Bipolar Mania
Hilary P. Blumberg, Emily Stern, Diana Martinez, Sally Ricketts, Jose de Asis,
Thomas White, Jane Epstein, P. Anne McBride, David Eidelberg, James H. Kocsis,
and David A. Silbersweig
Background: Executive control of cognition, emotion, and behavior are disrupted in the manic state of bipolar disorder. Whereas frontal systems are implicated in such dysfunction, the localization of functional brain abnormal-ities in the manic state is not well understood.
Methods: We utilized a high-sensitivity H2 15
0 positron emission tomography technique to investigate regions of increased brain activity in mania, compared to euthymia, in bipolar disorder.
Results:The principal findings were manic state-related increased activity in left dorsal anterior cingulate, and left head of caudate.
Conclusions:The findings suggest that the manic state of bipolar disorder may be associated with heightened activ-ity in a frontal cortical–subcortical neural system that includes the anterior cingulate and caudate. Biol Psy-chiatry 2000;48:1045–1052 ©2000 Society of Biological Psychiatry
Key Words:Bipolar disorder, mania, affective disorders, tomography emission-computed, gyrus cinguli, caudate nucleus
Introduction
C
lassic manic symptoms include sustained heightened mood, as well as associated abnormalities in atten-tional and executive functions, such as distractibility and excessive maladaptive behaviors. There are a number of nonimaging studies suggesting that manic states are asso-ciated with greater right than left hemisphere prefrontal and subcortical impairment, and greater ventral than dorsal cortical impairment (reviewed in Bear 1986; Sackeim et al 1982; Starkstein and Robinson 1997; Wexler 1980).Al-though human brain lesion studies can help to localize regions of decreased functioning, post-lesion compensa-tion and reorganizacompensa-tion can complicate the interpretacompensa-tion of findings. Furthermore, the elucidation of dysregulated or heightened activity in associated brain regions, which might also underlie manic symptoms, has been limited by available experimental methods. Functional neuroimaging techniques allow for the investigation, in vivo, of such possible manic state-related abnormalities in regional brain function.
Functional neuroimaging techniques have been applied to a greater extent to the study of depression, than of mania, in mood disorders. Positron emission tomography (PET) studies of depression have demonstrated abnormal-ities in ventral, rostral and dorsal anterior cingulate cortex (AC), dorsolateral prefrontal cortex, as well as caudate, which receives significant projections from these cortical structures (Baxter et al 1989; Bench et al 1993; DeAsis et al 1999; Drevets et al 1997; George et al 1997; Mayberg et al 1997).
The regional brain involvement in mania is less clear. There are findings that support possible frontal lobe, temporal lobe, and basal ganglia involvement in primary mania, although the associated studies differ in the imag-ing techniques and normalization procedures used, the delineation of the frontal lobe regions examined, and whether increases or decreases were found in these regions (Table 1).
Decreased anterior–posterior gradients and general frontal activity have been reported in mania (al-Mousawi et al 1996; O’Connell et al 1995; Rubin et al 1995). More specifically, mania-associated increased activity has been reported in dorsal and ventral AC (Drevets et al 1995, 1997; Goodwin et al 1997), and increased activity has been reported in more lateral prefrontal cortices (Baxter et al 1989).
There has been evidence to suggest possible involve-ment of the temporal polar cortex in mania; however; there have been conflicting findings in this area. Decreased right basotemporal activity has been reported, with a trend toward a negative correlation between mania ratings and
From the Functional Neuroimaging Laboratory, Department of Psychiatry, The Weill Medical College of Cornell University, New York (HPB, ES, DM, SR, JdA, TW, JE, PAM, DE, JHK, DAS) and the Department of Neurology, North Shore University Hospital–New York University School of Medicine, Manhas-sett (DE).
Address reprint requests to Hilary P. Blumberg, M.D., Veterans Administration Connecticut Healthcare System, Department of Psychiatry 116A, 950 Camp-bell Avenue, West Haven CT 06516.
Received November 24, 1999; revised May 23, 2000; accepted June 14, 2000.
