www.elsevier.com / locate / bres
Research report
Changes in electrophysiological properties of cat hypoglossal
motoneurons during carbachol-induced motor inhibition
*
Simon J. Fung , Jack Yamuy, Ming-Chu Xi, John K. Engelhardt, Francisco R. Morales,
Michael H. Chase
Department of Physiology and the Brain Research Institute, UCLA School of Medicine, 53-263 CHS, Los Angeles, CA 90095, USA Accepted 12 September 2000
Abstract
The control of hypoglossal motoneurons during sleep is important from a basic science perspective as well as to understand the bases for pharyngeal occlusion which results in the obstructive sleep apnea syndrome. In the present work, we used intracellular recording techniques to determine changes in membrane properties in adult cats in which atonia was produced by the injection of carbachol into the pontine tegmentum (AS-carbachol). During AS-carbachol, 86% of the recorded hypoglossal motoneurons were found to be postsynapti-cally inhibited on the basis of analyses of their electrical properties; the electrical properties of the remaining 14% were similar to motoneurons recorded during control conditions. Those cells that exhibited changes in their electrical properties during AS-carbachol also displayed large-amplitude inhibitory synaptic potentials. Following sciatic nerve stimulation, hypoglossal motoneurons which responded with a depolarizing potential during control conditions exhibited a hyperpolarizing potential during AS-carbachol. Both spontaneous and evoked inhibitory potentials recorded during AS-carbachol were comparable to those that have been previously observed in trigeminal and spinal cord motoneurons under similar experimental conditions as well as during naturally occurring active sleep. Calculations based on modeling the changes that we found in input resistance and membrane time constant with a three-compartment neuron model suggest that shunts are present in all three compartments of the hypoglossal motoneuron model. Taken together, these data indicate that postsynaptic inhibitory drives are widely distributed on the soma-dendritic tree of hypoglossal motoneurons during AS-carbachol. These postsynaptic inhibitory actions are likely to be involved in the pathophysiology of obstructive sleep apnea. 2000 Elsevier Science B.V. All rights reserved.
Theme: Motor systems and sensorimotor integration
Topic: Spinal cord and brainstem
Keywords: Postsynaptic inhibition; Dendritic inhibition; REM sleep; Apnea
1. Introduction obstructive sleep apnea syndrome [32]. In an attempt to understand the mechanism responsible for the reduction in Obstructive sleep apnea occurs because of a narrowing motoneuron activity during AS, hypoglossal nerve record-of the upper airway which is due, in part, to a decrease in ings were obtained during the suppression of motoneuron the activity of the tongue and laryngeal dilator muscles; activity that occurs following the pontine injection of this sleep disorder arises during quiet (NREM) sleep and carbachol; it was concluded that the activity of hypoglossal becomes exacerbated during active sleep (AS; also called motoneurons is reduced under these conditions [17,20]. It REM sleep) [23]. Electromyographic studies suggest that has been hypothesized that a disfacilitory mechanism is hypoglossal motoneurons innervating the tongue muscles responsible for the carbachol-induced suppression in hypo-are less active during AS than during quiet sleep in both glossal nerve activity because serotonin levels were found healthy subjects [33] and in patients diagnosed with to be reduced in this motor nucleus [21] and the pre-motor serotonergic raphe neurons also reduced their firing under these conditions [41]. However, when the endogenous *Corresponding author. Tel.:11-310-825-3348.
E-mail address: sjfung@ucla.edu (S.J. Fung). effects of serotonin are blocked, there is still suppression
of hypoglossal motoneurons [22]. Therefore, other pro- Halothane was then discontinued and the anesthesia was cesses, specifically postsynaptic inhibition, must play a key maintained usinga-chloralose (initial dose: 60 mg / kg, i.v., role in the suppression of hypoglossal motoneurons during supplemental dose: 20 mg / kg, i.v.) throughout the rest of
AS-carbachol and consequently may also be involved in the experiment. Gallamine triethiodide (Flaxedil , 1–2 the pathophysiology of the obstructive sleep apnea mg / kg, i.v.) was employed to improve stability during
syndrome. intracellular recording. Arterial blood pressure (mean5120
The presence of AS-specific glycinergic postsynaptic mm Hg), end-tidal carbon dioxide (3.5–5%) and core inhibition in trigeminal and spinal motoneurons has been temperature (37.5–38.58C) were monitored continuously. shown in intracellular studies carried out in
chronically-implanted, freely-behaving cats [8,24,29], which explains 2.2. Recording, stimulation and data analysis the suppression of the somatomotor reflex during AS
[7,15]. In addition postsynaptic inhibition of somatic Intracellular recordings from hypoglossal motoneurons motoneurons (with concurrent motor suppression) has been were obtained using glass microelectrodes filled with a observed in a pharmacological (cholinergic) model of AS solution of either 3 M KCl or 2 M K-citrate; tip resistance following carbachol microinjection in the nucleus pontis ranged between 10 and 35 MV. Intracellular current oralis (NPO) in both decerebrate [27] and a-chloralose injections were carried out through the recording mi-anesthetized cats [19,42]. We have designated this active croelectrode by using an Axoclamp 2A preamplifier (Axon sleep-like state as AS-carbachol [44]. Instruments, Inc.) operating in current clamp mode. The In a preliminary report based upon intracellular record- reference electrode consisted of an Ag /AgCl wire that was ing techniques, we presented an overview of changes in wrapped with saline-soaked gauze and placed subcuta-membrane properties of hypoglossal motoneurons during neously. The low-gain (103) and high-gain (1003) DC AS-carbachol in decerebrate anda-chloralose anesthetized outputs of the preamplifier were monitored on an oscillos-cats [43]. In the present work, we present complete, cope and stored on a video cassette recorder using a PCM rigorous analyses of these data within the framework of adapter. The intracellular data were digitized off-line at 10 linear cable theory using a three-compartment electrical ms / bin for action potentials and at 50ms / bin for signals network neuron model. These data lead us to conclude that used to measure other membrane properties.
