Association of TaqIB polymorphism in the cholesteryl ester
transfer protein gene with plasma lipid levels in a healthy Spanish
population
Dolores Corella
a, Carmen Sa´iz
a, Marisa Guille´n
a, Olga Portole´s
a, Francisco Mulet
a,
Jose´ I. Gonza´lez
a, Jose´ M. Ordova´s
b,*
aGenetic and Molecular Epidemiology Unit,Public Health Department,School of Medicine,Uni6ersitat de Vale`ncia,Valencia,Spain bJM-USDA Human Nutrition Research Center on Aging at Tufts Uni6ersity,711Washington St.,Boston,MA 02111, USA
Received 14 June 1999; received in revised form 25 October 1999; accepted 5 November 1999
Abstract
Genetic variants at the cholesteryl ester transfer protein (CETP) locus have been associated with CETP activity and mass, as well as plasma high density lipoprotein cholesterol (HDL-C) and apolipoprotein A-I levels. We have examined allele frequencies and lipid associations for the common CETP TaqIB polymorphism in a sample of 514 healthy subjects (231 men, mean age 37.4 years, and 283 women, mean age 35.7 years) residing in Valencia (Spain). The frequency of the less common TaqIB2 allele (0.351; 95% CI: 0.322 – 0.380) was significantly lower than those reported for Northern European populations. Consistent with previous studies, we found a significant association of the TaqIB polymorphism with HDL-C levels. Homozygotes for the B1 allele had lower HDL-C levels than subjects carrying the B2 allele (P trendB0.001 and 0.002, for men and women, respectively). No statistically significant genotype effects were observed for any of the other lipid measures. Multivariate models including TaqIB genotype, body mass index, smoking, alcohol, physical activity, marital status and education were fitted to predict HDL-C levels. The TaqIB polymorphism was consistently an independent predictor of HDL-C levels (PB0.001), and explained 5.8% of its variance. To evaluate gene-environmental interactions, first order interaction terms were tested into the multivariate model. No statistically significant interactions between the TaqIB genotypes and smoking, alcohol, physical activity or education were detected. In conclusion, we observed a significant association of the TaqIB polymorphism with HDL-C levels, which remained consistent across different levels of behavioral factors. Moreover, we found that the TaqIB2 allele frequency was lower in our sample than in other European populations, which could be a contributing factor to the unexpectedly high prevalence of coronary heart disease observed in the region of Valencia. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Cholesteryl ester transfer protein; Genetic polymorphisms; High density lipoproteins; Coronary heart disease
www.elsevier.com/locate/atherosclerosis
1. Introduction
The inverse association between high density lipo-protein – cholesterol (HDL-C) levels and coronary heart disease (CHD) risk has been known for over 25 years [1,2]. However, the mechanisms underlying this rela-tionship [3,4] and the factors responsible for the wide distribution of HDL-C concentrations observed in the general population are not yet fully understood.
Plasma HDL-C levels are determined by a variety of environmental and genetic factors. Epidemiological studies have demonstrated positive associations be-tween HDL-C concentrations and alcohol consump-tion, estrogens, and exercise, whereas a negative association has been observed with tobacco smoking [5,6]. Our understanding of the genetic factors deter-mining HDL-C levels in the general population is more limited. Preliminary twin and family studies indicated that 40 – 60% of the variation of HDL-C between indi-viduals was determined by genetic factors [7].
CETP is a glycoprotein that plays an important role in the transport of excess cholesterol from peripheral
* Corresponding author. Tel.: +1-617-5563102; fax: + 1-617-5563103.
E-mail address:[email protected] (J.M. Ordova´s).