Table 1. Results of Regional Functional Neuroimaging Studies of Primary Mania in Resting States
Group Technique na Frontal findings Temporal findings
Basal ganglia findings
Correlation to
mania scores Comments Baxter et al 1989 FDG PET 6 (6) Increased left
anterolateral compared to depression
Differences may be accounted for by the metabolic reduction in the depressed phase
Migliorelli et al 1993 HMPAO SPECT 5 (5) Decreased right basotemporal
Negative trend with right basotemporal
All subjects female, normalized to cerebellum
Drevets et al 1995 FDG PET 3 (3) Increased right ventral AC
Increased right ventral striatum
O’Connell et al 1995 IMP SPECT 11 Decreased frontal (in four of 11 subjects)
Increased temporal (in 11/11)
Increased right.left basal ganglia (in 5/11)
Positive with right temporal
Abnormalities on visual inspection
Rubin et al 1995 rCBF 11 Decreased anterior cortical rCBF in depression and mania
Preliminary suggestion of left
.right rCBF in mania compared to depression in inferior prefrontal cortex al-Mousawi et al 1996 FDG PET 15 (2) Decreased
anterior-posterior gradient
Increased right temporal and decreased left amygdala
Normalized to the area-weighted mean of all regions of interest
Drevets et al 1997 FDG PET 4 (3) Increased ventral AC
Goodwin et al 1997 EMZ SPECT 7 (7) Increased dorsal AC Subjects manic after lithium discontinuation
Gyulai et al 1997 IMP SPECT 3 Increased right. left anterior temporal (in 2/3) Blumberg et al 1999 H2150 PET 5 Decreased orbitofrontal
FDG, 18 fluorodeoxyglucose; PET, positron emission tomography; HMPAO, 99mTchexamethylpropyleneamine oxime; SPECT, single photon emission computed tomography; AC, anterior cingulate; IMP, isopropyliodoamphetamine; rCBF, regional cerebral blood flow; EMZ, 99mTc-exametazime.
a
Numbers in parentheses represent the number of unmedicated subjects. H.P.
Blumberg
et
al
BIOL
PSYCHIATRY
right basotemporal cerebral blood flow (CBF) (Migliorelli et al 1993); however, increased right temporal activity (al-Mousawi et al 1996; Gyulai et al 1997; O’Connell et al 1995) and decreased left amygdala activity (al-Mousawi et al 1996) have also been reported, and right temporal increases have been shown to positively correlate with mania scores (O’Connell et al 1995).
Abnormalities have been reported in bipolar mania in the basal ganglia. There was a report of increased uptake in the basal ganglia, greater in the right hemisphere (O’Connell et al 1995). Another preliminary report sug-gests that there may be increased right ventral striatal activity (Drevets et al 1995).
We previously reported manic state-related decreased orbitofrontal activity (Blumberg et al 1999). We suggested that dysfunction in ventral cortices might be associated with increased activity in associated cortical and subcor-tical structures with significant connectivity to these re-gions. As noted above, the nonimaging literature suggests that an imbalance with right more than left, and ventral more than dorsal, frontal lesions are associated with mania. It is plausible that, conversely, increased left dorsal frontal activity may contribute to a similar regional imbal-ance, in the absence of gross lesions. The small functional neuroimaging literature of mania to date has not resolved these issues. In this article we describe analyses performed to examine increases in brain activity in mania compared to euthymia with particular interest in lateralized frontal systems.
Methods and Materials
Subjects with Bipolar I Disorder, non–rapid-cycling, established by Structured Clinical Interview (First et al 1995) included five manic subjects (M; four female, one male; mean age 34.2 years, SD512.2), and six euthymic subjects (E; four female, two male; 32.5 years, SD511.0). Manic subjects met DSM-IV criteria for a current manic episode. Euthymic subjects did not meet DSM-IV criteria for a current manic or depressive episode and had a score of,6 on the 29-item Hamilton Depression Rating Scale (Hamilton 1960). Subjects were without comorbid Axis I
or II Disorders, except distant substance abuse (.5 years in 3 M, 3 E). All subjects were right-handed (Oldfield 1971), spoke English as a first language, and were without neurologic or medical illness. Patient groups did not differ significantly in estimated premorbid verbal intelligence quotient (Grober and Sliwinski 1991): M5111.666.0, E5118.767.6. The manic and euthymic groups also did not differ significantly in illness duration: M 5 14.2 years6 14.9, E 5 12.06 5.6. Subjects denied family history of psychotic or anxiety disorder. Medica-tions included mood stabilizers (lithium [4 M, 5 E], valproic acid [2 M, 2 E], and carbamazepine [1 M, 2 E]), antipsychotic agents (3 M), benzodiazepines (4 M, 1 E), and antidepressants (2 E). No subject required acute sedation for scanning. After complete description of the study to the subjects, written informed consent was obtained in accordance with hospital institutional review board protocols.