hypoglossal motoneurons are subjected to postsynaptic Hypoglossal motoneurons were identified by antidromic inhibition during AS-carbachol and that these inhibitory activation via stimulating either the ipsilateral hypoglossal processes are directed both to the soma and dendrites of nerve or its branches (single rectangular pulses, 150 ms hypoglossal motoneurons. duration, 2–7 V intensity). The antidromic spike amplitude was measured from the baseline membrane potential to the peak of the spike. The resting membrane potential was 2. Materials and methods obtained by subtracting the DC potential of the cell from the extracellular potential registered upon withdrawal of 2.1. Surgical preparation the electrode from the cell.
To measure the afterhyperpolarization potential (AHP), All experimental procedures were conducted in accord direct action potentials were evoked by intracellular stimu-with the Guide to the Care and Use of Laboratory Animals lation with a 500 ms depolarizing current pulse that was (7th edition, National Academy Press, Washington, DC, injected through the recording microelectrode. The AHP 1996) and approved by the Animal Research Committee of amplitude was measured from the negative peak to the the UCLA Office for the Protection of Research Subjects. baseline membrane potential preceding the stimulus. The Experiments were performed on 18 adult cats according time course of the AHP was evaluated using the AHP to procedures previously described in detail [43]. Briefly, half-width (i.e., its duration at half maximal amplitude). under halothane anesthesia (4%), tracheal intubation and The rheobase was defined as the current necessary to catheterization of the right carotid artery and external discharge an action potential in at least 90% of the trials jugular vein were performed. In order to antidromically with a 50 ms depolarizing current pulse at a repetition rate identify hypoglossal motoneurons, bipolar stimulating cuff of 1 / s. The motoneuron input resistance (R ) was mea-in electrodes were implanted bilaterally around the hypo- sured using low intensity hyperpolarizing (or depolarizing) glossal nerve in nine cats. In the other nine cats, stimulat- current pulses (0.5–4.0 nA, 50–100 ms). The Rin was ing cuff electrodes were implanted on the medial and calculated by dividing the maximal voltage drop by the lateral nerve branches of the hypoglossal nerve, which magnitude of the current injected (‘‘direct method’’) innervate the tongue protruder and retractor muscles, [1,27].
con-stant of the slow process (ta, see Ref. [13] for details), the hypoglossal motoneuron was developed. Anatomical mea-membrane time constant (tb), and the first equalizing time surements of horseradish peroxidase-filled hypoglossal constant (tc) were obtained from the negative reciprocal of motoneurons in the cat indicate that the ratio of the soma the slopes of the linear portions of the semilogarithmic membrane to total membrane area per neuron is approxi-plots of voltage against time [12,27,43,45]. mately 2.5% [14]. We therefore designed a model in which In 12 cats, sciatic nerve stimulation (single rectangular the soma compartment contained 2.5% of the total cell pulses, 150 ms duration, 0.5–0.7 Hz) was employed to capacitance with the remaining 97.5% divided equally evoke postsynaptic potentials in hypoglossal motoneurons between two (proximal and distal) dendritic compartments. during the control and AS-carbachol states [43]. In addi- In addition to these anatomical constraints, we required tion, spinal monosynaptic responses to the sciatic nerve that the passive electrical properties of this model be stimulus were recorded using a second pair of electrodes uniform under control conditions and that the model located 5 mm distal to the stimulating electrodes. Multiple exhibits the average values for R ,in tb and tc that were samples (n530–100 for AHP, Rin and time constants; observed under control conditions. Accordingly, we were
n58–36 for sciatic evoked postsynaptic and spinal reflex able to calculate the values for the lumped electrical responses) of the voltage responses to current pulses and elements in our three-compartment model that are listed in sciatic stimulation were averaged. the legend of Fig. 7. The effect of AS-carbachol in this In order to induce motor suppression comparable to the model was evaluated by reducing the electrical resistance atonia of AS, carbachol (1 mg in 0.25 ml saline) was in one or more of the compartments. The resistances were injected into the NPO (P3 L2 H-3.5; [4]) contralateral to reduced only to the degree necessary to produce the 17.5% the impaled hypoglossal motoneuron [19,42]. reduction in the average Rin that was observed in real For statistical evaluation of the membrane properties hypoglossal motoneurons during AS-carbachol (see Re-during AS-carbachol (see Table 1), a temporal window (3 sults). These new values of electrical resistance, presented h post-injection based on prior studies using this prepara- in Table 2, were then used to calculate theoretical values tion [19,42]) was adopted for cells scored under the AS- for tb. These time constants were determined using La-carbachol condition. The statistical significance of differ- place transforms to solve the three simultaneous differen-ences between the mean values of the measured membrane tial equations that describe this three-compartment model properties pre- vs. post-carbachol administration was [10].
