www.elsevier.com / locate / bres
Short communication
Immunocytochemical study on the distribution of p53 in the
hippocampus and cerebellum of the aged rat
a a a a
Yoon Hee Chung , Chung-Min Shin , Myeung Ju Kim , Byung-Kwon Lee ,
a a,b,c ,
*
Kyeong Han Park , Choong Ik Cha,
a
Department of Anatomy, Seoul National University College of Medicine, 28 Yongon-Dong, Chongno-Gu, Seoul 110-799, South Korea
b
Neuroscience Research Institute, Medical Research Center, Seoul National University, 28 Yongon-Dong, Chongno-Gu, Seoul 110-799, South Korea
c
Biomedical Research Center, KNIH, Seoul, South Korea
Accepted 29 August 2000
Abstract
A role for p53-mediated modulation of neuronal viability has been suggested by the finding that p53 expression is increased in damaged neurons in models of ischemia and epilepsy. P53 gene upregulation precedes apoptosis in many cell types, and a potential role for this molecule in apoptosis of neurons has already been demonstrated in Alzheimer’s disease. Recent studies suggest that p53-associated apoptosis may be a common mechanism of cell loss in several important neurodegenerative diseases. In the present study, we examined changes in p53-immunoreactive (IR) neurons in the brains of aged rats for the first time employing immunocytochemical and in situ hybridization methods. P53-IR neurons were found in the CA1 region of hippocampus, septal region and cerebellum in the aged rats, but there was no p53-IR cell in the brains of adult rats. In the hippocampus of the aged rat, p53-IR cells predominated in the stratum oriens and pyramidal layers, while the molecular layer contained relatively few p53-IR cells. The most prominent population of immunoreactive labeling in cerebellar cortex was localised within the cell bodies of Purkinje cells and dendrites in molecular layers. Upregulation of p53 in the Purkinje cells observed in this study suggests that significant loss of Purkinje cells with aging may be regulated with several apoptosis-controlling factors including p53 and oxidative stress mechanism. Further investigations are required to establish whether direct functional relations exist between p53 and the apoptotic neuronal death in normal aging or Alzheimer brains. 2000 Elsevier Science B.V. All rights reserved.
Theme: Motor systems and sensorimotor integration
Topic: Cerebellum
Keywords: p53; Hippocampus; Cerebellum; Aging; Apoptosis; Immunocytochemistry; in situ hybridization
The tumor suppressor gene p53 encodes a nuclear c-jun, cyclin D1, and p53, have been implicated in phosphoprotein involved in the control of cell growth. apoptosis [2,6,8,13,23,24].
Normally, tissue levels of p53 protein and transcriptional Enormous interest in p53 has developed in recent years activity are very low [14], but can be induced by various because of a high percentage of human malignancies that DNA-damaging agents, such as UV light, X-radiation and are associated with p53 mutations. A number of studies chemotherapy [7,16,17]. Several lines of evidence have led have shown that overexpression of wild-type p53 protein to the idea that terminally differentiated neurons undergo arrests the cell cycle at the G1-S interphase. P53-mediated apoptosis when prompted to re-enter an aberrant cell cycle. cell cycle arrest following genomic damage is an important In this regard, both apoptosis and mitosis share certain mechanism of cell growth control that probably facilitates morphological features. In addition, genes that play a role DNA repair prior to replication. Under certain conditions, in modulating cell proliferation, such as c-myc, c-fos, nuclear accumulation of wild-type p53 protein results in apoptosis. Accordingly, transfection of p53 constructs into cells that normally lack endogenous p53 expression causes *Corresponding author. Tel.:182-2-740-8205; fax:182-2-745-9528.
E-mail address: [email protected] (C.I. Cha,). apoptosis [21]. Further, p53 induction in vivo results in
tumor regression in nude mice that is morphologically Animals used by in situ hybridization were killed by consistent with apoptosis [22]. These examples indicate decapitation and the brains were quickly removed, frozen that increased p53 expression may not only serve as a and sliced at 12 mm on a cryostat. These sections were cellular marker of apoptosis, but also play an active role in fixed, acetylated, dehydrated and treated with chloroform. the cell death process. Recent studies suggested that p53- Hybridization was performed with digoxigenin-11-UTP associated apoptosis may be a common mechanism of cell (BM) labeled antisense p53 cRNA probe, and visualized loss in several important neurodegenerative diseases [4]. with NBT / BCIP (Promega).