A General Electric ADVANCE Positron Emission Tomograph was operated in three-dimensional mode (Lewellen et al 1996). Four scans, in one study session, were performed in the resting condition with eyes closed, lights dimmed, and low levels of ambient noise. Regional cerebral blood flow (rCBF) was mea-sured as an index of local neuronal activity (Raichle 1987). H2
150
delivery and data acquisition were performed according to a previously published technique (Silbersweig et al 1993). Re-alignment of images across runs within subject was performed to correct for slight head movement, and spatial smoothing (15 mm full-width-at-half-maximum) was performed. Head movement was significantly less than the resolution of smoothing for all subjects. Regional CBF was normalized to a mean of 50 mL/100 gm/min. Group effects were identified with multiple linear regression analysis. Image processing and analyses were per-formed with statistical parametric mapping (SPM ’96, Wellcome Department of Cognitive Neurology, University College Lon-don, UK) according to the General Linear Model, and probability estimates were determined according to the Theory of Random Fields (Friston et al 1995).
Results
Mean mania scores (Altman et al 1994) for the manic group and the euthymic group were 21.86 SD 6.7 and 2.56 4.5, respectively. The groups differed significantly on 7 of the 10 mania items (p, .05; Student’sttest, two
Table 2. Brain Regions of Increased Activity in the Manic State of Bipolar Disorder
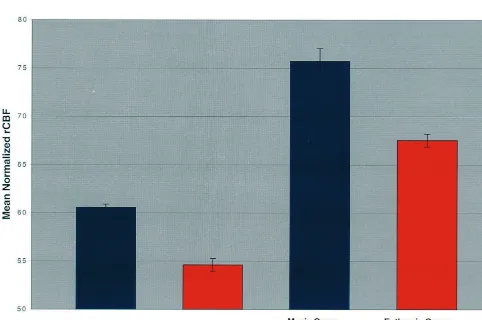
Brain area (Brodmann area) x y z Z Mania rCBF Euthymia rCBF
Left, dorsal AC (24/32) 218 12 34 4.69a 60.54
60.33 54.6160.66
Left caudate head 216 20 0 4.20a 75.76
61.32 67.5260.66
Right, dorsal AC (24/32) 24 6 38 3.32 60.4960.89 57.1060.49
Right ventral AC (32) 10 26 28 3.13 77.2960.63 74.2260.76
The brain areas represent regions of increased activity in the manic state, compared to the euthymic state, above significance thresholds ofp,.001, uncorrected for multiple comparisons. Coordinates of the maxima within a region of increased regional cerebral blood flow (rCBF) are in millimeters relative to the anterior commissure in standardized stereotactic space (Talairach and Tournoux 1988); x is the lateral distance from the midline (positive, right), y is the anteroposterior distance from the anterior commissure (positive, anterior), and z is the height relative to the anterior commissure–posterior commissure plane (positive, dorsal).Zis the significance level converted to the unit normal distribution. rCBF, mean adjusted rCBF6SD in mL/100 g/min. AC, anterior cingulate.
a
tailed), including measures of mood elevation, irritability, pressured speech, racing thoughts, distractibility, grandi-osity, and sleep disturbance. Although there were trends toward increases in mania, there were no significant differences on measures of hyperactivity, increased en-ergy, and impaired judgment. The lack of difference between groups on these items may reflect a bias in selecting manic subjects without psychomotor agitation that would render them unable to complete scanning, and in choosing only those subjects judged by an attending physician to have capacity to make an informed decision to participate in the study.
Increased mania-associated rCBF was detected in the bilateral dorsal AC, the right ventral AC, and in the left head of the caudate at a threshold of p , .001, uncorrected (Table 2). Applying correction for multiple comparisons, significant findings were restricted to the left dorsal AC and left head of caudate (p , .05, corrected for multiple comparisons; Figures 1 and 2).
Figure 1. Sagittal section 16 mm left of the midsagittal plane demonstrating increased activity in the head of the caudate, and in left dorsal anterior cingulate in mania, compared to euthymia. Regional cerebral blood flow functional results (p, .001) are displayed in color and superimposed on a structural T1-weighted
magnetic resonance imaging template.
