www.elsevier.com / locate / bres
Interactive report
Long-term light history modulates the light response kinetics of
1
luminosity (L)-type horizontal cells in the roach retina
*
A. Jenkins, M.W. Hankins
Imperial College School of Medicine, Division of Neuroscience and Psychological Medicine, Department of Integrative and Molecular Neuroscience, Fulham Palace Road, London W6 8RF, UK
Accepted 17 October 2000
Abstract
We have examined the effects of prolonged periods of darkness on the responses of luminosity-type horizontal cells (L-HCs) in the freshwater cyprinid, Rutilus rutilus. Two groups of retinae were compared, those recorded after 10 min dark adaptation (SA) and those recorded after 3 h dark adaptation (LA). The results suggest that long-term light history does not modify the general responsiveness of the L-HCs in this species. However, there are apparent changes in the receptive field of the cells and modifications to the kinetics of the light-evoked response. The kinetics changes involve both a delay in the onset of light response and a selective effect on the hyperpolarizing light-ON response. Thus the mean time constant (t) for the SA cells was 32.462.39 ms (n562), whilst that for the LA cells was 53.463.03 ms (n561). These effects occur in the absence of changes in the relative spectral sensitivity or threshold sensitivity of the HCs. The results suggest that in some vertebrate retinae, prolonged darkness (light-history) may regulate long-term plasticity in the kinetics of the cone–HC pathway. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Retina and photoreceptors
Keywords: Retina; Horizontal cell; Adaptation; Plasticity
1. Introduction tal cell (HC) receptive field [13,14]. By analogy, this effect
was interpreted in relation to changes in the activity of the There is growing interest in the mechanisms that dopaminergic interplexiform neurones, which have been subserve long-term plasticity of the retinal network. shown to regulate HC coupling [3,20]. However, recent Numerous studies in the vertebrate retina suggest that studies have established that dopamine release is not prolonged dark-adaptation has a profound effect on the enhanced by periods of prolonged darkness in the daytime responsiveness of cone pathways. Following prolonged [27].
dark adaptation, it was first shown that there are ana- In a detailed study of the white perch retina (Morone tomical changes in the cone pedicles, which were proposed americana), a similar phenomenon was described to be correlated with the loss of colour opponency and [21,29,30], although the extent of dark-suppression was cone function at the ganglion cell level of the goldfish significantly more pronounced than in carp. This suggests retina [16]. Subsequently it was demonstrated in the that the expression of dark-suppression may have a goldfish that prolonged darkness was associated with species-dependent component. It has also been established suppression of responsiveness of the L-type horizontal in the white perch that there are significant changes in the cells [32]. Furthermore, it was shown that such dark- kinetics of the light response after prolonged darkness suppression was associated with a reduction in the horizon- [12]. However, these changes were associated with profound changes in the threshold sensitivity and
1 chromatic sensitivity, suggesting they are mediated Published on the World Wide Web on 7 November 2000.
through rod–cone interaction.
*Corresponding author. Tel.:144-208-846-7521.