evaluated using the unpaired, two-tailed Student’s t-test and the Mann–Whitney U-test. Statistical significance was
set at P,0.05. The P-values reported in this study are 3. Results derived from the Student’s t-test.
For the analysis of the frequency of inhibitory synaptic Data were collected from 115 motoneurons in which potentials, only cells recorded with citrate-filled microelec- antidromic action potentials were recorded following trodes were used because these inhibitory potentials are stimulation of the (a) hypoglossal nerve trunk (hypoglossal chloride and voltage dependent [9,26,27,43]. Data seg- motoneurons, n568), (b) medial branch of the hypoglossal ments (10 s duration) of high-gain records of the mem- nerve (protruder motoneurons, n524), and (c) lateral brane potential were digitized (50 ms bin width) and branch of the hypoglossal nerve (retractor motoneurons, analyzed off-line, as documented previously [9,25,27,42]. n523). The antidromic spike amplitudes were 60 mV or In order to investigate the location of the membrane greater throughout the period in which membrane prop-conductance changes, a three-compartment model of the erties were measured (Table 1). No statistically significant
Table 1
a
Changes in electrophysiological properties of hypoglossal motoneurons
Control AS-Carbachol
Resting membrane potential, mV 263.160.8 (65) 263.461.1 (38)
AP amplitude, mV 73.460.8 (73) 72.361.0 (42)
Table 2
a
Simulation of electrophysiological data using a three-compartment model of hypoglossal motoneurons
Control Location of shunts
Soma Soma Proximal Soma Proximal Distal
1 1 1
Proximal Proximal Distal
1
Distal
† † †
R (M1 V) 189.10 30.91 118.38 189.10 134.40 189.10 189.10
† † † †
R (M2 V) 9.46 9.46 5.92 5.60 6.72 6.53 9.46
† † †
R (M3 V) 9.46 9.46 9.46 9.46 6.72 6.53 3.63
Rin(MV) 7.88 6.50 6.50 6.50 6.50 6.50 6.50
tb(ms) 6.96 6.24 5.39 5.16 4.95 4.85 4.11
%DRin
]] – 1.69 0.78 0.68 0.61 0.58 0.43
%Dtb
a †
The resistance values as indicated by in one or more of the soma and dendritic compartments of this model are those required to simulate the observed changes in Rin during AS-carbachol. Note that the last column contains the closest match to the observed changes in both Rinandtb. See Methods and legend of Fig. 7 for calculation of individual values in this Table.
differences in membrane properties were found between consistent with those observed when cells that were protruder, retractor, and undifferentiated hypoglossal recorded independently during AS-carbachol and control motoneurons during control and AS-carbachol states. conditions (see below).
Therefore, data from all motoneurons recorded under the
same condition were pooled and considered as a general 3.1. Inhibitory postsynaptic potentials (IPSPs) in population of hypoglossal motoneurons for the present hypoglossal motoneurons
analysis. A total of 73 (40 undifferentiated, 14 protruder,
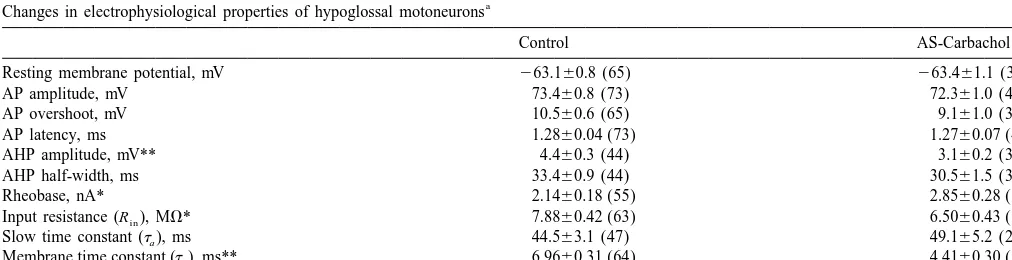
19 retractor) motoneurons were recorded during control Under control conditions, there were only small-am-(pre-drug) conditions, whereas 42 (28 undifferentiated, 10 plitude fluctuations in the membrane potential of hypo-protruder, 4 retractor) were recorded during the 3 h period glossal motoneurons, as illustrated in Fig. 1A; IPSP noise of AS-carbachol. Two motoneurons were recorded con- occurred rarely (mean frequency50.1360.03 Hz, range5
tinuously during both control conditions and during AS- 0–0.6 Hz, n541 cells; Fig. 1C). In addition, sciatic stimuli carbachol; they exhibited changes in membrane properties elicited a depolarizing potential (Fig. 1D; n521 cells).