Based on the increased expression of p53 seen with In the adult rats (control group), no p53-IR cell was DNA damage, p53 has been proposed to be a component found in any region of the central nervous system (Table of cell cycle checkpoints that inhibit the progression of 1).
cells through the cell cycle until damaged DNA is repaired. In contrast, p53-IR neurons were found in the hip-Inhibition of p53 function has been reported to extend the pocampus, septal region and cerebellum of the aged rat proliferative lifespan of human fibroblast, suggesting a (Table 1). P53-IR cells were found in all laminae of the significant role in cellular senescence and a possible link CA1 region of hippocampus except for the molecular layer between senescence and DNA damage. Recently, de la of Ammon’s horn, where they were sparse. P53-IR cells Monte et al. [4] reported p53-immunoreactivity in the predominated in the stratum oriens and pyramidal layers, human aged brain. while the molecular layer contained relatively few p53-IR In the present study, we examined changes in p53-IR cells (Fig. 1). Both the stratum oriens and pyramidal layer neurons in the brains of aged rats for the first time harbored many p53-IR cells with broad differences in employing immunocytochemical and in situ hybridization somal morphology and dendritic branching patterns. In methods. Ammon’s horn, they ranged from small and fusiform or Twelve adult (4–6 month old) and 15 aged (20–29 multipolar, to medium or large multipolar neurons. Some month old) Sprague–Dawley rats were examined in this neurons in the pyramidal layer were bipolar, with short or study. The authors conformed to the Seoul National long spinous dendrites that were oriented perpendicular to University Ethical Committee Guidelines for Laboratory the pial surface. The morphology of p53-IR cells ranged Animals. The animals were perfused transcardially with from small, round bipolar cells with sparsely branching cold phosphate buffered saline (PBS, 0.02 M, pH 7.4), and dendrites to relatively large cells with widely branching then with ice-cold 4% paraformaldehyde for 10 min at a dendrites trees.
flow rate of 50–60 ml / min. Brains were removed immedi- In the septal region, p53-IR cells were scattered through-ately and sliced into blocks 4–6 mm thick. These were out the rostral septal area, mostly in the lateral septal immersed in a cold fixative for 6–12 h and then washed in division (intermediate part of lateral septal nucleus) adja-a series of cold sucrose solutions of increadja-asing concen- cent to the lateral ventricle. P53-IR neurons were medium trations. Frozen sections were cut at 40mm in the coronal in size and multipolar or fusiform in shape. P53-IR cells plane, and were incubated using the free-floating method were also found in the cerebellum. The most prominent for 48–72 h at 48C in primary antiserum containing Triton population of immunoreactive labeling in cerebellar cortex X-100 (0.3%), bovine serum albumin (0.5 mg / ml) and was localized within the cell bodies of Purkinje cells and normal goat serum (3 drops / 10 ml). Monoclonal mouse dendrites in molecular layers (Fig. 2)
anti-human p53 protein antibodies (DAKOE, Code No. M In agreement with immunocytochemistry, p53 mRNA 7001, Lot 056 at a dilution of 1:100) were used as primary signals were also localized in the CA1 region of hippocam-antibodies and visualized according to the avidin–biotin pus, cerebellar hemisphere and the vermis of the aged rat. complex (ABC) method, using an ABC kit (VectastainE). P53 mRNA in situ hybridization showed intensely positive Sections were then developed for peroxidase reactivity signals in the pyramidal layer of the CA1 region and with 3,39-diaminobenzidine (DAB), and after visualization Purkinje cell layer of cerebellum. However, the stratum were mounted on gelatin–coated slides.