tissues to the liver. This glycoprotein mediates the transfer of cholesteryl esters from HDL or LDL into triglyceride-rich lipoproteins, and thereby stimulates re-verse cholesterol transport [8]. The CETP mRNA
en-codes a polypeptide of Mr53000, which is
N-glycosilated at four sites, giving rise to the mature form of CETP of Mr74000 [9]. The CETP gene encom-passes 16 exons and it has been assigned to chromo-some 16 (16q21) near the LCAT locus. Sib pair linkage analyses have suggested that variation in HDL-C be-tween individuals was related to the inheritance of alleles at or near the cholesteryl ester transfer protein (CETP) gene [10]. Several polymorphisms and rare variants have been detected [11,12], and some of these have been shown to have a significant effect on plasma lipid levels [12 – 14]. A common polymorphism detected using TaqI (TaqIB) has been shown to be a silent base change affecting the 277th nucleotide in the first intron of the CETP gene [15]. The allele carrying the cutting site for the TaqI enzyme is called B1, whereas the one in which the cutting site is missing is known as B2. This polymorphism has been consistently associated with plasma levels of HDL-C, individuals carrying the B2 allele having the highest levels of HDL-C [16 – 18]. However, this association might be population specific [14,19] and highly influenced by environmental factors such as alcohol consumption and tobacco smoking [17,20,21]. Therefore, the aim of this study was to estimate allele frequencies of the TaqIB polymorphism, and to investigate the relationship between this poly-morphism and plasma lipid levels taking into account other biological and environmental factors in a healthy population from Valencia on the Mediterranean coast of Spain. This region presents an unusually high rate of cardiovascular disease that, at the present time, is the highest in the country [22]. The results of this research could contribute to our understanding of the genetic and environmental factors associated with cardiovascu-lar risk.
2. Methods
2.1. Subjects and study design
This work is part of a broader population survey on cardiovascular risk factors in the Valencia Community, aimed to ascertain the prevalence of both genetic and environmental CHD risk factors in this population. In this paper we present data obtained in a cross-sectional study with 514 healthy, unrelated subjects (231 men and 283 women) residing and working in Valencia. These subjects were randomly selected from more than 5000 employees examined in a Medical Center (Mutua de Accidentes de Trabajo y Enfermedades Profesionales). A random sample was selected and invited to
partici-pate in this study. Informed consent was only obtained in 63% of those invited. More men than women refused to provide a blood sample for the genetic analysis. Previously validated questionnaires were distributed at the time of the medical check-up, and participants were invited to fill them. Non-Caucasians, pregnant and hysterectomized women, individuals taking any lipid lowering drug or those who were diagnosed of any type of cardiovascular disease, were excluded from the study at this stage. The final group size used in this study included 514 participants with a mean age of 36.3 years (range: 17 – 66).
2.2. Sample and data collection
A venous blood sample was collected in the morning during the medical check-up into EDTA-containing glass tubes after overnight fasting. The same day, aliquots of the whole blood tubes were transported to the Central Laboratory of the Medical Center for plasma lipid analyses, and to the Laboratory of the Genetic and Molecular Epidemiology Unit, for DNA isolation and genotyping.
Anthropometric measurements were taken using standard techniques: weight with light clothing by digi-tal scales; height without shoes by fixed stadiometer. Body mass index (BMI) was calculated as the ratio of weight to the square height (kg/m2).
Blood pressure was taken with a calibrated mercury sphygmomanometer following the WHO MONICA protocol with the average of two consecutive readings of the first and fifth Korotkoff sounds for systolic and diastolic blood pressure (SBP and DBP), respectively.
2.3. Questionnaire
types of alcoholic beverages consumed in Spain, which presumably covered all types of alcoholic beverages usually consumed. For each item, the questionnaire included seven frequency categories, and requested
in-formation on the number of glasses/day. For each item,
glasses/day were calculated by adding the weekend
consumption to the workday consumption. For each alcoholic beverage, mean of daily ethanol consumption (in grams) was calculated by multiplying the amount consumed (in ml) by the percentage of ethanol supplied by each specific beverage. From the reported alcoholic beverages, alcohol consumption was categorized as a drinker variable according to the amount of alcohol consumed. Men and women were classified into three groups according to the population tertiles: No
con-sumption (alcohol concon-sumption=0 g/day), Moderate
(B10.5 g alcohol/day for men, and B5.5 g/day for
women), and High consumption (\10.6 g alcohol/day
for men, and \5.6 g alcohol/day for women). In some
analyses, only two categories were considered:
non-drinkers (alcohol consumption=0) and drinkers
(sub-jects with any amount of alcohol consumed).