During AS-carbachol, hypoglossal motoneurons could spike during AS-carbachol (Table 1). The mean antidromic be divided into two populations based upon the presence or latency and the mean resting membrane potential of absence of the following effects. A majority (86%, n536 hypoglossal motoneurons were also similar during the two cells) of the hypoglossal motoneurons exhibited (a) bar- conditions (Table 1).
rages of large-amplitude (approximately 1 mV) hyper- The following changes in electrophysiological properties polarizing synaptic potentials (Fig. 1B, asterisks) that were observed in motoneurons that exhibited spontaneous, occurred at a mean frequency of 5.5961.03 Hz (range5 and / or sciatic evoked IPSPs during AS-carbachol. The two 0.6–23.7 Hz, n533 cells; Fig. 1C) and / or (b) sciatic nerve cells that were recorded continuously also exhibited similar stimulus-evoked IPSPs similar to those that are present in changes in these membrane properties during AS-carbach-masseter motoneurons during AS-carbachol (Fig. 1E, n5 ol.
13 cells [18]). The occurrence of such hyperpolarizing
potentials (at a longer delay than control depolarizing 3.2.2. Afterhyperpolarization potential (AHP)
potentials) during AS-carbachol is consistent with the There was a 29.6% reduction in the mean AHP am-previously documented phenomenon termed ‘‘reticular plitude during AS-carbachol (P,0.01; Table 1). Fig. 2C response-reversal’’ [7,18]. The remaining motoneurons presents a small-sized AHP in this representative hypo-(14%, n56) did not show any effect as judged by the glossal motoneuron during AS-carbachol, compared with absence of hyperpolarizing potentials and a lack of hy- the large-sized AHP observed in another motoneuron perpolarizing response to sciatic stimulation; in addition, recorded during control conditions (Fig. 2A). Compared to there were no significant difference in membrane prop- control conditions (Fig. 2B), there was a shift to the left of erties between this population of cells and control cells. the frequency distribution of AHP amplitudes recorded from motoneurons during AS-carbachol (Fig. 2D); in 3.2. Electrophysiological properties of hypoglossal addition, the time course of the AHP, as assessed by its
motoneurons half-width, did not exhibit any change. Since the mem-brane potential was unaffected during AS-carbachol, the 3.2.1. Antidromic spike and resting membrane potential 29.6% decrement in mean AHP amplitude cannot be due to Compared to control conditions, no significant changes proximity to the reversal potential for the ionic current were seen in the amplitude and overshoot of the antidromic responsible for the AHP. Other factors (e.g., changes in the
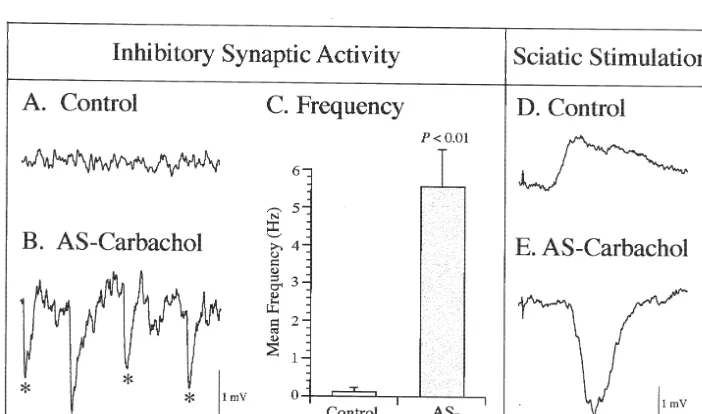
Fig. 3. Frequency distribution of rheobase measured under control (A; n555 cells) and AS-carbachol (B; n530 cells) conditions. The arrows represent the mean values of each population (2.14 and 2.85 nA for cells during control and AS-carbachol conditions, respectively).
cell R ) must be involved in the decrement of AHPin indicates that there is a clear reduction in the excitability of amplitude (see Discussion). motoneurons during AS-carbachol. Since the mean resting potential did not change during AS-carbachol (Table 1), 3.2.3. Rheobase the observed 33.2% increase in rheobase is probably due, The data presented in Table 1 and the frequency in part, to a decrease in cell Rinproduced by an increase in distribution plots in Fig. 3 demonstrate that there was a membrane conductance (see below).