E-mail address: [email protected] (M.W. Hankins). Preliminary experiments on the L-HCs in the roach
A. Jenkins, M.W. Hankins / Brain Research 887 (2000) 230 –237 231
retina (Rutilus rutilus) recorded through a 24-h diurnal tion. Cells were impaled with no photic stimuli and cycle, reported no significant modulation of the HC light initially characterized using a single paired red and green response amplitude, but noted significant changes in the flash of moderate intensity (650 nm, 531 nm, Imax56.7
22
light response kinetics [9]. L-HCs in the roach, like most mW cm , 450–500 ms). These responses provided the cyprinids receive a dominant LWS (red) cone input, with a data for the principal amplitude and kinetic analysis with a weak MWS (green) cone input [2]. This raises the question minimal light exposure (SA, n569; LA, n579). The as to whether long-term light history or circadian / diurnal responses of the cells were then additionally characterized regulation might drive the changes in cone-driven L-HC using a range of spectral or spatial stimuli. These responses kinetics. Interestingly, long-term light-history appears to provided information confirming the relative spectral sen-affect the temporal response of the human photopic (cone) sitivity, and allowed us to assess the extent of the HC electroretinogram [7]. Thus it was shown that the b-wave receptive fields of individual cells. After the experiments, implicit time of the photopic cone ERG exhibits a pro- detailed measurements of the individual light evoked nounced sinusoidal oscillation, the period of oscillation responses (S-potential) were performed in accordance to being|24 h with peak temporal response in the middle of those outlined in Fig. 1. This involved simple amplitude
the day. This raises the possibility that the temporal measurement of the principal components, together with a response of cone pathways is modulated according to range of kinetic measurements on the rate of hyperpolari-long-term light-history. zation and depolarization. An assessment of receptive field We have therefore examined the effect of long-term dark size was made using two methods. Firstly, the amplitude of adaptation on the light responses of L-HCs cells in the the annulus response for each cell was extrapolated to an roach retina. Some of this work has been reported in equivalent amplitude response spot area (Fig. 1c). Second-abstract form [5]. ly, the ratio of the amplitude of the annulus and spot (annulus / spot) was calculated for each cell to give a measure of the relative strength of surround response.
2. Methods
Experiments were performed on the isolated freshwater 3. Results
cyprinid Rutilus rutilus (roach) retina. The fish were
maintained in outdoor holding tanks under natural day– 3.1. The amplitude of the light response and the night ambient lighting. The experiments were performed receptive field properties
during the middle 3 h of subjective day. The fish were
dark-adapted according to one of two regimes. In the case We examined the general light responsiveness of the of short dark adaptation (SA) the fish were placed in L-HCs cells using a number of approaches. First we absolute darkness for 10 min, in long dark adaptation (LA) examined the HC response amplitudes to the initial single
22
they were placed in darkness for 3 h. After this period they red stimulus (650 nm, 4.1 mm o.d., 6.7 mW cm ). We were killed (T50) and the retinae isolated under IR found no significant difference in the amplitudes in the two illumination. After the preadaptation both groups of retinae groups; thus the response of the SA cells was 19.6360.86 experienced the same conditions throughout the recording mV and that for the LA cells was 20.2760.80 mV (mean61 period. The retinae were placed photoreceptor side upward S.E.M.). For a population of cells, we examined the V / in a moist oxygenated plexiglass recording chamber. The log I relation and found no significant differences in chamber was semi-enclosed to allow electrode penetration amplitude at any of the intensities (Fig. 2a). This was also from above. The experimental recording period then lasted confirmed by measuring the 1 / 2Vmax stimulus intensities from T55 min to T520 min. Recordings were made from for the individual cells, and the mean intensity for SA luminosity-type horizontal cells (L-HCs) using KCl-filled retinae (21.260.2 log units) was not significantly different glass micropipettes (80–100 MV), and conventional re- from the LA retinae (21.360.27 log units). We also cording methods. Cell recordings were digitized and examined the dark resting membrane potential (E ) for them
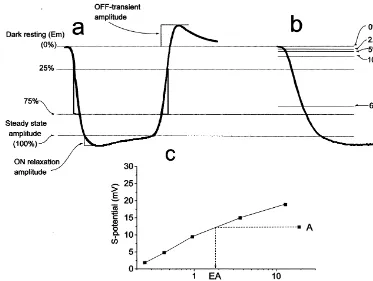
Fig. 1. Measurement of HC light response components. (a) Schematic of the light response waveform of an L-HC to a|500-ms stimulus. Amplitude measurements were made relative to dark resting membrane potential of the steady state light response at 450 ms. ON-relaxation amplitude was measured from the light peak to the steady state level. The OFF-transient peak was measured relative to E . Hyperpolarizing and depolarizing rates of change werem measured within the pseudo-linear range (25–75%) of the light response (S). (b) Time to reach 2.5, 5, 10, 50 and 66% of the steady light response was also measured for each cell. (c) Method used to determine the equivalent area of the annulus response. The amplitude of the annulus light response of each cell (A) was translated to an equivalent area response (EA) by extrapolation from its response / spot size curve.
