www.elsevier.comrlocaterapplanim
Cereal aversion in behaviourally resistant house
mice in Birmingham, UK
R.E. Humphries
a,1, R.M. Sibly
a,), A.P. Meehan
ba
Department of Biochemistry and Physiology, School of Animal and Microbial Sciences, UniÕersity of Reading, P.O. Box 228, Whiteknights, Reading RG6 6AJ, UK
b
Research and DeÕelopment DiÕision, Rentokil Initial, Felcourt, East Grinstead, West Sussex RH19 2JY, UK Accepted 26 August 1999
Abstract
In 1986, house mice in a small defined area of inner Birmingham were reported as not taking a variety of rodenticides from bait containers, a phenomenon labelled ‘behavioural resistance’. This study investigated behavioural resistance by comparing the food preferences of West Midlands
Ž . Ž .
behaviourally resistant WMBR mice with those of normal BC mice. Nine bait boxes each
Ž
containing one of nine different foods cheese, chicken, tuna fish, peanut butter, canary seed, Cat
Ž . .
stars, wheat, PCD MOD pellets and Non-tox were introduced to 12 WMBR and seven BC sites
ŽExperiment 1 . The experiment was repeated in the laboratory with six pens of WMBR and six of.
Ž .
BC mice Experiment 2 , and to investigate whether the preferences had a genetic basis the
Ž .
offspring were similarly assayed Experiment 3 . In each experiment the consumption of each food was measured over 7 days and the droppings around the bait boxes were counted to assess mouse activity. Food neophobia was noted in some populations of BC mice. Tested in the wild and in the laboratory WMBR mice showed an aversion to foods containing cereals, as did their offspring. These results, with other lines of evidence, strongly suggest that cereal aversion in WMBR mice has a physiologicalrgenetic basis. Since cereal aversion allows WMBR mice to survive cereal-based rodenticidal baits, we conclude that WMBR mice have genetically based behaviours that allow them to survive poisoning regimes that kill other strains.q2000 Elsevier
Science B.V. All rights reserved.
Keywords: House mouse; Food preferences; Neophobia; Behavioural resistance; Bait avoidance
)Corresponding author. Tel.:q44-118-931-8461; fax:q44-118-931-0180; e-mail: [email protected] 1
Now at: Faculty of Veterinary Science, University of Liverpool, Veterinary Teaching Hospital, Leahurst, Neston, CH64 7TE, UK.
0168-1591r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
( ) R.E. Humphries et al.rApplied Animal BehaÕiour Science 66 2000 323–333 324
1. Introduction
Ž .
In 1986, pest control operatives reported that house mice Mus domesticus in localised inner-city areas of London and Birmingham in the UK had stopped taking a
Ž .
variety of rodenticide baits from bait containers Humphries et al., 1992 . Bait avoidance was identified in the field wherever house mice continually avoided taking both acute
Ž .
rodenticides rapid mortality, single-dose poisons, such as Alphachloralose and chronic
Ž .
rodenticides delayed mortality, multiple-dose poisons, such as Bromadiolone . The continued existence of the mice was established from fresh droppings, catches on sticky-boards, damage to goods and structures, the presence of tracks and active burrows, andror sightings of live and dead mice. This phenomenon was labelled ‘behavioural resistance’, although there was initially no evidence that the behaviour was inherited.
Ž .
Throughout initial attempts to catch West Midlands behaviourally resistant WMBR mice for the experiments reported here, no mice were captured in live-capture traps
Ž .
baited with cereals Humphries et al., 1992 , which at the time was surprising since
Ž
house mice generally prefer cereals to non-cereal foods Southern, 1954; Rowe et al.,
.
1974 . In urban environments however, mice can obtain much of their food from
Ž .
garbage Schein and Orgain, 1953; personal observation , and some trapping success
Ž
was achieved when traps were baited with either tuna fish or chicken Humphries et al.,
.