A sample of sections was reacted without primary antiserum, and a different sample was exposed to primary
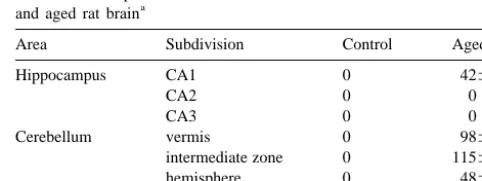
Table 1
antiserum that had been preincubated for 24 h with p53 The number of p53-immunoreactive neurons in each area of the control
a
protein. Sections from these samples did not exhibit any of and aged rat brain immunoreactivity described in this report. Sections from
Area Subdivision Control Aged
each adult and aged group were stained together
eliminat-Hippocampus CA1 0 42611
ing conflicts between different experimental conditions. We
CA2 0 0
selected five slides in each area of the adult (n512) and
CA3 0 0
aged rats (n515), and counted all the p53-IR neurons in Cerebellum vermis 0 98618 each corresponding area of the brain. Visual assessment intermediate zone 0 115623
hemisphere 0 48615
and densitometric measurement using a NIH image
pro-Septal region 0 2468
gram (Scion Image) determined the number and extent of
a
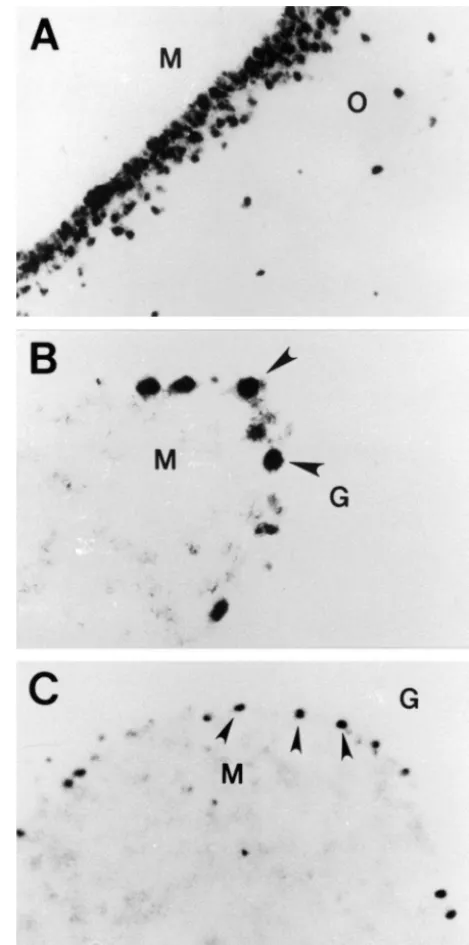
Fig. 1. P53-IR neurons in the lateral (A) and medial (B) portion of CA1 region in the hippocampus of the aged rat. P53-IR cells predominated in the stratum oriens and pyramidal layers, while the molecular layer contained relatively few p53-IR cells. M: Molecular layer, P: Pyramidal layer, O: Stratum oriens. Scale bar550mm (A, B).
oriens of the CA1 region showed relatively lower p53 mRNA level than immunoreactivity (Fig. 3).
In this study, we demonstrated that the immunoreactivi-ty of p53 was upregulated. Immunocytochemistry showed intensely stained p53-IR neurons in the hippocampus, septal region and cerebellum in the aged rat, but no p53-IR
cells were observed in the control group (Table 1). Fig. 3. Localization of p53 mRNAs in the CA1 region of hippocampus Hippocampal pyramidal cells and cerebellar Purkinje cells (A), cerebellar hemisphere (B) and the vermis (C) of the aged rat. Note are among the most vulnerable in the nervous system to that p53 mRNA in situ hybridization showed intensely positive signals in pyramidal layer of the CA1 region (A) and Purkinje cell layers of hypoxia and ischemia. Likewise, hippocampal pyramidal
cerebellum (arrowheads: B, C). M: Molecular layer, O: Stratum oriens, G: cells and cerebellar Purkinje cells are among the largest
Granular layer. Scale bar550mm (A, C); 25mm (B). neurons in the nervous system; their dendritic fields are
among the most highly arborized; their projection distances are lengthy; and their metabolic requirements are among the highest [20]. So there is possibility that decreased
blood flow in the aged rat brain is one of the causative factors to the up-regulation of p53 with aging. Recently, de la Monte et al. [4] reported p53-immunoreactivity in the human aged brain.