Discussion
DSM-IV criteria for a manic episode include a sustained heightened emotional state, as well as accompanying symptoms of distractibility, rapid loosely connected thoughts, and behaviors associated with increased motiva-tional drives (American Psychiatric Association 1994). We report here greater manic, versus euthymic, state-related activity in the AC and caudate. The greater activity in these structures may relate to behavioral abnormalities observed in mania.
The AC is associated with attentional, emotional, and cognitive functions. The AC has significant reciprocal connections to the amygdala (Vogt et al 1992), a brain structure thought to be central to emotional processing. Stimulation of AC can lead to euphoria and a sense of well-being, as well as alterations in rate, volume, and degree of perseveration in speech (reviewed in Devinsky et al 1995). Cingulate seizures have been associated with ictal emotional changes, and interictal irritability, emo-tional lability, and sexual behaviors (Devinsky et al 1995). Rats will self-stimulate in this brain region in a manner enhanced over time and thought to be associated with kindling (Corbett and Stellar 1983), a process theorized to develop in limbic brain regions in bipolar disorder. Anti-convulsant agents, also used as mood stabilizing agents in bipolar disorder, have been demonstrated to interfere with this process (Post et al 1982).
The principal finding of this study was in the dorsal region of the AC. This region of the AC has relatively organized and granular cytoarchitecture, and the related behavioral functions are of higher order (Devinsky et al 1995), than the ventral AC. The dorsal AC has been associated with cognitive functions, such as the appropri-ate directing of attention, conflict monitoring, and re-sponse selection (Botvinick et al 1999; Carter et al 1998; Peterson et al 1999; Posner and Rothbart 1998). Decreased activity in the left dorsal AC has been described in association with depressive cognitive abnormalities (Bench et al 1993; DeAsis et al 1999; George et al 1997). The more ventral regions of the AC region have a more agranular cytoarchitecture and the related functions are more primitive. The ventral AC has efferent connections to autonomic, endocrine, and visceral effectors (Nauta 1971), and is thought to regulate the associated functions. The rostral AC has also been associated with the expres-sion of internal states, including vocalizations and social behaviors (Devinsky et al 1995). The ventral AC region has been demonstrated to activate more to emotional stimuli, in contrast to the dorsal AC, which may activate more during cognitive tasks (Whalen et al 1998). One region of increased activity in this study, the ventral AC, is a region that overlaps that previously reported to have
increased activity in mania (Drevets et al 1997), represent-ing a possible convergence of findrepresent-ings.
This study also demonstrated increased activity in the left head of the caudate in mania, ipsilateral to the increased activity in the dorsal AC. The AC has excitatory efferent input to the head of the caudate (Eblen and Graybiel 1995; Yeterian and van Hoesen 1978). The modulatory caudate input back to the AC has intervening connections via the globus pallidus and thalamus (Alex-ander et al 1986). The known structural connectivity between the cingulate and caudate suggests that the increased activity occurs within the context of this corti-cal–subcortical neural system. Increased activity has pre-viously been reported in mania in the basal ganglia (Drevets et al 1995; O’Connell et al 1995) and warrants further investigation.
We speculate that increased AC activity could relate to heightened, dysregulated attentive and cognitive pro-cesses, such as manic-type distractibility and maladaptive excessive behaviors. An alternative possible interpretation for increased left AC activity in the manic state relates to the behavioral constraint required during scanning. The scanning requirement of decreasing inappropriate behav-ioral outputs, such as psychomotor movements and speak-ing, may require additional efforts on the part of manic subjects. Heightened AC and caudate activity could be associated with this demand.
The findings here are preliminary and require replica-tion, as this study is limited by the relatively small number of subjects and their medication status. More manic subjects were receiving both antipsychotic and benzodiaz-epine medications, and more euthymics were receiving antidepressants. In each group all but one subject was on lithium at the time of study. Goodwin et al (1997) reported decreases in anterior cingulate and right caudate CBF after lithium withdrawal, whereas development of mania on lithium withdrawal was associated with increased AC CBF. There have been reports of increased caudate activ-ity and basal ganglia size associated with antipsychotic medication (Buchsbaum et al 1987; Chakos et al 1994; Holcomb et al 1996), and decreased rostral AC activity with antidepressant treatment (Mayberg et al 1997); how-ever, there is not clear evidence to suggest a contribution to the lateralized effects seen in this study. Both antipsy-chotic and benzodiazepine medication have been reported to be associated with decreased AC activity (Holcomb et al 1996; Veselis et al 1997), which would have predicted the opposite outcome in the AC in the manic group. Findings in ventral AC and striatum have previously been reported in three unmedicated manic subjects, although the results were lateralized to the right hemisphere (Drevets et al 1995).