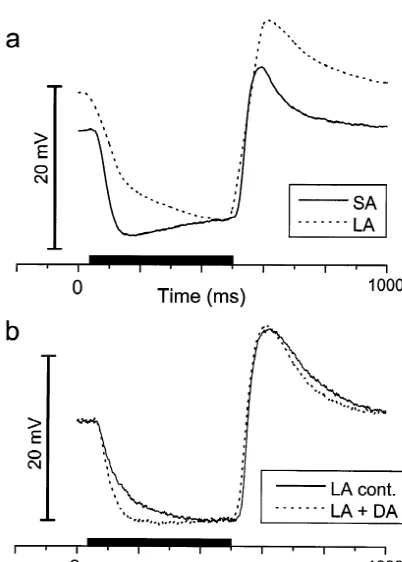
group of retinae obtained under infra-red illumination in response to a single red-flash stimulus (650 nm), normal-the middle of normal-the night. The data from normal-the night-HCs were ized the response to the steady-state light response level not significantly different from the SA and LA retinae for each cell and averaged these waveforms. The results (Fig. 2a,b). are shown in Fig. 4b, and establish that there are signifi-Analysis of the cell responses to a range of spot and cant changes in the waveform, most clearly apparent in the annulus stimuli were used to assess the general extent of speed of hyperpolarization at light-ON. SA cells were HC receptive field. The results show there are highly significantly faster than those recorded in LA retinae. Cells significant (P,0.001) differences in the mean extent and from the LA retinae were characterized by a slower rate of distribution of receptive fields according to their long-term hyperpolarization, reflected by a long delay in reaching preadaptation history (Fig. 3). The annulus / spot ratios maximal amplitude. Furthermore, these differences in ON-were significantly larger in the LA retinae, consistent with kinetics were also apparent when cells were recorded in an increased sensitivity to the peripheral stimulus. In the presence of a rod-saturating (500 nm) background. addition, when we examined the equivalent isoluminant Typical single light responses for SA and LA cells are area (see methods) for the annulus stimulus (0.9 mm i.d., given in Fig. 4a.
2
4.1 mm o.d.) we found this value to be 1.9560.35 mm in Using the waveform parameters outlined in Fig. 1, we
2
the SA cells and 5.6860.59 mm in the LA cells (n531, a performed a detailed analysis of the light responses significant difference at P,0.001, t-test). recorded in the two groups of retinae. The latencies were examined at a number of %-response increments. The data 3.2. Changes in the L-HC light response (S-potential) revealed that the onset of hyperpolarization was
signifi-waveform cantly delayed in the LA cells compared to that of the SA
A. Jenkins, M.W. Hankins / Brain Research 887 (2000) 230 –237 233
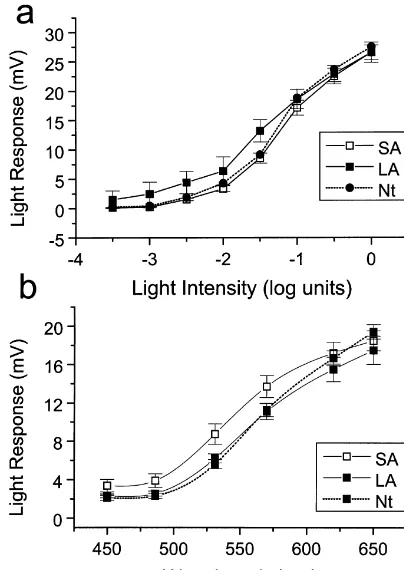
Fig. 3. Frequency distributions of the annulus / spot ratios for the SA and LA cells. In this case the cell responses to a spot (o.d. 4.1 mm) and Fig. 2. (a) V / log I response curves. Mean steady-state HC light response annulus (i.d. 0.9 mm, o.d. 4.1 mm) were used to calculate an annulus / amplitude (mV) is plotted as a function of stimulus intensity. SA (h) and spot ratio for each cell. Note that in the LA cells the distribution and LA (j) are plotted together with the standard errors of means (61 mean ratio is shifted to higher values, corresponding to an increased S.E.M.). Data are also shown for L-HCs recorded from retinae obtained in sensitivity to the peripheral annulus stimuli. The mean ratio for the SA the middle of the night under infra-red (? ? ?). The data were statistically group was 0.5560.03 (n533), whilst that for the LA group was 0.860.02 tested (t-test and Bonferroni correction) and none of the differences (n530). This difference was found to be significant at the P,0.001 level. between SA and LA were found to be significant at the P,0.05 level The data are consistent with an increased effective HC-receptive field in (n519). Stimulus 650 nm, spot 4.1 mm diameter (o.d.), Log 0.2.193 the LA condition.