Fig. 2. P53-IR Purkinje cells in cerebellar hemisphere (A) and the vermis Recent studies have provided supportive evidence for a (B) of the aged rat. Note that vast majority of immunoreactivity is
relationship between p53 gene activation and neuronal confined to the Purkinje cell layer and molecular layer. P: Purkinje cell
death in the CNS. Following middle cerebral artery layer, M: Molecular layer, G: Granular layer. Scale bar525mm (A); 50
in regions of severe neuronal damage [15]. Similar results Acknowledgements have been obtained following photochemical brain injury
[18]. Thus, p53 induction may be a response to a variety of This study was supported by Seoul National University insults that result in permanent neuronal loss. Using the Hospital Research Fund (800-20000239). This study was transgenic mice expressing a p53 null allele, Crumrine et supported in part by year 2000 BK21 project for Medicine, al. [3] has shown that loss of p53 affords relative protec- Dentistry and Pharmacy.
tion against focal ischemic neuronal damage. In addition, cerebellar granule cells in p53 null mice are resistant to
radiation-induced apoptosis [26]. Furthermore, the absence References or suppression of p53 expression has been shown to
protect cultured neurons from excitotoxin-mediated [27] [1] C. Barlow, P.A. Dennery, M.K. Shigenaga, M.A. Smith, J.D. Morrow, L.J. Roberts 2nd, A. Wynshaw-Boris, R.L. Levine, Loss of and spontaneous cell death [5]. Conversely, overexpression
the ataxia–telangiectasia gene product causes oxidative damage in of p53, mediated by adenoviral gene delivery, induced
target organs, Proc. Natl. Acad. Sci. USA 96 (1999) 9915–9919. neuronal cell death with features characteristic of apoptosis
[2] F. Colotta, N. Polentarutti, M. Sironi, A. Mantovani, Expression and [10]. These results indicate that p53 induction is a marker involvement of c-fos and c-jun protooncogenes in programmed cell of neuronal apoptosis and suggest that the p53 protein is death induced by growth factor deprivation in lymphoid cell lines, J. actively involved in the death pathway. Biol. Chem. 267 (1992) 18278–18283.
[3] R.C. Crumrine, A.L. Thomas, P.F. Morgan, Attenuation of p53 One hypothesis of aging is that it is a consequence of
expression protects against focal ischemic damage in transgenic the accumulation of oxidative damage to cells, an
inevit-mice, J. Cereb. Blood Flow Metab. 14 (1994) 887–891.
able consequence of leakage of reactive oxygen species [4] S.M. de la Monte, Y.K. Sohn, N. Ganju, J.R. Wands, P53- and during normal metabolism. Several reports indicate that CD95-associated apoptosis in neurodegenerative diseases, Lab In-p53 regulates sensitivity to oxidative damage in CNS vest. 78 (1998) 401–411.
[5] O. Eizenberg, A. Faber–Elman, E. Gottlieb, M. Oren, V. Rotter, M. neurons [26,27] and cerebellar Purkinje cells are highly
Schwartz, p53 plays a regulatory role in differentiation and apop-sensitive to oxidative stress [1,19]. So up-regulation of p53
tosis of central nervous system-associated cells, Mol. Cell Biol. 16 in the Purkinje cells observed in this study suggests that (1996) 5178–5185.
significant loss of Purkinje cells with aging may be [6] R.S. Freeman, S. Estus, E.M. Johnson, Analysis of cell cycle-related regulated with several apoptosis-controlling factors includ- gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death, Neuron 12 (1994) 343– ing p53 and oxidative stress mechanism. Recently,
Ina-355. mura et al. [9] reported increased number of p53-positive
[7] P.A. Hall, P.H. McKee, H.D. Menage, R. Dover, D.P. Lane, High neurons in the Purkinje cell layer using organotypic slice levels of p53 protein in UV-irradiated normal human skin, Oncogene culture exposed to bleomycin and indicated that p53 is 8 (1993) 203–207.
involved in DNA strand break-induced apoptosis of fully [8] J. Ham, C. Babij, J. Whitfield, C.M. Pfarr, D. Lallemand, M. Yaniv, L.L. Rubin, A c-Jun dominant negative mutant protects sympathetic postmitotic central nervous system neurons.
neurons against programmed cell death, Neuron 14 (1995) 927–939. Apoptotic-like cell death has been described in
associa-[9] N. Inamura, T. Araki, Y. Enokido, C. Nishio, S. Aizawa, H. tion with various human neurodegenerative disorders such Hatanaka, Role of p53 in DNA strand break-induced apoptosis in as amyotrophic lateral sclerosis, Parkinson’s disease, Al- organotypic slice culture from the mouse cerebellum, J Neurosci. zheimer’s disease and Huntington’s disease [4,25]. Recent- Res. 60 (2000) 450–457.