significant increase in rheobase between motoneurons
during AS-carbachol compared with control conditions 3.2.4. Input resistance (R )IN
(33.2%, P,0.01; Table 1). This change in rheobase Compared to control conditions (Fig. 4B), cells during
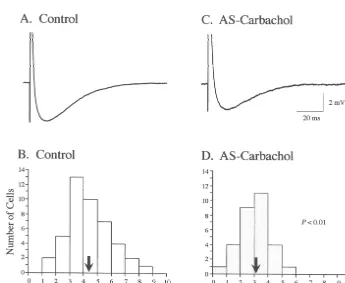
AS-carbachol (Fig. 4D) exhibited a 17.5% reduction in the motoneuron during AS-carbachol. Overall, as shown in mean Rin (P,0.05; Table 1). Fig. 4C illustrates the low Fig. 6A and B, the shift to the left of the frequency
Rin measurement from a typical hypoglossal motoneuron distribution of tb measurements from the AS-carbachol during AS-carbachol, compared to the high Rin from group results in a 36.6% reduction in meantb, compared to another motoneuron under control conditions (Fig. 4A). that measured under control conditions (P,0.01; Table 1). Based upon our previous findings that the threshold This result suggests that there is an increase in the voltage is constant [43], the 17.5% decrease in mean Rin membrane conductance of these cells during AS-carbachol can account for 21.2%, or approximately two-thirds, of the [30].
33.2% increase in the rheobase that was observed during The bottom panels of Fig. 6 illustrate the frequency
AS-carbachol. distribution oftc measurements from motoneurons during
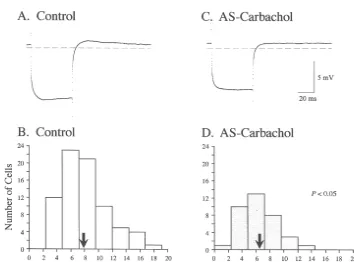
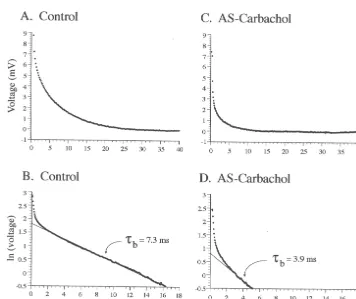
control (Fig. 6C) and AS-carbachol (Fig. 6D) states. These 3.2.5. Time constants measurements were made after the tb component was The top two traces in Fig. 5 illustrate the passive voltage ‘‘peeled’’ away from voltage decay responses (for details decay that results after the exponential process responsible see Ref. [12]). There was a 35.9% decrease in the meantc
for sag (theta process) is subtracted from the raw voltage (P,0.01; Table 1), which is similar to the 36.6% decrease response of two representative motoneurons recorded observed in meantb during AS-carbachol. The changes in under control (Fig. 5A) and AS-carbachol (Fig. 5C) tc indicate an increased rate of redistribution of charge conditions, respectively. Upon plotting these corrected along the somatodendritic tree [30] that could contribute to voltage decay responses using semilogarithmic coordi- the observed increase in the conductance of hypoglossal nates, a regression line was fitted to the linear portion of motoneurons during AS-carbachol.
the plots, as shown in Fig. 5B and D. The negative
reciprocal of the slope of these linear regression lines yield 3.3. Changes in Rin andtb in a model hypoglossal
thetb measurements for these representative motoneurons motoneuron
under control (Fig. 5B) and AS-carbachol (Fig. 5D)
conditions. Note the shortened tb exhibited by the The changes in Rin and tb that resulted from placing
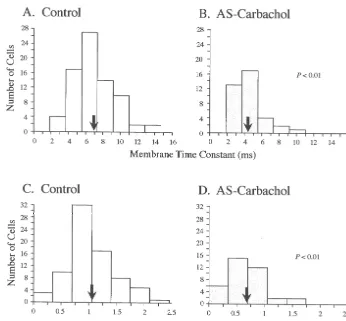
Fig. 6. Decrease in membrane time constant (tb) and equalizing time constant (tc) during AS-carbachol. Time constant analyses were based upon 64 and 37 hypoglossal motoneurons under control (A,C) and AS-carbachol (B,D) conditions, respectively. Top (A,B) and bottom (C,D) panels are frequency distributions oftb and tc, respectively. Comparison between the means (arrows; control tb 6.96 ms, AS-carbacholtb 4.41 ms; control tc 1.06 ms, AS-carbacholtc0.68 ms) indicates that there was a significant reduction in both time constants during AS-carbachol.
shunts in one or more of the compartments of this model (Fig. 7) are shown in Table 2. The data are presented, from left to right, in order of decreasing ratio of the percentage of change in Rin to the percentage of change in tb (this ratio is presented in the bottom row of Table 2). A decrease in this ratio indicates that dendritic compartments make a greater contribution to changes in conductance. The closest match to the observed changes in Rin andtbof hypoglossal motoneurons occurs when all of the conduct-ance change is located in the distal dendritic compartment.