bipolar manic states. Elevated emotional states in healthy subjects have been reported in association with increased AC activity. For example, Ketter et al (1997) demon-strated increased rCBF in AC associated with procaine-induced euphoria in healthy subjects. In a study of transient psychologically induced emotional states (via emotional memory and face presentation) also in healthy subjects, AC rCBF has been demonstrated to increase significantly in sad states, and there was a trend toward an increase in happy states (George et al 1995). Increased anterior cingulate activity is also not specific to the bipolar diagnosis, and has also been demonstrated in symptomatic states of anxiety disorders, such as obsessive-compulsive disorder and phobias (Rauch et al 1994, 1995). These and our findings, seen in the light of the behavioral neurosci-entific literature, suggest that the AC is a vulnerable part of an important final common pathway of emotional regulation.
As this study was designed to examine relative regional changes in CBF, mean CBF measures were normalized for each subject, and global changes were not assessed. There has been a previous report to suggest possible global brain changes in mania. Kishimoto et al (1987), reported gen-eralized increases in 11C-glucose uptake in three medica-tion-free manic patients compared to control subjects. Other groups have not found an increase in global activity (Rubin et al 1995; Schwartz et al 1987; Silfverskiold and Risberg 1989).
Concurrent volumetric measurements were not per-formed in this study. A possible contribution of dorsal AC volume change across mood states can be a subject for further study. There have been reports of volumetric differences in ventral AC grey matter (Drevets et al 1997), and a conflicting literature on the possibility of caudate volume abnormalities in mood disorders (Aylward et al 1994; Dupont et al 1995; Krishnan et al 1992; Lenze and Sheline 1999). This literature suggests possible trait ab-normalities; however, volumetric state abnormalities that would have greater implications for the current work are less clear.
The most significant areas of increased manic state-related activity were in the left dorsal AC and head of caudate. This finding, and our previously reported finding of decreased orbitofrontal activity in mania (Blumberg et al 1999), are consistent with theories that relative right lower than left, and ventral less than dorsal, prefrontal activity is associated with manic states (Bear 1986; Sack-eim et al 1982; Starkstein and Robinson 1997; Wexler 1980). This has been theorized to extend to relative impairment in activity in related right hemisphere subcor-tical structures, including caudate, thalamus, and medial diencephalon (Mega et al 1997; Starkstein and Robinson 1997). Much of the reasoning for these theories is based
upon manic behavior described in association with brain lesions. The data in this functional neuroimaging study suggest that greater left dorsal AC and caudate activity may contribute to an equivalent net imbalance in brain function associated with the manic state in bipolar disorder.
Supported by the DeWitt-Wallace Fund in the New York Community Trust.
The authors thank the volunteers for their participation, and Vijay Dhawan, PhD, Abdelfatihe Belakhlef, PhD, and Claude Marguleff, MA for their expert technical assistance, and Hong Pan, PhD for his statistical input.
The findings of this study were presented at the 53rd annual meeting of the Society of Biological Psychiatry, May 27–31, 1998, Toronto.
References
Alexander GE, DeLong MR, Strick PL (1986): Parallel organi-zation of functionally segregated circuits linking basal gan-glia and cortex.Annu Rev Neurosci9:357–381.
al-Mousawi AH, Evans N, Ebmeier KP, Roeda D, Chaloner F, Ashcroft GW (1996): Limbic dysfunction in schizophrenia and mania. A study using 18F-labelled fluorodeoxyglucose and positron emission tomography.Br J Psychiatry169:509 – 516.
Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM (1994): The clinician-administered rating scale for mania (CARS-M): Development, reliability and validity.Biol Psy-chiatry36:124 –134.
American Psychiatric Association. (1994):Diagnostic and Sta-tistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Press.
Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, et al (1994): Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder.Am J Psychiatry151:687– 693.
Baxter LR Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al (1989): Reduction of prefrontal cortex glucose metabolism common to three types of depression.Arch Gen Psychiatry46:243–250.