13 22 21
10 quanta cm s . (b) Relative spectral sensitivity. The amplitude of the light response (mV) is plotted as a function of stimulus wavelength
additional waveform components, including a light-ON
(l, nm). Data are shown for the spectral sensitivity for the SA (h) and
LA (j). Data are also shown for L-HCs recorded from retinae obtained relaxation (roll-back) before the response settles to the in the middle of the night under IR (? ? ?). We found no significant steady state (450 ms), and / or a light-OFF transient (de-differences in the SA and LA spectral response at any of the wavelengths
polarizing overshoot). Where present, we measured the
tested (t-test, Bonferroni correction). Stimulus spot: 4.1 mm, nSA537,
22 amplitudes of these components in accordance with the nLA525 (Imax6.7mW cm ).
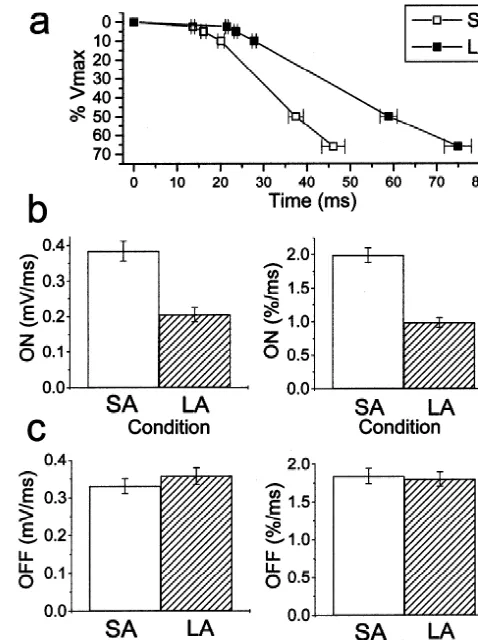
definition outlined in Fig. 1. When we examined the mean amplitudes of both these components in the two groups of light response (25–75%). This was performed both on the preadaptation conditions, we found no significant differ-raw data (mV/ ms) and normalized data (% / ms). Both sets ences in their relative expression (Table 1). Furthermore of data show that there is a significant difference (P, the percentage of cells that expressed either of these 0.001) in the rate of polarization (Fig. 5b). Time-constants components did not vary according to the light-history. (t) were also calculated for the light-ON using the time
points between threshold (2.5%) and 66% response. These 3.3. Horizontal cell responses in the superfused retinal show that the meant for the SA cells was 32.462.39 ms preparations
(n562), whilst that for the LA cells was 53.463.03 ms
(n561) and again these results were significant at the We performed a number of experiments utilizing SA and
prepara-Fig. 5. Kinetics of the HC light response. (a) Delay in the onset of the light response. In this graph the time taken to reach 2.5, 5, 10, 50 and 66% of the steady-state amplitude are shown (61 S.E.M.). The differ-Fig. 4. HC light response waveform. (a) The S-potential light response ences between the SA and LA cells were all significant at the P,0.001 for a typical cell from the SA group (———) and the LA group (? ? ?). level. The latency to 2.5% threshold was 13.6260.41 ms for the SA cells The waveforms are normalized to the steady state light level. Stimuli 650 and 21.4860.33 ms for the LA cells (mean61 S.E.M.). (b) The rate of nm, spot 4.1 mm. (b) the averaged normalized waveform range (61 hyperpolarization at light-ON. A comparison of the absolute (mV/ ms) and S.E.M. error spreads) plotted for the total cell populations in the study. normalized (% / ms) rates for the SA and LA cells, these differences were Note the clear shift in the kinetics of the light-ON hyperpolarization in both significant at the P,0.001 level. (c) The rates, absolute and the LA group combined with a significant delay to reach steady state normalized, for the depolarization at light-OFF, showing no significant (nSA574, nLA587). difference between the SA and LA cell populations.