Physical activity was estimated by questions about regular leisure-time physical sports (aerobics, basket-ball, bicycling, gymnastics, running, soccer, squash, swimming, tennis, volleyball, and others), as well as the average number of h per week spent in each activity. According to the type and time [23], subjects were categorized as Sedentary (no physical exercise),
Moder-ate (one sport less than 3 h/week) and High (one sport
more than 3 h/week or more than two sports). For
regression analyses, physical exercise was also dichoto-mized into Sedentary versus Active (Moderate plus High). In addition to the type and time spent per week in physical exercise, another question about regular daily walking more than 20 min, with two possible answers ‘yes’ and ‘no’, was also included.
Marital status, classified as married, single and di-vorced, was dichotomized as being Single (living alone and divorced) or Living with a partner. Education was initially coded into five categories: Non-schooling, Pri-mary school, Secondary school, University-1 (short cy-cle) and University-2 (long cycy-cle), and afterwards recoded into three levels: Primary, Secondary and Uni-versity (short+long cycles).
2.4. Laboratory analyses
Plasma total cholesterol and tryglicerides were deter-mined by a Technicon Chem 1 assay (Technicon Instru-ments, Tarrytown, NY), and HDL-C was measured in the supernatant after precipitation of apolipoprotein B — containing lipoproteins with heparin-manganese chloride. LDL-C was calculated according to the equa-tion of Friedewald et al. [24] for samples with serum triglyceride concentrations below 400 mg per deciliter.
2.5. DNA extraction and genotyping
Genomic DNA was extracted from white blood cells by phenol extraction. A fragment of 535 base pairs in the intron 1 of CETP gene was amplified by polymerase chain reaction (PCR). Each amplification was per-formed using 500 ng of genomic DNA in a volume of
50 ml containing 40 pmol of each oligonucleotide (U:
CACTAGCCCAGAGAGAGGAGTGCC and L: CT-GAGCCCAGCCGCACACTAAC), 0.2 mM dNTPs,
1.5 mM MgCl2, 10 mM Tris pH 8.4 and 0.25 U of Taq
polymerase (Gibco BRL, Paisley, UK). The PCR con-ditions were 95°C for 5 min, and subsequently 28 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 45 s, and finally at 72°C for 5 min. The PCR products were subject to restriction enzyme analysis by digestion with 4 units of the restriction endonuclease TaqI for 16ml of
PCR sample at 65°C for 2 h, in the buffer recom-mended by the manufacturer (Pharmacia Inc. Sweden).
3. Statistical analysis
Questionnaires, biochemical and genetic data were key-entered twice and processed to avoid errors in coding and interpretation. Normal distribution for all continuous variables was checked by graphical methods and by hypotheses tests. Triglycerides and alcohol in-take were markedly skewed, and these variables were logarithmically and squared root transformed, respec-tively, to improve normality for statistical testing. To assess mean differences of lipid and anthropometric
variables between genders, Student’s ttest for
indepen-dent samples was used after determining the homogene-ity of variances by the Levene statistic. Chi square tests were conducted to compare differences in percentages. This statistical test was also performed to examine whether the genotype frequencies were in Hardy – Wein-berg equilibrium [25]. For multiple comparisons of means between genotypes, one way analysis of variance
(ANOVA) was performed. P values for linear trends
between categories were also calculated using the ANOVA analysis by partition of the between-groups sums of squares into trend components. Once estab-lished that differences exist among means, to determine which means differ with correction for multiple com-parisons, Bonferroni test was applied. To test the null hypotheses of no association between CETP genotypes and HDL-C, controlling by one or more potential
environmental and genetic confounders or effect
CETP genotypes, multiple linear regression analysis with dummy variables (for categorical and interaction terms) was applied. In order to improve statistical power, data from men and women were analyzed to-gether, including a dummy variable for gender into the regression model. A core model with gender, age, BMI, CETP TaqIB genotypes, and a set of variables for tobacco smoking and alcohol consumption, was first identified using forward and backward procedures. The selection criterion for choosing the core model was the following: a variable or interaction term was retained into the model if the global term was statistically sig-nificant (PB0.05). Regression diagnostics (analysis of
residuals, influence of outliers and colinearity) were employed to check the assumptions and to assess the
Table 2
CETP TaqIB polymorphisms in the population by gendera
Women
All Men
n(%) n(%) n(%)
Genotypes
117 (41.3%) B1B1 222 (43.2%) 105 (45.5%)
100 (43.3%)
223 (43.4%) 123 (43.5%) B1B2
B2 Allele 0.351 0.329 0.369
(0.329–0.398) (0.286–0.372)
95% (C.I.) (0.322–0.380)
aDifferences by gender across CETP/TaqIB polymorphisms were
nonsignificant. Chi squarePvalue=0.332.