Bear DM (1986): Hemispheric asymmetries in emotional func-tion: A reflection of lateral specialization in cortical-limbic connections. In: Doane BK, Livingston KE, editors. The Limbic System: Functional Organization and Clinical Disor-ders.New York: Raven, 29 – 42.
Bench CJ, Friston KJ, Brown RG, Frackowiak RSJ, Dolan RJ (1993): Regional cerebral blood flow in depression measured by positron emission tomography: The relationship with clinical dimensions.Psychol Med23:579 –590.
Blumberg H, Stern E, Ricketts S, Martinez D, de Asis J, McBride A, et al (1999): Rostral and orbital prefrontal cortex dysfunc-tion in the manic state of bipolar disorder.Am J Psychiatry 156:1986 –1988.
Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection-for-action in anterior cingulate cortex.Nature402:179 –181.
Cooper-Langston K, Kessler R (1987): Positron emission tomography studies of basal ganglia and somatosensory cortex neuroleptic drug effects: Differences between normal controls and schizophrenic patients.Biol Psychiatry22:479 – 494.
Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance.Science280:747–749. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G,
Bogerts B, et al (1994): Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs.Am J Psychiatry151:1430 –1436.
Corbett D, Stellar JR (1983): Neurological reactivity during medial prefrontal cortex stimulation: Effects of self-stimula-tion experience.Physiol Behav31:771–776.
DeAsis J, Silbersweig D, Alexopoulos G, Blumberg H, Kalayam B, Eidelberg D, Stern E (1999): Decreased hippocampal and anterior cingulate activation in geriatric depression. Biol Psychiatry45:5115–5116.
Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour.Brain118:279 –306. Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T,
Vannier M, Raichle ME (1997): Subgenual prefrontal cortex abnormalities in mood disorders.Nature386:824 – 827. Drevets WC, Price JL, Videen TO, Todd RD, Raichle ME
(1995): Metabolic abnormalities in the subgenual prefrontal cortex and ventral striatum in mood disorders.Neurosci Abstr 21:260.
Dupont RM, Jernigan TL, Heindel W (1995): Magnetic reso-nance imaging and mood disorders—localization of white matter and other subcortical abnormalities.Arch Gen Psychi-atry52:747–755.
Eblen F, Graybiel (1995): Highly restricted origins of prefrontal cortical inputs to striosomes in the macaque monkey.J Neu-rosci15:5999 – 6013.
First MB, Spitzer RL, Gibbon M, Williams JBW (1995): Structured Clinical Interview for DSM-IV Axis I & II Disor-ders (Version 2.0). New York: New York State Psychiatric Institute, Biometrics Research.
Friston KJ, Holmes A, Worsley K, Poline J, Frith C, Heather J, Frackowiak RSJ (1995): Statistical parametric maps in func-tional imaging: A general linear approach.Hum Brain Mapp 2:189 –210.
George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, et al (1995): Brain activity during transient sadness and happi-ness in healthy women.Am J Psychiatry152:341–351. George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA,
Pazzaglia PJ, et al (1997): Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop).J Neuropsychiatry Clin Neurosci9:55– 63. Goodwin GM, Cavanagh JTO, Glabus MF, Kehoe RF, O’Carroll
RE, Ebmeier KP, et al (1997): Uptake of 99mTc-exametaz-ime shown by single photon emission computed tomography before and after lithium withdrawal in bipolar patients: associations with mania.Br J Psychiatry170:426 – 430. Grober E, Sliwinski M (1991): Development and validation of a
model for estimating premorbid verbal intelligence in the elderly.J Clin Exp Neuropsychol13:933–949.
Gyulai L, Alavi A, Broich K, Reilly J, Ball WB, Whybrow PC (1997): I-123 Iofetamine single-photon emission tomography in rapid cycling bipolar disorder: A clinical study. Biol Psychiatry41:152–161.
Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry23:56 – 62.
Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA (1996): Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol.Am J Psychiatry153:41– 49.
Ketter TA, Andreason PJ, George MS, Lee C, Gill DS, Parekh PI, et al (1997): Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences.Arch Gen Psychiatry53:59 – 69.
Kishimoto H, Takazu O, Ohno S, Yamaguchi T, Fujita H, Kuwahara H, et al (1987): 11C-glucose metabolism in manic and depressed patients.Psychiatry Res22:81– 88.
Krishnan KR, McDonald WM, Escalona PR, Doraiswamy PM, Na C, Husain MM, et al (1992): Magnetic resonance imaging of the caudate nuclei in depression: Preliminary observations. Arch Gen Psychiatry49:553–557.