[10] J. Jordan, M.F. Galindo, J.H. Prehn, R.R. Weichselbaum, M. ly, two laboratories reported that p53 is involved in the
Beckett, G.D. Ghadge, R.P. Roos, J.M. Leiden, R.J. Miller, p53 pathogenesis of Alzheimer’s disease. La Ferla et al. [12]
expression induces apoptosis in hippocampal pyramidal neuron have derived transgenic mice with intracellular expression cultures, J. Neurosci. 17 (1997) 1397–1405.
of Abspecifically in neurons with the hope of defining the [11] Y. Kitamura, S. Shimohama, W. Kamoshima, Y. Matsuoka, Y. successive steps, which may reflect Alzheimer’s disease Nomura, T. Taniguchi, Changes of p53 in the brains of patients with Alzheimer’s disease, Biochem. Biophys. Res. Comm. 232 (1997) pathogenesis. Interestingly, they found that Ab can
acti-418–421. vate the p53 dependent apoptotic pathway and that
ex-[12] F.M. La Ferla, C.K. Hall, L. Ngo, G. Jay, Extracellular deposition of tracellular deposition of Ab occurs secondary to neuronal beta-amyloid upon p53-dependent neuronal cell death in transgenic cell death. Another indirect evidence supporting the in- mice, J. Clin. Invest. 98 (1996) 1626–1632.
volvement of the p53 in the pathogenesis of Alzheimer’s [13] D.P. Lane, Cancer. p53, guardian of the genome, Nature 358 (1992) 15–16.
disease is the report of Kitamura et al. [11]. They
[14] A.J. Levine, p53, the cellular gatekeeper for growth and division, measured the level of p53 in the temporal cortices in eight
Cell 88 (1997) 323–331.
control and eight Alzheimer’s disease cases, and they [15] Y. Li, M. Chopp, Z.G. Zhang, C. Zaloga, L. Niewenhuis, S. Gautam, found that the level of p53 was increased approximately p53-IR protein and p53 mRNA expression after transient middle two-fold in the Alzheimer’s disease brain compared to cerebral artery occlusion in rats, Stroke 25 (1994) 849–856.
[16] S.W. Lowe, H.E. Ruley, T. Jacks, D.E. Housman, p53-dependent control brains.
apoptosis modulates the cytotoxicity of anticancer agents, Cell 74 Further investigations are required to establish whether
(1993) 957–967.
[18] H. Manev, A. Kharlamov, D.M. Armstrong, Photochemical brain [23] Y. Shi, J.M. Glynn, L.J. Guilbert, T.G. Cotter, R.P. Bissonnette, D.R. injury in rats triggers DNA fragmentation, p53 and HSP72, Neurore- Green, Role for c-myc in activation-induced apoptotic cell death in port 5 (1994) 2661–2664. T cell hybridomas, Science 257 (1992) 212–214.
[19] K. Nakaso, M. Kitayama, H. Fukuda, K. Kimura, T. Yanagawa, T. [24] R.J. Smeyne, M. Vendrell, M. Hayward, S.J. Baker, G.G. Miao, K. Ishii, K. Nakashima, K. Yamada, Oxidative stress-related proteins Schilling, L.M. Robertson, T. Curran, J.I. Morgan, Continuous c-fos A170 and heme oxygenase-1 are differently induced in the rat expression precedes programmed cell death in vivo, Nature 363 cerebellum under kainate-mediated excitotoxicity, Neurosci. Lett. (1993) 166–169.
282 (2000) 57–60. [25] C.B. Thompson, Apoptosis in the pathogenesis and treatment of [20] J. Rogers, S.F. Zornetzer, F.E. Bloom, R.E. Mervis, Senescent disease, Science 267 (1995) 1456–1462.
microstructural changes in rat cerebellum, Brain Res. 292 (1984) [26] K. Wood, R.J. Youle, The role of free radicals and p53 in neuron
23–32. apoptosis in vivo, J. Neurosci. 15 (1995) 5851–5857.
[21] J.J. Ryan, R. Danish, C.A. Gottlieb, M.F. Clarke, Cell cycle analysis [27] H. Xiang, D.W. Hochman, H. Saya, T. Fujiwara, P.A. Schwartzkroin, of p53-induced cell death in murine erythroleukemia cells, Mol. Cell R.S. Morrison, Evidence for p53-mediated modulation of neuronal Biol. 13 (1993) 711–719. viability, J. Neurosci. 16 (1996) 6753–6765.