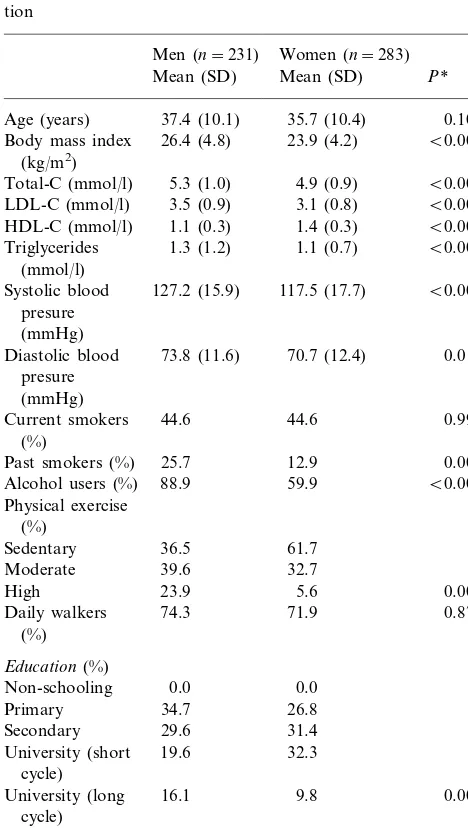
Table 1
Demographic, biochemical and life-style characteristics of the popula-tion
Men (n=231) Women (n=283) Mean (SD) Mean (SD) P*
0.102 Age (years) 37.4 (10.1) 35.7 (10.4)
Body mass index 26.4 (4.8) 23.9 (4.2) B0.001
(kg/m2)
Triglycerides 1.3 (1.2) 1.1 (0.7) B0.001
(mmol/l)
B0.001
127.2 (15.9)
Systolic blood 117.5 (17.7) presure
(mmHg)
0.017 73.8 (11.6) 70.7 (12.4)
Diastolic blood
Past smokers (%) 25.7 12.9 0.002
B0.001
Alcohol users (%) 88.9 59.9 Physical exercise
Daily walkers 71.9 0.872
(%)
University (short 19.6 32.3 cycle)
University (long 16.1 9.8 0.002
cycle)
*Pvalue in the comparison between men and women. Student’s test for comparison of means, and Chi square tests for percentages.
accuracy of computations. Once the core model was identified for the total sample, separate models with the same variables were computed for men and women. Finally, additional life-style variables (physical activity, marital status and educational level) were included into the core model defined in the previous stage. A variable or interaction term was maintained in the final regres-sion model as independent term or as control variable on the basis of statistical significance (PB0.05 orPB
0.20, respectively). All analyses were done using the Statistical Package for Social Sciences (SPSS, version 8.0) for windows.
4. Results
The main characteristics (age, BMI, lipid levels, blood pressure, tobacco smoking, alcohol consumption, physical activity, education, and marital status) of the study subjects (231 men and 283 women) are shown in Table 1. The CETP TaqIB genotype could be deter-mined unequivocally in all of them. Genotypes and allele frequencies of this polymorphism are shown in Table 2. The genotype distribution did not differ for
men and women (P=0.332) and no deviation from the
Hardy – Weinberg equilibrium was observed (P=
0.410). The calculated B2 allele frequencies were 0.351 (95% CI: 0.322 – 0.380). The allele frequency of the B2 allele was significantly lower than that reported by Kauma et al. [20] in Finland, who detected an allele
frequency for B2=0.436, with a calculated 95% CI of
0.406 – 0.466; and also statistically different from those in an average population from 11 areas of Europe [18]. In this case, the reported allele frequency for B2 was 0.440 (95% CI, 0.416 – 0.464).