4. Discussion
In the present study, data obtained from a number of Fig. 7. Diagram illustrating the three-compartment model of the hypo- complementary experiments suggest that hypoglossal glossal motoneuron. The model was developed according with the
motoneurons are postsynaptically inhibited during AS-following considerations: (1) that the soma compartment contained 2.5%
carbachol, and by extension, during the atonia of naturally of the total cell capacitance, (2) that the remaining 97.5% capacitance
was divided equally between the two dendritic compartments, (3) that the occurring AS. Hypoglossal motoneurons that were re-passive electrical properties were uniform under control condition; and, corded during AS-carbachol exhibited IPSPs (spontaneous (4) that the mean values of R ,in tb and tc that were observed in real and evoked) and became less excitable than those recorded motoneurons were recreated by modeling. Accordingly, the following
during control conditions, as indicated by their increased values were obtained for the electrical elements of the modelled
rheobase. The data also demonstrate a significant increase motoneuron under control conditions: Q15Q252.88 MV, R15189.10,
indicated by the reduced R ,in tb and tc [30] during AS- cant change in rheobase or in the other electrophysiologi-carbachol. The two-fold larger percentage change in tb cal properties which were measured during AS-carbachol. (36.6%) compared with the percentage change in Rin Therefore, it is possible that during AS-carbachol not all (17.5%) during AS-carbachol is consistent with our model- hypoglossal motoneurons are subjected to a uniform ing results, which indicate that there are distributed pattern of postsynaptic inhibition; we are exploring this changes in the membrane conductance of hypoglossal possibility in studies that are currently being conducted.
motoneurons. Multiple factors could account for the increase in
According to the theoretical work of Carlen and Durand membrane conductance in the present study. The observed [5], a reduction in Rin reliably reflects a conductance bombardment of hypoglossal motoneurons by IPSPs can increase that occurs mainly on the soma and proximal cause an increase in membrane conductance during AS-dendrites. Their model also predicts that a reduction intb carbachol. Because IPSP activity during AS-carbachol is reflects the magnitude of the increase in membrane con- chloride-dependent and strychnine-sensitive [43], and is ductance regardless of the location (proximal and distal also similar to the AS-specific IPSPs that are present in from the intrasomatic point of current injection) of such a lumbar and trigeminal motoneurons [9,29,34], it is likely conductance increase. Experimentally, disproportionate that a glycine receptor-mediated postsynaptic inhibitory changes intband R , similar to the magnitude observed inin mechanism is engaged by AS-specific premotor inter-the present study, have been reported in rat lumbar neurons to produce the inhibition observed in hypoglossal sympathetic ganglion cells in vitro [31]. According to motoneurons during this state. This is in accord with Redman et al. [31], such disproportionate changes in tb findings, using ultrastructural techniques, of glycinergic and Rin can be explained by modification in the resting boutons in apposition to all compartments of the spinal potassium conductance at distal dendritic sites. In the motoneuron membrane, including somatic, proximal and present study, since the percentage changes in tb and tc distal dendrites [28,38].
exceeded the percentage change in R , conductancein The observed increase in membrane conductance might increases during AS-carbachol are likely to occur at distal also be due partially to the withdrawal of excitatory dendritic sites. This conclusion is supported by our addi- serotonergic drive to hypoglossal motoneurons during AS-tional calculations based on a three-compartment model of carbachol. Serotonin is known to excite motoneurons and hypoglossal motoneurons. decrease their membrane conductance in vivo [39,40] and The ratio of the percentage of change in Rin to per- in vitro [3,16,35]. It is possible that the withdrawal of this centage of change intb is 1.1 for spinal cord motoneurons excitatory drive, as evidenced during AS [36] and AS-[27,42] and 1.2 for masseter motoneurons [19]. Using the carbachol [41], could increase the membrane conductance results from our three-compartment model that are pre- of the motoneuron. While we cannot rule out the possi-sented in Table 2, these ratios suggest that conductance bility of a presynaptic contribution to the cessation of changes in the soma and proximal dendritic compartments hypoglossal motoneuron activity during AS-carbachol, our are responsible for the Rin and tb changes that were data shows that a majority of hypoglossal motoneurons are observed in these motoneurons. This result is in agreement clearly inhibited by postsynaptic processes during this with previous conclusions that were based on the reversal state. In addition, a process of disfacilitation is difficult to of AS-dependent IPSPs following either the injection of reconcile with the carbachol-induced decrease in AHP chloride ions or the injection of hyperpolarizing current amplitude that was observed in the present study. In fact, [11,26]. In hypoglossal motoneurons, the ratio of per- our observation is the exact opposite to that expected had centage change in R / percentage change inin tbwas 0.48. A serotonin withdrawal been a dominant factor involved in ratio on this order of magnitude could only be obtained in the change in electrical properties [2,3,16,35,40]. On the our model simulations when there is a significant shunt other hand, the observed changes in rheobase, R ,in tb, and present in the distal dendritic compartment. tc, together with spontaneous and evoked IPSPs, are all The fact that we were able to observe individual IPSPs consistent with the presence of a postsynaptic inhibitory in most hypoglossal motoneurons during AS-carbachol mechanism during AS-carbachol. Furthermore, changes in suggests that some inhibitory synapses are located elec- these membrane activities and electrophysiological prop-trotonically close to the recording electrode, presumably erties accord with those present in all somatic motoneurons on the soma or proximal dendrites. This fact, together with that have been studied during naturally occurring AS the small differences between the predicted and observed [8,24,29] as well as those examined during AS-carbachol values of the tb whenever shunts are present in both [19,27,42].
con-[16] C.F. Hsiao, P.R. Trueblood, M.S. Levine, S.H. Chandler, Multiple ductance in both somatic and dendritic membrane
compart-effects of serotonin on membrane properties of trigeminal ments suggests that postsynaptic inhibition is widely
motoneurons in vitro, J. Neurophysiol. 77 (1997) 2910–2924. distributed on the soma-dendritic tree of hypoglossal [17] H. Kimura, L. Kubin, R.O. Davies, A.I. Pack, Cholinergic stimula-motoneurons. Thus, we suggest that hypoglossal tion of the pons depresses respiration in decerebrate cats, J. Appl. motoneurons, as well as spinal and trigeminal motoneurons Physiol. 69 (1990) 2280–2289.