1992, 1996 .
Here, we investigate the food preferences and aversions of WMBR mice in the field
ŽExperiment 1 and in the laboratory Experiment 2 using a ‘cafeteria’ experimental. Ž .
Ž .
design in which nine foods were presented simultaneously. ‘Non-tox’ a cerealroil mix was included as one of the test foods because it had been extensively used as a bait base by Rentokil throughout Birmingham city centre for 9 years prior to the experiment. In Experiment 3, the offspring of the wild-caught mice were assayed to test whether the WMBR food aversions are genetic or learnt. To provide a comparison with normal mice,
Ž .
seven nearby populations BC mice were investigated at sites where cereal-based baits
Ž .
were readily taken. Note was taken of any initial aversions to novel foods neophobias since these are of special interest in rodent control.
2. Materials and methods
2.1. Experiment 1
rodenticides were removed, disposable latex gloves being worn whenever food or droppings were handled. A 5=1 m area adjacent to a wall containing droppings but not
Ž .
food sources was selected at each site. On the first day of the experiment day 0 droppings were removed from these areas and nine cardboard mouse bait boxes
Ž10=4=4 cm were placed against the 5-m length of wall at intervals of 50 cm. Each.
bait box contained 4 g of one of the first nine foods listed in Table 1. All foods were crushed, chopped up or minced in a food processor into pieces less than 0.3 g to minimise hoarding. Foods removed from the boxes in the previous 24 h were recorded on days 1, 2, and 7. Evaporation was allowed for by recording the weight losses from boxes to which mice could not gain access. Each container was topped up daily with fresh food so that 4 g of each food was available to the mice, and the number of droppings in the experimental areas was counted. In one of the BC sites takes on some days were near maximal so that food preferences could not be established, and this site was therefore not included in the analysis. Food consumption and dropping counts were comparable at the remaining WMBR and BC sites.
2.2. Experiments 2 and 3
Six male and six female WMBR mice were live-trapped from eight shops, restaurants and offices in Birmingham city centre, and the same numbers of BC mice were live-trapped from six farms in Berkshire. The substantial problems encountered in
Ž
trapping and maintaining the WMBR mice are described by Humphries et al. 1992;
.
1996 . No site contributed more than three mice, and if two mice came from the same site they were kept as a pair. All animals weighed over 14 g, were sexually mature but not pregnant, and had hind-foot lengths of at least 16 mm on capture, and WMBR and
Ž
BC mice did not differ in body weight. The mice were kept in large cages 55=39=19
. Ž .
cm or pens as below in the period between trapping and the start of the experiments
Žmean times14 days, no difference between WMBR and BC pairs of mice and fed on.
Ž . Ž .
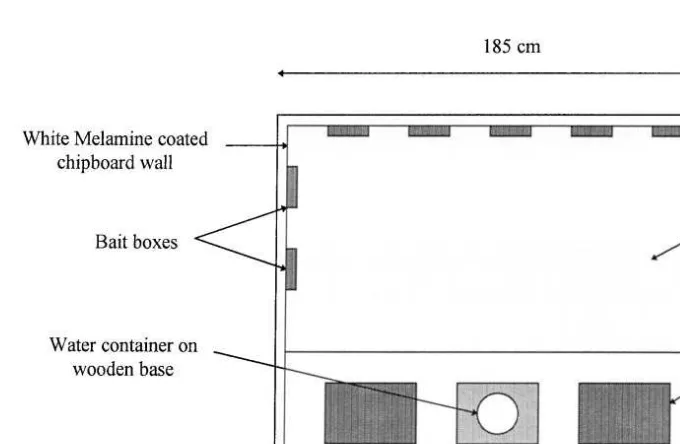
tuna fish and PCD MOD pellets Table 1 . Pens were made of sheet aluminium
Ž185=185=90 cm and contained two wooden mouse boxes 30. Ž =24=12 cm filled.