Table 3 shows plasma lipid levels by CETP TaqIB genotypes and gender in the study participants. Only HDL-C levels differed statistically between the three
B2 carriers had significantly higher HDL-C levels as compared with B1B1 homozygotes. This allele effect
was present in both genders (P trendB0.001 and P
trend=0.002, in men and women, respectively). No
statistically significant associations were observed be-tween other lipid variables and the CETP TaqIB poly-morphism. After Bonferroni corrections, compared with the B1B1 genotype, the mean HDL-C level in men
was 5.8% (P\0.05) and 20.6% (PB0.05) higher in the
B1B2 and B2B2 genotype, respectively. In women these
increases were 5.3% (P\0.05) and 13.5% (PB0.05).
To assess the influence of covariates on this association, we examined the distribution of BMI, age, tobacco smoking, alcohol consumption, physical activity,
mari-tal status and education across CETP TaqIB polymor-phism. No statistically significant differences for any of these variables across genotypes were observed (data not shown).
To investigate the influence of the measured environ-mental factors and the CETP TaqIB polymorphism on HDL-C levels, we carried out multivariate modeling with dummy variables as described in Section 2. Table 4 presents the results of the core model (with age, BMI, tobacco smoking and alcohol consumption as indepen-dent variables) for all subjects, and for men and women separately. From the various fitted models, a continu-ous variable (cig/day) was the most statistically
signifi-cant indicator (P=0.003) of tobacco smoking related
Table 3
Plasma lipid levels by CETP/TaqIB genotypes and gender in the total sample
B1B2
Mean (SD) Mean (SD) Mean (SD)
0.217 4.90 (0.83) 5.01 (1.02) 4.74 (0.91)
Women
Men 3.44 (0.82) 3.63 (0.89)
LDL-C (mmol/l) 3.18 (0.70) 0.067 0.219
Men 1.02 (0.26)b 1.08 (0.24)b
HDL-C (mmol/l) 1.23 (0.16)a,c 0.004 0.001
0.008 0.002 Women 1.33 (0.30)b 1.40 (0.27)b 1.51 (0.25)a,c
Men 1.86 (1.65) 1.47 (1.16)
Triglycerides (mmol/l) 1.30 (0.85) 0.418 0.339
Women 0.88 (0.48) 0.95 (0.58) 0.79 (0.33) 0.279 0.526 Men 38.6 (9.63)
Age (years) 36.0 (10.13) 38.0 (11.64) 0.219 0.823
0.658
*Pvalue obtained in the ANOVA test for the global comparison between genotypes. a, b, c:Pvalues obtained in the Bonferroni post-hoc test: a:PB0.05 compared with B1B1 genotype; b:PB0.05 compared with B2B2 genotype; c:PB0.05 compared with B1B2 genotype.
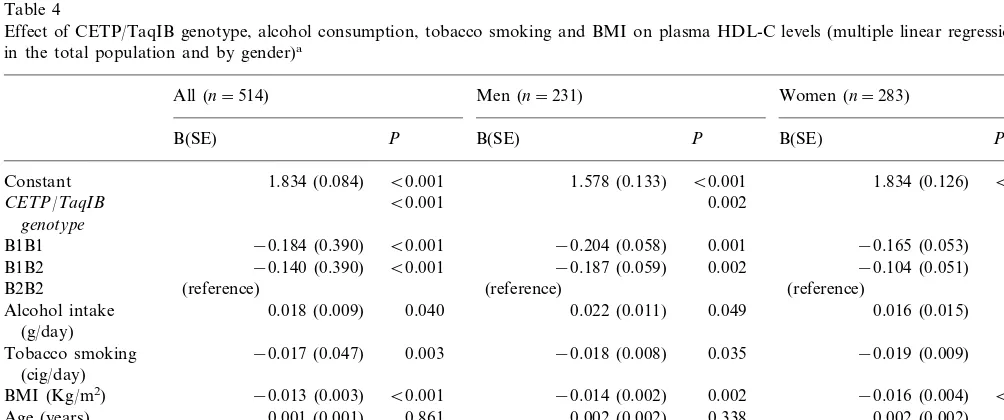
Table 4
