal. Chem. 50 12 , 1675–1679. Facchini, M.C., Fuzzi, S., Zappoli, S., Andracchio, A., Gelencser, A., Kiss, G., Krivacsy, Z., Meszaros, E.,´ ´ ´ ´
Hansson, H.C., Alsberg, T., Zebuhr, Y., 1999. Partitioning of the organic aerosol component between fog¨
droplets and interstitial air. J. Geophys. Res. 104, 26821–26832.
Filella, M., Buffle, J., van Leeuwen, H.P., 1990. Effect of physico-chemical heterogeneity of natural complexants: Part I. Voltammetry of labile metal–fulvic complexes. Anal. Chim. Acta 232, 209–223. Fuzzi, S., Orsi, G., Bonforte, G., Zardini, B., Franchini, P.L., 1997. An automated fog water collector suitable
for deposition networks: design, operation and field tests. Water, Air, Soil Pollut. 93, 383–394. Havers, N., Burba, P., Lambert, J., Klockow, D., 1998. Spectroscopic characterization of humic-like
substances in airborne particulate matter. J. Atmos. Chem. 29, 45–54.
Mukai, H., Ambe, Y., 1986. Characterization of a humic acid-like brown substance in airborne particulate
Ž .
matter and tentative identification of its origin. Atmos. Environ. 20 5 , 813–819.
Piotrowicz, S.R., Springer-Young, M., Puig, J.A., Spencer, M.J., 1982. Anodic stripping voltammetry for
Ž .
evaluation of organic-metal interactions in seawater. Anal. Chem. 54 8 , 1367–1371.
Spokes, L.J., Campos, M.L.A.M., Jickells, T.D., 1996. The role of organic matter in controlling copper
Ž .
speciation in precipitation. Atmos. Environ. 30 23 , 3959–3966.
Went, F.W., 1966. On the nature of Aitken condensation nuclei. Tellus 2, 549–556.
Wilson, S.A., Huth, T.C., Arndt, R.E., Skogerboe, R.K., 1980. Voltammetric methods for determination of
Ž .
metal binding by fulvic acids. Anal. Chem. 52 9 , 1515–1518.
´
Zappoli, S., Andracchio, A., Fuzzi, S., Facchini, M.C., Gelencser, A., Kiss, Gy., Krivacsy, Z., Molnar, A.,´ ´ ´
Meszaros, E., Hansson, H.C., Rosman, K., 1999. Inorganic, organic and macro-molecular components of´ ´