Ž .
with paper wool as bedding Fig. 1 . At the front of the pens there were two metal food
Ž .
trays 26=16=2.5 cm , each placed on a piece of wood in order to prevent sawdust being scattered into the trays and to enable any spillage of food to be easily collected
Ž
and recorded. Vitamin K1 enriched water from a standard chick drinker made of glass
.
and plastic was available ad libitum. Three pieces of white Melamine-coated chipboard
Žeach measuring 180=30=1.75 cm were pushed firmly against the back and two side.
walls of the pens. All the pens were floored with approximately 5 cm of sawdust firmly patted down in order that any burying or hoarding of food in the sawdust could be easily detected.
In Experiment 2, a male and a female were placed in each of 12 pens on day 0, WMBR pens being paired with BC pens on the basis of date of capture. The pens may seem very large, but we had only limited success in keeping WMBR mice in smaller enclosures. To control the food eaten in the week prior to testing for preferences, the
Ž .
()
R.E.
Humphries
et
al.
r
Applied
Animal
Beha
Õ
iour
Science
66
2000
323
–
333
326
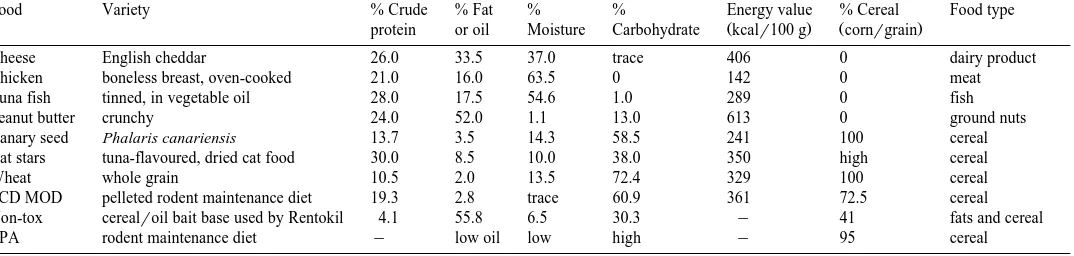
Table 1
The nutritional composition and cereal content of the 10 experimental foods, of which the first nine were presented in bait boxes. After McCance and Widdowson
Ž1991 , with additional information supplied by food manufacturers.. yindicates information not available
Food Variety % Crude % Fat % % Energy value % Cereal Food type
Ž . Ž .
protein or oil Moisture Carbohydrate kcalr100 g cornrgrain
Cheese English cheddar 26.0 33.5 37.0 trace 406 0 dairy product
Chicken boneless breast, oven-cooked 21.0 16.0 63.5 0 142 0 meat
Tuna fish tinned, in vegetable oil 28.0 17.5 54.6 1.0 289 0 fish
Peanut butter crunchy 24.0 52.0 1.1 13.0 613 0 ground nuts
Canary seed Phalaris canariensis 13.7 3.5 14.3 58.5 241 100 cereal
Cat stars tuna-flavoured, dried cat food 30.0 8.5 10.0 38.0 350 high cereal
Wheat whole grain 10.5 2.0 13.5 72.4 329 100 cereal
PCD MOD pelleted rodent maintenance diet 19.3 2.8 trace 60.9 361 72.5 cereal
Non-tox cerealroil bait base used by Rentokil 4.1 55.8 6.5 30.3 y 41 fats and cereal
Fig. 1. Experimental design showing layout of bait boxes in Experiments 2 and 3. Each box contained 4 g of one of the first nine foods in Table 1.
down to reduce hoarding, and placed in the two metal food trays at the front of the pens
ŽFig. 1 . The 24-h consumption was recorded on days 1, 2, 3, 6 and 7. At the end of the.
seventh day all droppings were removed from the back half of the pens and nine bait boxes containing foods were introduced into them as in Experiment 1, except that the between-box spacing was reduced and the side walls were used as well as the back wall
ŽFig. 1 . Food order in the bait boxes was randomised but the same order was used for.
paired WMBR and BC pens. To allow for seasonal variations in day length the boxes were introduced 1 h before sunset. The bait boxes were provisioned daily at 0900–1000 h and food consumption was recorded and droppings counted as in Experiment 1. After 14 days, the mice were caught and weighed, and any hoarded food was weighed.