tions, and in four out of six cells studied we found that 10 our experiments with the SA and LA retinae in superfused
mM DA selectively and reversibly affected the light-ON preparations (Fig. 6).
kinetics in L-HCs recorded from LA retinae (Fig. 6b). When we examined the steady-state amplitudes of the light responses of L-HCs and their V / log I functions, we found no difference in the cells recorded from the two conditions (Fig. 2a). Furthermore, there was no significant
4. Discussion change in the relative spectral sensitivity (Fig. 2b), such as
A. Jenkins, M.W. Hankins / Brain Research 887 (2000) 230 –237 235
Table 1
a Analysis of the ON-relaxation and OFF-transient components of the HC light response
Parameter Condition Mean n % Expression t-test P-value
amplitude6S.E.M. of$1 mV
ON relaxation SA 2.1760.30 74 55.4 21.01 0.31
(mV) LA 1.7860.25 87 48.2
OFF overshoot SA 2.3460.21 74 70.3 1.28 0.20
(mV) LA 2.8060.27 87 65.5
a
The data show the mean amplitudes of the components, together with the percentage of cells that express the component. We found that long-term light history had no significant effect on the expression of either the ON-relaxation or the OFF-transient
that in Rutilus rutilus there is little significant expression other species to date [1]. Phylogenetically the white perch of the dark-suppression phenomenon seen so clearly in and hybrid bass belong to the Moronidae family (perch), other species. The absence of dark suppression is also not whilst the goldfish, carp and roach belong to the distinct related to the time of day, since the amplitudes and spectral Cyprinidae (carp). Collectively, this may suggest that the sensitivity of L-HCs was the same when experiments were expression of dark-suppression is partly species dependent, performed at night (Fig. 2a,b). Whilst it has been shown, and that in Rutilus rutilus we have a retina where this for example, that long periods of darkness have a strong effect is minimal. Recently, we have recorded L-HC influence on the amplitude of light responses in the white responses in the carp (Cyprinus carpio) retina under perch retina [29], the extent of dark-suppression appears identical conditions to those reported in this study. In the less profound in the carp and goldfish retina. In contrast, carp, we see a significant dark-suppression of HC re-studies of the hybrid bass (Morone sp.) have suggested that sponses in the LA condition, consistent with that reported dark-suppression in this species is stronger than in any in previous studies [32].
In contrast, with the minimal effect of long-term light history on general HC responsiveness, we did find that both the receptive field and general kinetics of the light response differed between the SA and LA conditions. Analysis of the annulus / spot ratio (Fig. 3) and the equivalent area index revealed a significant difference in the extent of HC effective receptive field. It appears that the long period of darkness in the LA group results in HCs that are more sensitive to the peripheral stimulus. It is likely that the changes in HC receptive field may reflect the strength of HC coupling which is regulated, at least in part, by retinal DA release in most vertebrate retinae [11,20]. Studies of light-dependent dopamine release in the cyprinid retina have provided somewhat contradictory results, and release appears to be partly dependent upon the diurnal status and long-term light history. Thus, in a recent study it was shown that steady light did not affect release, but flickering light increased dopamine release 2-fold. During prolonged darkness, the release of dopamine increased slowly over 2 h, but only when the experiments were performed at night [27].