Lenze EJ, Sheline YI (1999): Absence of striatal volume differ-ences between depressed subjects with no comorbid medical illness and matched comparison subjects. Am J Psychiatry 156:1989 –1991.
Lewellen TK, Kohlmyer RS, Miyaoka RS, Kaplan MS, Stearns CW, Schubert SF (1996): Investigation of the performance of the General Electric ADVANCE positron emission tomo-graph in 3D mode.IEEE Trans Nucl Sci43:2199 –2207. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman
JS, Tekell JL, et al (1997): Cingulate function in depression: A potential predictor of treatment response. Neuroreport 8:1057–1061.
Mega MS, Cummings JL, Salloway S, Malloy P (1997): The limbic system: An anatomic, phylogenetic, and clinical per-spective.J Neuropsychiatry Clin Neurosci9:315–330. Migliorelli R, Starkstein SE, Teson A, de Quiros G, Vazquez S,
Leiguarda R, et al (1993): SPECT findings in patients with primary mania.J Neuropsychiatry Clin Neurosci5:379 –383. Nauta WJH (1971): The problem of the frontal lobe: A
reinter-pretation.J Psychiatr Res8:167–187.
O’Connell RA, van Heertum RL, Luck D, Yudd AP, Cueva JE, Billick SB, et al (1995): Single-photon emission computed tomography of the brain in acute mania and schizophrenia. J Neuroimaging5:101–104.
Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh Handedness Inventory. Neuropsychologia 9:97–113.
Peterson BS, Skudlarski P, Gatenby C, Zhang H, Anderson AW, Gore JC (1999): An fMRI study of Stroop word-color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems.Biol Psychiatry45: 1237–1258.
Posner MI, Rothbart MK (1998): Attention, self-regulation and consciousness.Philos Trans R Soc Lond B Biol Sci353:1915– 1927.
Raichle ME (1987): The nervous system. In: Mountcastle VB, editor.Handbook of Physiology,Sec 1, Vol V. Bethesda, MD: American Physiological Society, 643– 674.
Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al (1994): Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disor-der using oxygen 15-labeled carbon dioxide and positron emission tomography.Arch Gen Psychiatry51:62–70. Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter
HC, et al (1995): A positron emission tomographic study of simple phobic symptom provocation.Arch Gen Psychiatry 52:20 –28.
Rubin E, Sackeim HA, Prohovnik I, Moeller JR, Schnur DB, Mukherjee S (1995): Regional cerebral blood flow in mood disorders: IV. Comparison of mania and depression. Psychi-atry Res61:1–10.
Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbu-hler JP, Geschwind N (1982): Hemisphere asymmetry in the expression of positive and negative emotions.Arch Neurol 39:210 –218.
Schwartz JM, Baxter LR, Mazziotta JC, Gerner RH, Phelps ME (1987): The differential diagnosis of depression. Relevance of positron emission tomography studies of cerebral glucose metabolism to the bipolar-unipolar dichotomy. JAMA 258: 1368 –1374.
Silbersweig DA, Stern E, Frith CD, Cahill C, Schnorr L, Grootoonk S, et al (1993): Detection of thirty-second cogni-tive activations in single subjects with positron emission
tomography: A new low-dose H2
150 regional cerebral blood
flow three-dimensional imaging technique. J Cereb Blood Flow Metab13:617– 629.
Silfverskiold P, Risberg J (1989): Regional cerebral blood flow in depression and mania.Arch Gen Psychiatry46:253–259. Starkstein SE, Robinson RG (1997): Mechanisms of
disinhibi-tion after brain lesions.J Nerv Ment Dis182:108 –114. Talairach J, Tournoux P (1988):Co-Planar Stereotaxic Atlas of
the Human Brain.New York: Thieme.
Veselis RA, Reinsel RA, Beattie BJ, Mawlawi OR, Feshchenko VA, DiResta GR, et al (1997): Midazolam changes cere-bral blood flow in discrete brain regions: An H2(15)O positron emission tomography study. Anesthesiology 87: 1106 –1117.
Vogt BA, Finch DM, Olson CR (1992): Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions.Cereb Cortex2:435– 443.
Wexler BE (1980): Cerebral laterality and psychiatry: A review of the literature.Am J Psychiatry137:279 –291.
Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL (1998): The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44:1219 –1228.