Experiment 3 followed the experimental protocol of Experiment 2 using mature offspring from the first and second litters of the mice used in Experiment 2. The mice used in Experiment 2 were maintained in the pens on a diet consisting of all 10 foods. The offspring were born in the pens and kept in them until separated from their parents
Ž . Ž .
( ) R.E. Humphries et al.rApplied Animal BehaÕiour Science 66 2000 323–333 328
3. Results
3.1. EPA consumption before the introduction of the bait boxes in Experiments 2 and 3
It was not possible to assess natural feeding in Experiment 1 before the introduction of the bait boxes. However, in the laboratory, in Experiment 2, only the cereal-based diet EPA was available in the week prior to bait box introduction, and during this time
Ž .
the wild-caught WMBR mice took consistently less EPA than BC mice Fig. 2A . BC
Ž .
mice ate less on day 1 than on day 2 Wilcoxon test, Ws21.0, ns6, ps0.036 but thereafter consistent amounts day to day. The pattern of EPA consumption by the offspring of the wild-caught mice in Experiment 3, was similar to that of their parents
ŽFig. 2B ..
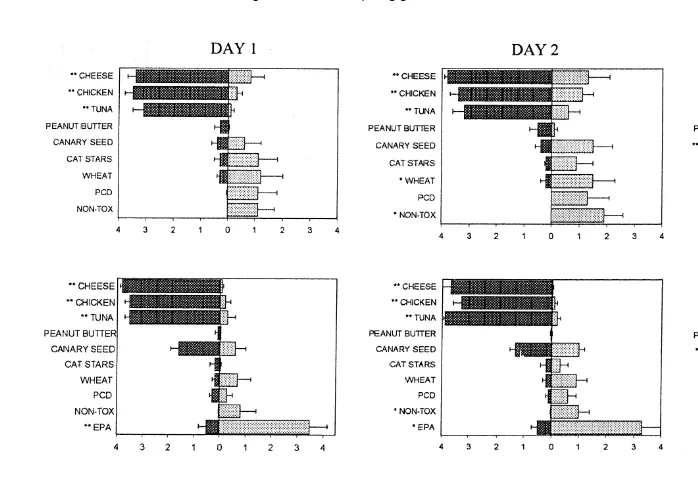
3.2. Food preferences and aÕersions
Food consumption on days 1, 2 and 7 after bait box introduction is shown for each experiment in Fig. 3. When the nine foods were introduced to wild mice in their natural environments in Experiment 1, WMBR mice took consistently more cheese, chicken and tuna fish than BC mice, and the amounts taken were close to the maximum available, 4
Ž .
g Fig. 3, top row . Only small amounts of the other foods were taken by WMBR mice and Non-tox was completely avoided in 11 of the 12 WMBR sites, not even tooth marks being observed. By contrast, BC mice showed no clear preferences.
A similar pattern was seen when the nine foods were introduced to wild mice in the
Ž .
laboratory in Experiment 2 Fig. 3, middle row . The WMBR mice again took consistently more cheese, chicken and tuna fish, and Non-tox was completely avoided by four of the six pairs of WMBR mice. By contrast, BC mice took relatively small amounts of the nine foods in bait boxes on day 1, but EPA was taken in large amounts. By day 7, however, BC mice were taking some of all of the foods.
Ž .
The pattern of food consumption of the offspring Fig. 3, bottom row developed so that by day 7 they were similar to those of their parents, except that WMBR offspring ate more PCD and Non-tox than their parents. WMBR offspring showed no clear preference for any particular food type on day 1, though they avoided canary seed and wheat.