The most striking difference between the SA and LA retinae came from our analysis of the light-ON response. There was an apparent increase in the latency to light-ON in the LA group (Fig. 5a). The latency (threshold 2.5%) for the SA cells was around 14 ms, whilst in the LA group this was prolonged by around 50% to 21 ms. The latencies
Fig. 6. Recordings from L-HCs in superfused retinal preparations. (a) a
comparison of typical light response waveforms of L-HCs recorded in SA we find in the SA condition are consistent with those
retina (———) and LA retina (? ? ?). Presentation of the light stimuli (650 previously reported in cone-driven HCs in the light-nm, spot 4.1 mm, 450 ms), is denoted by the solid bar. (b) The effects of adapted retina [18,28]. Our results suggest that long-term 10 mM dopamine (DA) applied in the superfusate upon the light
light history has a pronounced influence on the
photo-responses of an L-HC recorded in an LA adapted retina. Note that DA
receptor input to the L-HCs. In addition to the effect on
evokes a selective effect upon the light-ON response, increasing the
rate of HC hyperpolarization (Fig. 5b). Both the absolute tion, or diurnal phase, can initiate a number of anatomical rate of hyperpolarization (mV/ ms) and the normalized changes in the cone–HC synapse. In addition to the slope (%-response / ms) were significantly slower in cells initiation of retino-motor movements, there are reported from the LA retina. This difference in the response is also modifications to the density of presynaptic cone ribbons apparent when the general waveforms of SA and LA cells [23–25] and postsynaptic HC spinules [26], both of which were compared (Fig. 4). In contrast, the rate of HC can occur within the time scale of 2 h in darkness. Whilst repolarization at light-OFF was not different in the SA and the precise relationship between the anatomical changes LA retinas (Fig. 5b). Our detailed analysis of the HC and the physiology of the cone–HC synapse remains response waveform therefore suggests that prolonged somewhat obscure, we cannot exclude these factors con-periods of darkness during the day results in a selective tributing to the long-term plasticity in HC response change in the kinetics of the light-ON hyperpolarization. kinetics which we have reported here.
We examined a potential correlation between the synaptic Interestingly we have examined the effects of dopamine delay (2.5% latency) and the rate of HC hyperpolarization (DA) upon the kinetics of L-HC light responses from LA and found no simple linear relationship. This suggests that retinae in superfused preparations. Previous studies have light history may affect at least two presynaptic com- shown that dopamine has rather inconsistent effects on ponents to the L-HCs. roach L-HCs in terms of membrane potential and cell The kinetics of the HC light response could be affected coupling [8], and this may explain some of the differences by changes in the passive membrane properties of the HC, in the properties of the roach retina. However, we have or by modification of the strength of HC–HC coupling shown that DA application to the LA retina can evoke [15]. However, such an effect should modify both the selective changes in the light-ON response (Fig. 6b). These light-ON and light-OFF kinetics, and this is clearly not results do not establish cause and effect, but imply that consistent with our results. Whilst the time constant for some of the differences between the SA and LA retinae light-ON in the SA group was 32.462.39 ms, that for the may involve retinal dopaminergic activity
LA group was 53.463.03 ms. In contrast, there was no We have described in detail the effect of long-term significant corresponding variation in the light-OFF time light-history upon the L-HCs. This has shown that pro-course. It is also important, that since we found no change longed darkness in the daytime does not significantly in the dark resting potential, the kinetic changes cannot be modulate the amplitude or sensitivity of the light response explained in terms of voltage-dependent activity in the HC of L-HCs cells in the roach retina. We suggest that this membrane. species shows no evidence of significant dark-suppression. The selective