[18] K.A. Kohlmeier, F. Lopez-Rodriguez, M.H. Chase, Strychnine [6,24], are postsynaptically inhibited during naturally
blocks inhibitory postsynaptic potentials elicited in masseter occurring AS.
motoneurons by sensory stimuli during carbachol-induced motor atonia, Neuroscience 78 (1997) 1195–1202.
[19] K.A. Kohlmeier, F. Lopez-Rodriguez, R.H. Liu, F.R. Morales, M.H. Chase, State-dependent phenomena in cat masseter motoneurons, Acknowledgements
Brain Res. 722 (1996) 30–38.
[20] L. Kubin, H. Kimura, H. Tojima, R.O. Davies, A.I. Pack, Suppres-These studies were supported by USPHS grants sion of hypoglossal motoneurons during the carbachol-induced MH43362, NS23426, NS09999 and HL60296. atonia of REM sleep is not caused by fast synaptic inhibition, Brain
Res. 611 (1993) 300–312.
[21] L. Kubin, C. Reignier, H. Tojima, O. Taguchi, A.I. Pack, R.O. Davies, Changes in serotonin level in the hypoglossal nucleus region References during carbachol-induced atonia, Brain Res. 645 (1994) 291–302.
[22] L. Kubin, H. Tojima, C. Reignier, A.I. Pack, R.O. Davies, Inter-[1] J.N. Barrett, W.E. Crill, Specific membrane properties of cat action of serotonergic excitatory drive to hypoglossal motoneurons motoneurones, J. Physiol. (Lond.) 239 (1974) 301–324. with carbachol-induced REM sleep-like atonia, Sleep 19 (1996)
187–195. [2] D.A. Bayliss, M. Umemiya, A.J. Berger, Inhibition of N- and P-type
calcium currents and the after-hyperpolarization in rat motoneurones [23] E. Lugaresi, F. Cirignotta, P. Montagna, E. Sforza, Snoring: patho-by serotonin, J. Physiol. (Lond.) 485 (1995) 635–647. genic, clinical, and therapeutic aspects, in: M.H. Kryger, T. Roth, W.C. Dement (Eds.), Principles and Practice of Sleep Medicine, [3] D.A. Bayliss, F. Viana, E.M. Talley, A.J. Berger, Neuromodulation
W.B. Saunders, Philadelphia, 1994, Chapter 61. of hypoglossal motoneurons: cellular and developmental
mecha-nisms, Respir. Physiol. 110 (1997) 139–150. [24] F. Morales, M.H. Chase, Postsynaptic control of lumbar motoneuron [4] A.L. Berman, The Brainstem of the Cat, A Cytoarchitectonic Atlas excitability during active sleep in the chronic cat, Brain Res. 225
with Stereotaxic Coordinates, University of Wisconsin Press, (1981) 279–295.
Madison, 1968. [25] F.R. Morales, P.A. Boxer, J.P. Jervey, M.H. Chase, A computerized [5] P.L. Carlen, D. Durand, Modelling the postsynaptic location and system for the detection and analysis of spontaneously occurring
magnitude of tonic conductance changes resulting from neuro- synaptic potentials, J. Neurosci. Meth. 13 (1985) 19–35.
transmitters or drugs, Neuroscience 6 (1981) 839–846. [26] F.R. Morales, M.H. Chase, Repetitive synaptic potentials respon-[6] S.H. Chandler, M.H. Chase, Y. Nakamura, Intracellular analysis of sible for inhibition of spinal cord motoneurons during active sleep,
synaptic mechanisms controlling trigeminal motoneuron activity Exp. Neurol. 78 (1982) 471–476.
during sleep and wakefulness, J. Neurophysiol. 44 (1980) 359–371. [27] F.R. Morales, J.K. Engelhardt, P.J. Soja, A.E. Pereda, M.H. Chase, [7] M.H. Chase, M. Babb, Masseteric reflex response to reticular Motoneuron properties during motor inhibition produced by mi-stimulation reverses during active sleep compared with wakefulness croinjection of carbachol into the pontine reticular formation of the or quiet sleep, Brain Res. 59 (1973) 421–426. decerebrate cat, J. Neurophysiol. 57 (1987) 1118–1129.