The total daily consumption of all foods before and after bait box introduction is shown in Fig. 2. When the bait boxes were introduced total food consumption by wild
Ž
WMBR mice increased dramatically Wilcoxon test comparing consumption on the days
.
before and after bait box introduction, Ws21.0, ns6, ps0.036 , and thereafter they took significantly more food than BC mice on most days. Their offspring showed a
Ž .
similar pattern, though less marked Fig. 2B . There was no significant difference in
Ž . Ž .
Fig. 2. Total food consumption grpair of mice before and after bait box introduction by A wild WMBR
Ž . Ž . Ž .
( ) R.E. Humphries et al.rApplied Animal BehaÕiour Science 66 2000 323–333 330
Fig. 3. Consumption of the foods presented in the preference tests. Within each panel the amount taken by
Ž . Ž .
WMBR mice is shown on the left dark bars , and that by BC mice on the right light bars . The panels in the top row refer to Experiment 1, those in the middle and bottom rows to Experiments 2 and 3. Columns of panels refer to days 1, 2 and 7. Units are grsite in Experiment 1, grpen in Experiments 2 and 3. Stars indicate Mann–Whitney test p-values for differences between WMBR and BC sites:U
p-0.05,UU
p-0.01,UUU
p -0.001. Bars indicate standard errors of the means.
total consumption over the 14 days of the experiment between pairs of WMBR mice and BC mice in either experiment. The amount of food found hoarded by WMBR and BC mice at the end of Experiments 2 and 3 was small, averaging 1.0 grpair of mice.
4. Discussion
4.1. Food preferences and aÕersions
Strong and persistent preferences of WMBR mice for cheese, chicken and tuna fish
Ž . Ž
and aversions to cereals canary seed and wheat and cereal-based foods Cat stars, PCD
. Ž .
and Non-tox were evident in the preference tests in all three experiments Fig. 3 , and cereal aversion was seen in the pre-bait box week of Experiments 2 and 3, when, offered
Ž
only the cereal-based food EPA, WMBR mice ate substantially less than BC mice Fig.
.
2 and lost weight. Although there were minor nutritional differences between the foods, the only consistent feature of those to which WMBR mice were averse was that they
Ž .
but when they were taken the cereal content of the diet was low. Thus, for instance,
Ž .
some PCD and Non-tox were taken by WMBR mice in Experiment 3 Fig. 3 , but the cereal content of these foods is lower than that of the other cereal-based foods. The WMBR mice food aversions are in marked contrast to the usual preferences of wild and
Ž
laboratory house mice, i.e., cereals, shown here by the BC mice Southern, 1954; Rowe
.
et al., 1974; Meehan, 1984 .
When the bait boxes with foods were introduced in Experiments 2 and 3, the food
Ž .
consumption by WMBR mice increased markedly Fig. 2 . Presumably, when they were offered foods they liked they took large amounts to compensate for the earlier period when they had eaten less. This compensation was such that over the 14 days of the experiments WMBR and BC mice did not differ in total consumption.
4.2. Genetics and ontogeny of cereal aÕersion
The similarity between parents and offspring in cereal aversion in Experiments 2 and
Ž .
3 Fig. 3 raises the question as to whether cereal aversion in WMBR mice has a genetic
Ž
basis or is learnt. Foetalrpre-natal learning is established in rodents Smotherman, 1982;
. Ž
Hepper, 1988, 1991 , as is learning between birth and weaning Galef and Henderson, 1972; Galef and Sherry, 1973; Bronstein and Crockett, 1976; Duveau and Godinot,
. Ž . Ž
1988 . However, following on from the experiments reported here Maczka 1993 MSc
. Ž . Ž .
thesis and Rout 1994 MSc thesis showed that the cereal aversion of our WMBR mice persisted through several generations. In an undergraduate project, Taylor et al.