effect upon light-ON might be explained What has been revealed is that prolonged darkness (light-by long-term light-history affecting the rate of glutamate history) has a profound effect on the kinetics of the HC reuptake in the retina. More recently it has been suggested light response. Interestingly, studies of the human elec-that the HC response waveform, whilst dependent upon troretinogram have shown that the latency of the photopic changes in the rate of glutamate release, is shaped by (cone) b-wave varies throughout the diurnal cycle [6]. glutamate reuptake in the synaptic cleft [4,22]. Thus, it has Furthermore, such temporal changes could be partially been shown that the application of dihydrokainate (DHK), initiated by prolonged darkness in the day. It has been a selective uptake inhibitor of retinal glutamate uptake, suggested that hyperpolarizing neurones, including HCs, slows the horizontal cell hyperpolarization at light-ON in a may play a role in shaping and terminating the b-wave dose-dependent manner, without affecting the repolariza- [17]. It is therefore tempting to suggest that the effects of tion at light-OFF [4]. Such an explanation might be long-term light history on light response kinetics may be a consistent with our observations in the LA condition, since general feature of cone pathways in some species. long periods in the dark prior to the experiments are likely
to increase the loading on the glutamate uptake system,
rendering the system less effective. Acknowledgements The kinetics of the HC light response is also regulated
by the activity of HC–cone feedback. Thus it has been This work was supported by an MRC studentship award shown that GABA can affect the temporal properties of the to Aaron Jenkins. The authors would also like to acknowl-HC light response in a number of species [19,31]. Further- edge the considerable initial encouragement received from more, it appears that the GABA feedback from HC to the late Professor Keith Ruddock.
cones is a novel example of positive feedback that acts to slow down the onset of the HC light response [10]. Whilst
in this study we have observed no shift in the HC resting References membrane potential, we cannot rule out feedback as a
candidate in the temporal changes initiated by long-term [1] W.H. Baldridge, R. Weiler, J.E. Dowling, Dark-suppression and light history. light-sensitization of horizontal cell responses in the hybrid bass
A. Jenkins, M.W. Hankins / Brain Research 887 (2000) 230 –237 237
[2] M.B. Djamgoz, J.E. Downing, H.J. Wagner, The cellular origin of an primate photopic electroretinogram: a role for hyperpolarizing unusual type of S-potential: an intracellular horseradish peroxidase neurons in shaping the b-wave, Vis. Neurosci. 11 (1994) 519–532. study in a cyprinid fish retina, J. Neurocytol. 14 (1985) 469–486. [18] H. Spekreijse, A.L. Norton, The dynamic characteristics of color-[3] J.E. Dowling, B. Ehinger, Synaptic organization of the amine- coded S-potentials, J. Gen. Physiol. 56 (1970) 1–15.
containing interplexiform cells of the goldfish and Cebus monkey [19] S. Stone, P. Witkovsky, The actions of gamma-aminobutyric acid, retinas, Science 188 (1975) 270–273. glycine and their antagonists upon horizontal cells of the Xenopus [4] L. Gaal, B. Roska, S.A. Picaud, S.M. Wu, R. Marc, F.S. Werblin, retina, J. Physiol. 353 (1984) 249–264.
Postsynaptic response kinetics are controlled by a glutamate trans- [20] T. Teranishi, K. Negishi, S. Kato, Dopamine modulates S-potential porter at cone photoreceptors, J Neurophysiol. 79 (1998) 190–196. amplitude and dye-coupling between external horizontal cells in the [5] M.W. Hankins, A. Jenkins, Prolonged dark adaptation — effects on carp retina, Nature 301 (1983) 243–246.
the responses of luminosity horizontal cells in the cyprinid retina, [21] K. Tornqvist, X.L. Yang, J.E. Dowling, Modulation of cone horizon-Invest. Ophthalmol. Vis. Sci. 39 (1998) S209. tal cell activity in the teleost fish retina. III. Effects of prolonged [6] M.W. Hankins, R.J. Jones, K.H. Ruddock, Diurnal variation in the darkness and dopamine on electrical coupling between horizontal
b-wave implicit time of the human electroretinogram, Vis. Neurosci. cells, J. Neurosci. 8 (1988) 2279–2288.