[8] M.H. Chase, S.H. Chandler, Y. Nakamura, Intracellular determi- [28] G. Ornung, O.P. Ottersen, S. Cullheim, B. Ulfhake, Distribution of nation of membrane potential of trigeminal motoneurons during glutamate-, glycine- and GABA-immunoreactive nerve terminals on sleep and wakefulness, J. Neurophysiol. 44 (1980) 349–358. dendrites in the cat spinal motor nucleus, Exp. Brain Res. 118 [9] M.H. Chase, P.J. Soja, F.R. Morales, Evidence that glycine mediates (1998) 517–532.
the postsynaptic potentials that inhibit lumbar motoneurons during [29] C. Pedroarena, P. Castillo, M.H. Chase, F.R. Morales, The control of the atonia of active sleep, J. Neurosci. 9 (1989) 743–751. jaw-opener motoneurons during active sleep, Brain Res. 653 (1994) [10] C.A. Desoer, E.S. Kuh, in: Basic Circuit Theory, McGraw-Hill, 31–38.
New York, 1969, pp. 527–581. [30] W. Rall, Core Conductor Theory and Cable Properties of Neurons, [11] J.K. Engelhardt, F.R. Morales, P.J. Soja, M.H. Chase, Location on Handbook of Physiology, Amer. Physiol. Soc, Bethesda, 1977.
alpha motoneurons of synapses responsible for spontaneous IPSPs [31] S.J. Redman, E.M. McLachlan, G.D. Hirst, Nonuniform passive occurring during active sleep or carbachol-induced atonia, Bio- membrane properties of rat lumbar sympathetic ganglion cells, J. physics J. 47 (1985) 52a. Neurophysiol. 57 (1987) 633–644.
[12] J.K. Engelhardt, F.R. Morales, P.E. Castillo, C. Pedroarena, I. Pose, [32] J.E. Remmers, W.J. deGroot, E.K. Sauerland, A.M. Anch, Patho-M.H. Chase, Experimental analysis of the method of ‘‘peeling’’ genesis of upper airway occlusion during sleep, J. Appl. Physiol. 44 exponentials for measuring passive electrical properties of mam- (1978) 931–938.
malian motoneurons, Brain Res. 675 (1995) 241–248. [33] E.K. Sauerland, R.M. Harper, The human tongue during sleep: [13] J.K. Engelhardt, F.R. Morales, J. Yamuy, M.H. Chase, Cable electromyographic activity of the genioglossus muscle, Exp. Neurol.
properties of spinal cord motoneurons in adult and aged cats, J. 51 (1976) 160–170.
Neurophysiol. 61 (1989) 194–201. [34] P.J. Soja, F. Lopez-Rodriguez, F.R. Morales, M.H. Chase, The [14] Y. Fukunishi, Y. Nagase, A. Yoshida, M. Moritani, S. Honma, Y. postsynaptic inhibitory control of lumbar motoneurons during the Hirose, Y. Shigenaga, Quantitative analysis of the dendritic architec- atonia of active sleep: effect of strychnine on motoneuron properties, tures of cat hypoglossal motoneurons stained intracellularly with J. Neurosci. 11 (1991) 2804–2811.
cats: correlation with level of behavioral arousal, Brain Res. 163 [42] M.C. Xi, R.H. Liu, J. Yamuy, F.R. Morales, M.H. Chase, Electro-(1979) 135–150. physiological properties of lumbar motoneurons in the alpha-[37] M. Uemura, K. Matsuda, M. Kume, Y. Takeuchi, R. Matsushima, N. chloralose-anesthetized cat during carbachol-induced motor
inhibi-Mizuno, Topographical arrangement of hypoglossal motoneurons: tion, J. Neurophysiol. 78 (1997) 129–136.
an HRP study in the cat, Neurosci. Lett. 13 (1979) 99–104. [43] J. Yamuy, S.J. Fung, M. Xi, F.R. Morales, M.H. Chase, Hypoglossal [38] A.N. van den Pol, T. Gorcs, Glycine and glycine receptor immuno- motoneurons are postsynaptically inhibited during carbachol-in-reactivity in brain and spinal cord, J. Neurosci. 8 (1988) 472–492. duced rapid eye movement sleep, Neuroscience 94 (1999) 11–15. [39] C.P. Vandermaelen, G.K. Aghajanian, Serotonin-induced depolariza- [44] J. Yamuy, J.R. Mancillas, F.R. Morales, M.H. Chase, C-fos
expres-tion of rat facial motoneurons in vivo: comparison with amino acid sion in the pons and medulla of the cat during carbachol-induced transmitters, Brain Res. 239 (1982) 139–152. active sleep, J. Neurosci. 13 (1993) 2703–2718.
[40] S.R. White, S.J. Fung, Serotonin depolarizes cat spinal motoneurons [45] J.E. Zengel, S.A. Reid, G.W. Sypert, J.B. Munson, Membrane in situ and decreases motoneuron afterhyperpolarizing potentials, electrical properties and prediction of motor-unit type of medial Brain Res. 502 (1989) 205–213. gastrocnemius motoneurons in the cat, J. Neurophysiol. 53 (1985) [41] G. Woch, R.O. Davies, A.I. Pack, L. Kubin, Behaviour of raphe cells 1323–1344.