Ž1996 investigated the physiological basis of the cereal aversion by comparing caecum.
mass and duodenal a-amylase activity in WMBR and BC mice. The results suggested
that low a-amylase activity in WMBR mice caused ingested carbohydrate to bypass
normal digestion and reach the caecum, causing caecal enlargement. Associated symp-toms could cause WMBR mice to select a low-carbohydrate diet, and hence avoid cereals and other carbohydrate-rich foods. The genetics of cereal aversion have been investigated by crossing WMBR mice with a laboratory strain of mice, and then
Ž
backcrossing the heterozygotes with the parental strains Holmes, C., 1995,
undergradu-.
ate project . The results suggested a multi-locus basis for cereal aversion in WMBR mice. Taking together these various lines of evidence, we conclude that cereal aversion in WMBR mice does have a physiologicalrgenetic basis. Since the trait allows WMBR mice to survive cereal-based rodenticidal baits, it seems that WMBR mice are truly ‘behaviourally resistant’, possessing genetically based behaviours that allow them to survive poisoning regimes that kill other strains.
There was no suggestion that bait boxes were avoided by WMBR mice since they readily took large amounts of food from bait boxes if they contained foods that they liked. However, it is interesting that in Experiment 1 WMBR mice completely avoided the bait base Non-tox in 11 of 12 sites. Since WMBR mice took some Non-tox in
Ž .
Experiment 3 Fig. 3 they were not innately completely averse to it, so the mice in Experiment 1 presumably learnt to avoid it by associating it with the Non-tox-based rodenticides Betalard and Bromard then widely used in WMBR sites. Implications of
Ž .
( ) R.E. Humphries et al.rApplied Animal BehaÕiour Science 66 2000 323–333 332
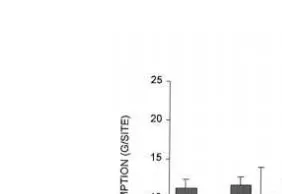
Ž .
Fig. 4. Total food consumption grsite from the nine bait boxes on study days at WMBR and BC sites in Experiment 1. Bars indicate standard errors of the means.
4.3. Food neophobia
House mice are generally regarded as inquisitive animals showing little neophobia,
Ž
i.e., initial avoidance of novel stimuli in familiar environments Southern, 1954; Wolfe,
.
1969; Meehan, 1984 although there are some reports of neophobia in both laboratory
Ž
and wild mice Misslin, 1982; Misslin and Ropartz, 1981; Kronenberger and Medioni,
´
.
1985; Brigham and Sibly, in press . WMBR mice took large amounts of food from the bait boxes from day 1 in all three experiments, and so, by definition, showed no evidence of neophobia. There was however, between-site variation in the initial re-sponses to the bait boxes of mice in the six BC sites in Experiment 1. Whereas the mice in three BC sites took food from the bait boxes from day 1, mice in the other three BC sites took nothing from bait boxes on day 1, though they did feed from them later, and
Ž
the average daily consumption from BC bait boxes increased with time Wilcoxon test
.
comparing days 1 and 3, Ws21.0, ns6, ps0.036, Fig. 4 . Similar results were obtained in Experiments 2 and 3. The average daily consumption of EPA by BC mice
Ž .
increased between days 1 and 2, before the introduction of the bait boxes Fig. 2 , and on days 1 and 2 after bait box introduction BC mice continued eating EPA rather than
Ž .
the foods in the bait boxes Fig. 3 . Again, there was variation between pens in the BC mice’s initial responses. In Experiment 2, two pairs of BC mice took large amounts of
Ž . Ž
food from the bait boxes on day 1 7.5 and 9.6 g , but three pairs took no food at all for
.
further details of all three experiments, see Humphries, 1994 . In all replicates dropping counts showed activity around the bait boxes from day 1, and since some of the foods have a strong smell, it is likely that the mice were aware of the boxes. We conclude that some but not all populations of BC mice were neophobic to new foods.