Effect of CETP/TaqIB genotype, alcohol consumption, tobacco smoking and BMI on plasma HDL-C levels (multiple linear regression analysis in the total population and by gender)a
All (n=514) Men (n=231) Women (n=283)
B(SE) P B(SE) P B(SE) P
B0.001 B0.001
1.834 (0.084) 1.834 (0.126)
Constant 1.578 (0.133) B0.001
0.009
B1B1 −0.204 (0.058) 0.001 −0.165 (0.053) 0.002
0.050
Alcohol intake 0.049 0.016 (0.015)
(g/day)
0.048
−0.017 (0.047) 0.003
Tobacco smoking −0.018 (0.008) 0.035 −0.019 (0.009)
(cig/day)
Age (years) 0.001 (0.001) 0.338
Gender B0.001
B0.001
Male −0.297 (0.030)
(reference) Female
R2 of the model 0.376 B0.001 0.143 B0.001 0.147 B0.001
aDependent variable: HDL-C (mmol/l); B=regression coefficient. SE=standard error; Regression coefficients for alcohol intake and tobacco
Table 5
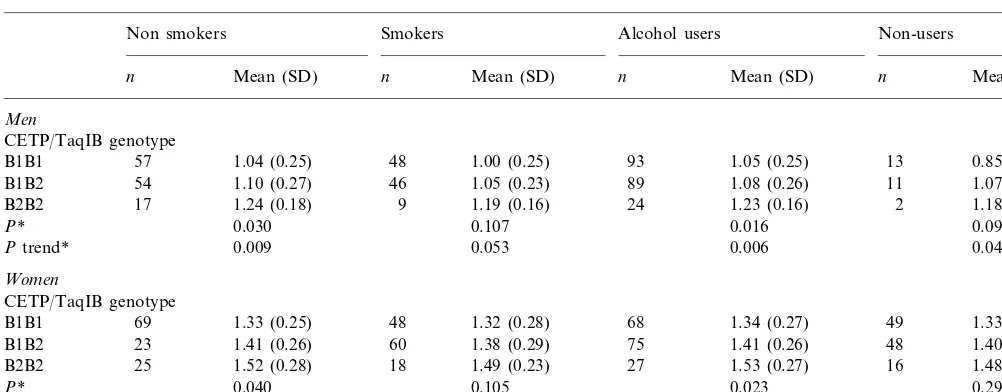
Mean plasma HDL-C according to CETP/TaqIB genotype, stratified by tobacco smoking, alcohol consumption and gender
Non smokers Smokers Alcohol users Non-users
Mean (SD)
n n Mean (SD) n Mean (SD) n Mean (SD)
Men
CETP/TaqIB genotype
1.04 (0.25) 48 1.00 (0.25) 93 1.05 (0.25) 13 0.85 (0.23)
B1B1 57
1.10 (0.27) 46 1.05 (0.23) 89
54 1.08 (0.26)
B1B2 11 1.07 (0.15)
17
B2B2 1.24 (0.18) 9 1.19 (0.16) 24 1.23 (0.16) 2 1.18 (0.17)
0.030 0.107 0.016 0.098
P*
0.009 0.053 0.006
Ptrend* 0.046
Women
CETP/TaqIB genotype
1.33 (0.25) 48 1.32 (0.28)
B1B1 69 68 1.34 (0.27) 49 1.33 (0.27)
1.41 (0.26) 60 1.38 (0.29) 75
23 1.41 (0.26)
B1B2 48 1.40 (0.29)
1.52 (0.28) 18 1.49 (0.23) 27
B2B2 25 1.53 (0.27) 16 1.48 (0.21)
0.040 0.105 0.023
P* 0.294
Ptrend* 0.012 0.045 0.006 0.113
*Pvalues obtained in the ANOVA test for the global comparison between genotypes.
to HDL-C in the total sample. For alcohol consump-tion, also a continuous variable (square root of daily ethanol intake in g/day) was better than the categorical variables created as described in Section 2. Interest-ingly, when type of alcoholic beverages was further explored (beer, red wine, white wine or hard liquor), red wine consumption was the most positive and
statis-tically associated with HDL-C levels (PB0.001), while
beer consumption presented a negative regression co-efficient (P\0.05). In this core model, when the whole
group was examined, we found that BMI, age, CETP
TaqIB polymorphism, total alcohol consumption (g/
day) and tobacco smoking (cig/day), were statistically
significant predictors of HDL-C levels, explaining 37.6% (adjusted r2
=0.376; PB0.001) of the variance.