15 (1998) 55–67. [22] C.A. Vandenbranden, J. Verweij, M. Kamermans, L.J. Muller, J.M. [7] M.W. Hankins, R.J.M. Jones, K.H. Ruddock, Diurnal variation in the Ruijter, G.F. Vrensen, H. Spekreijse, Clearance of neurotransmitter b-wave component of the human electroretinogram (ERG), J. from the cone synaptic cleft in goldfish retina, Vis. Res. 36 (1996)
Physiol. 483 (1995) 42. 3859–3874.
[8] M.W. Hankins, K.H. Ruddock, Hyperpolarization of fish retinal [23] L. Vollrath, A. Meyer, F. Buschmann, Ribbon synapses of the horizontal cells by kainate and quisqualate, Nature 308 (1984) mammalian retina contain two types of synaptic bodies — ribbons
360–362. and spheres, J. Neurocytol. 18 (1989) 115–120.
[9] S.E.J. Jeffery, R.M.J. Jones, K.H. Ruddock, Diurnal variations in the [24] L. Vollrath, I. Spiwoks-Becker, Plasticity of retinal ribbon synapses, electrophysiological activity in the isolated retina of a cyprinid fish Microsc. Res. Tech. 35 (1996) 472–487.
(Roach), J. Physiol. 459 (1993) 61. [25] H.J. Wagner, Darkness-induced reduction of the number of synaptic [10] M. Kamermans, F. Werblin, GABA-mediated positive autofeedback ribbons in fish retina, Nat. New Biol. 246 (1973) 53–55.
loop controls horizontal cell kinetics in tiger salamander retina, J. [26] H.J. Wagner, M.B. Djamgoz, Spinules: a case for retinal synaptic Neurosci. 12 (1992) 2451–2463. plasticity [see comments], Trends Neurosci. 16 (1993) 201–206. [11] E.M. Lasater, J.E. Dowling, Dopamine decreases conductance of the [27] R. Weiler, W.H. Baldridge, S.C. Mangel, J.E. Dowling, Modulation
electrical junctions between cultured retinal horizontal cells, Proc. of endogenous dopamine release in the fish retina by light and Natl. Acad. Sci. USA 82 (1985) 3025–3029. prolonged darkness, Vis. Neurosci. 14 (1997) 351–356.
[12] S.C. Mangel, W.H. Baldridge, R. Weiler, J.E. Dowling, Threshold [28] M. Yamada, Y. Shigematsu, M. Fuwa, Latency of horizontal cell and chromatic sensitivity changes in fish cone horizontal cells response in the carp retina, Vis. Res. 25 (1985) 767–774. following prolonged darkness, Brain Res. 659 (1994) 55–61. [29] X.L. Yang, K. Tornqvist, J.E. Dowling, Modulation of cone horizon-[13] S.C. Mangel, J.E. Dowling, Responsiveness and receptive field size tal cell activity in the teleost fish retina. I. Effects of prolonged of carp horizontal cells are reduced by prolonged darkness and darkness and background illumination on light responsiveness, J. dopamine, Science 229 (1985) 1107–1109. Neurosci. 8 (1988) 2259–2268.
[14] S.C. Mangel, J.E. Dowling, The interplexiform-horizontal cell [30] X.L. Yang, K. Tornqvist, J.E. Dowling, Modulation of cone horizon-system of the fish retina: effects of dopamine, light stimulation and tal cell activity in the teleost fish retina. II. Role of interplexiform time in the dark, Proc. R. Soc. London B: Biol. Sci. 231 (1987) cells and dopamine in regulating light responsiveness, J. Neurosci. 8
91–121. (1988) 2269–2278.
[15] V.V. Maximov, A.L. Byzov, Horizontal cell dynamics: what are the [31] X.L. Yang, S.M. Wu, Effects of prolonged light exposure. GABA, main factors?, Vis. Res. 36 (1996) 4077–4087. and glycine on horizontal cell responses in tiger salamander retina, [16] J.P. Raynauld, J.R. Laviolette, H.J. Wagner, Goldfish retina: a J. Neurophysiol. 61 (1989) 1025–1035.