Acknowledgements
References
Brigham, A.J., Sibly, R.M., in press. A review of the phenomenon of neophobia. In: Cowan, D.P., Feare, C.J.
ŽEds. , Advances in Vertebrate Pest Management. Filander Verlag, Furth, Germany..
Bronstein, P.M., Crockett, D.P., 1976. Exposure to the odour of food determines the eating preference of rat pups. Behav. Biol. 18, 387–392.
Duveau, A., Godinot, F., 1988. Influence of the odourization of the rearing environment on the development of odour-guided behaviour in rat pups. Physiol. Behav. 42, 265–270.
Galef, B.G. Jr., Henderson, P.W., 1972. Mother’s milk: a determinant of the feeding preference of weaning rat pups. J. Comp. Physiol. Psychol. 78, 213–219.
Galef, B.G. Jr., Sherry, D.F., 1973. Mother’s milk: a medium for transmission of cues reflecting the flavour of mother’s diet. J. Comp. Physiol. Psychol. 83, 374–378.
Hepper, P.G., 1988. Adaptive fetal learning: prenatal exposure to garlic affects postnatal preferences. Anim. Behav. 36, 935–936.
Hepper, P.G., 1991. Transient hypoxic episodes: a mechanism to support associative fetal learning. Anim. Behav. 41, 477–480.
Ž
Humphries, R.E., 1994. Investigations into possible behavioural resistance in inner-city house mice Mus
.
domesticus Rutty in the UK. PhD thesis, University of Reading.
Humphries, R.E., Meehan, A.P., Sibly, R.M., 1992. The characteristics and history of behavioural resistance in
Ž . Ž .
inner-city house mice Mus domesticus in the UK. In: Borrecco, J.E., Marsh, R.E. Eds. , Proc. 15th
Vertebrate Pest Conf., March, University of California, 161–164.
Humphries, R.E., Sibly, R.M., Meehan, A.P., 1996. The characteristics of behavioural resistance and bait avoidance in house mice in the UK. Proc. BCPC Pests and Diseases Conf., Brighton, November, 1, 157–164.
Ž
Kronenberger, J.P., Medioni, J., 1985. Food neophobia in wild and laboratory mice´ Mus musculus
. Ž .
domesticus . Behav. Processes 2 1 , 53–60.
Ž .
Maczka, T.M., 1993. The inheritance of unusual food preferences in the urban house mouse Mus domesticus . MSc thesis, University of Reading.
Meehan, A.P., 1984. Rats and Mice: Their Biology and Control. Rentokil, East Grinstead, Sussex. Misslin, R., 1982. Some determinants of the new object reaction of the mouse. Biol. Behav. 3, 209–214. Misslin, R., Ropartz, P., 1981. Responses in mice to a novel object. Behaviour 78, 169–177.
Ž
Rout, A.L., 1994. A comparative study of trapping and feeding behaviour in two strains of house mice Mus
.
domesticus . MSc thesis, University of Reading, UK.
Ž .
Rowe, F.P., Bradfield, A., Redfern, R., 1974. Food preferences of wild house mice Mus musculus L. . J.
Ž .
Hyg. Cambridge 73, 473–478.
Schein, M.W., Orgain, H., 1953. A preliminary analysis of garbage as food for Norway rats. Am. J. Trop. Med. Hyg. 2, 1117–1130.
Smotherman, W.P., 1982. Odour aversion learning by the rat fetus. Physiol. Behav. 29, 769–771. Southern, H.N., 1954. Control of Rats and Mice, Vol. 3. Clarendon Press, Oxford, pp. 99–115.
Taylor, R.C., Stephens, A.G., Sibly, R.M., 1996. The physiological basis of dietary preference in West Midlands behaviourally resistant house mice. Proc. Nutr. Soc. 55, 223.