After adjusting for age, BMI, tobacco smoking and alcohol consumption, TaqIB polymorphism was
inde-pendently associated (PB0.001) with plasma HDL-C
levels, and was able to explain 5.8% of the variance in the HDL-C in the population. Considering B2B2 indi-viduals as reference, B1B2 subjects showed a mean
decrease of HDL-C levels of −0.140 mmol/l (PB
0.001), and B1B1 subjects a mean decrease of −0.184
mmol/L (PB0.001). This effect was comparable to the
gender effect, which lowers HDL-C by −0.297 mmol/l
(PB0.001) in men, compared with women (PB0.001).
When first order interaction terms between the poly-morphism and environmental variables (tobacco and alcohol) were tested, no statistically significant terms
(P=0.715 for tobacco smoking andP=0.455 for
alco-hol consumption; see Table 5 for HDL-C and TaqIB genotype by smoking and alcohol drinking status) were found, and they were not included in the previously reported core model. In the separated analysis for men and women (Table 4), the CETP TaqIB polymorphism
was also independently associated with HDL-C levels
in both men and women (PB0.01).
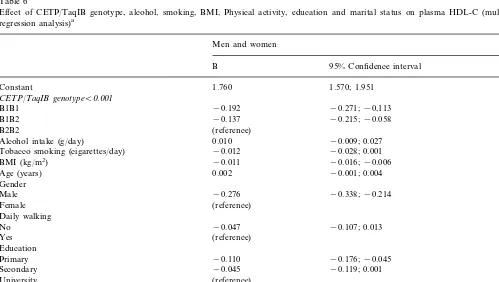
When additional life-style variables (physical activity, education and marital status) were added to the core model (Table 6), CETP TaqIB polymorphism also re-mained independently and consistently (as judged by regression coefficients) associated with HDL-C levels
(PB0.001). Educational level was strongly associated
with HDL-C (P=0.005). Individuals with higher
edu-cation (university as reference) presented the highest HDL-C. Marital status was also independently and
statistically associated with HDL-C (P=0.048).
Physi-cal exercise did not enter into the final model either as a three-level variable (sedentary, moderate and high), or as a dichotomized variable (sedentary or active). Daily walking was associated with a mean increase of
0.047 mmol/l in HDL-C without reaching statistical
significance (P=0.126). With regard to tobacco
smok-ing, the introduction into the core model of educational level and marital status, there was an important change
in its statistical significance (from P=0.003, to P=
0.070). A similar trend was observed for alcohol
con-sumption (from P=0.040 toP=0.317). Finally, when
separate models for men and women were computed (results not shown), the effect of CETP TaqIB poly-morphism on HDL-C levels, controlling for alcohol, tobacco, physical activity, education and marital status, remained statistically significant in both genders.
5. Discussion
environmental factors, in a random healthy population from the Mediterranean region of Spain. Our study was designed to have homogeneous ethnic origin and place of residence. The sample size was sufficiently large to provide good estimates of prevalence at a global level and by gender, but generally did not permit a valid sub-stratification of variables at the stratum level. Moreover, the study population consisted of young, healthy subjects to minimize the effects of medication and aging on serum lipid profiles.
The increasing recognition of genetic-environmental interactions in the etiology of most diseases [26 – 28], and the important advances in the DNA technology, make it necessary to include genetic information into epidemiological studies to identify how some polymor-phic traits are associated with several phenotypic traits, taking into account environmental factors [26 – 30]. Along those lines, in the present study we have ana-lyzed the common TaqIB polymorphism on the CETP gene in the context of a population survey to ascertain the prevalence of genetic and environmental cardiovas-cular risk factors in the Valencia Community. This region has been characterized by having a higher inci-dence of cardiovascular disease than other Spanish regions. Interestingly, allele frequencies of the CETP TaqIB polymorphism found in this region were differ-ent from those reported in other Caucasoid populations
from North Europe [20,21,31], the South of Europe [18] or North-America [32], and were similar to those re-ported in Koreans [33]. In this Mediterranean popula-tion, allelic frequency for the B2 allele (0.357; 95% CI: 0.317 – 0.396) was the lowest among those reported in Caucasian populations. A recent paper [18], investigat-ing the associations of the TaqIB polymorphism with plasma lipoproteins in 14 university student popula-tions from 11 European countries, concluded that the overall frequency of the B2 allele in these countries was 0.44 (95%CI: 0.406 – 0.466), with no significant varia-tion across Europe. This finding must be interpreted with caution because of the small sample size of the
population sampled in each country (B50). The
au-thors found the following allele frequencies: 0.46 for the Baltic region (Tallinn, Estonia; Helsinki and Oulou, Finland), 0.44 for the UK region (Glasgow, Belfast and Bristol, UK), 0.38 for the Middle region (Aarhus, Den-mark; Hamburg, Germany; Gent, Belgium, and Zurich, Switzerland) and 0.41 for the South region (Lisbon, Portugal; Reus, Spain; Naples, Italy and Athens, Greece). Adding our estimated allelic frequency for the B2 allele in the east of Spain to the previously reported data, a north to south decline in the prevalence of this allele can be observed. This geographical variation may be less important than the clearly described north to south decline in the o4 allele frequency at the
apolipo-Table 6
Effect of CETP/TaqIB genotype, alcohol, smoking, BMI, Physical activity, education and marital status on plasma HDL-C (multiple linear regression analysis)a
Men and women
P 95% Confidence interval
B
1.760 1.570; 1.951
Constant B0.001
CETP/TaqIB genotypeB0.001
B1B1 −0.192 −0.271;−0,113 B0.001
0.001
−0.215;−0.058
B1B2 −0.137
(reference) B2B2
0.010
Alcohol intake (g/day) −0.009; 0.027 0.317
−0.028; 0.001 0.070
Tobacco smoking (cigarettes/day) −0.012
BMI (kg/m2) −0.011 −0.016;−0.006 B0.001
Age (years) 0.002 −0.001; 0.004 0.186
B0.001
Gender
−0.338;−0.214 B0.001
Male −0.276
(reference) Female
Daily walking 0.126
−0.107; 0.013 0.126
−0.047 No
Yes (reference)
Education 0.005
−0.110
Primary −0.176;−0.045 B0.001
−0.045
Secondary −0.119; 0.001 0.051
(reference) University
0.048 Marital status
0.052
Single 0.001; 0.121 0.048
Married (reference)
R2 of the model 0.396 B0.001
protein E gene for continental Western Europe [34]. Interpopulation differences in the prevalence of genetic traits have been related to the variation in the preva-lence of traditional cardiovascular risk factors, and also to the mortality rates. It has been hypothesized that
higher frequency of theo4 allele in Northern European
countries could account for some of the higher CHD incidence in those countries as compared to Southern Europe [35]. While the incidence of CHD in Mediter-ranean populations is lower than in North-European ones, the TaqIB2 allele is more prevalent in the north-ern populations. In order to reconcile this apparent paradox, one can hypothesize that there is an evolu-tionary selection for the protective B2 allele in individu-als in northern countries due to the more atherogenic environment present in those countries. This selection might not exist in Southern Europe because the pres-ence of environmental protective factors make these subjects less dependent upon the ‘genetic protection’ provided by the B2 allele through its association with elevated HDL-C levels. Conversely, if the B2 allele is negatively involved in cardiovascular mortality, the higher mortality rates observed in the Valencia Com-munity as compared with other geographically defined populations in Spain might be determined by the low prevalence of this allele. Currently, there are no pub-lished data in our country to contrast this hypothesis. Despite the different prevalence for the B2 allele, in the present study an independent and consistent associ-ation of CETP TaqIB polymorphism with HDL-C levels was found. No statistically significant differences in other lipid levels were observed across TaqIB geno-types. The association with HDL-C has been reported before by several studies [14,16,17,20,36 – 39]. Some of them also found a relation with LDL-C, tryglicerides or apolipoprotein A-I levels [18,36,39]. CETP activity was measured in various investigations and related to TaqIB genotype [18,20,37,39]. However, the relation-ship between plasma CETP activity and HDL-C levels has not been clarified [40], and the lack of an associa-tion between CETP activity and the TaqIB polymor-phism has also been reported by other investigators [41]. In the present study, data on CETP activity were not available. The mechanism by which the TaqIB polymorphism may affect CETP activity or HDL-C levels is not known. It is very unlikely that this poly-morphism located in an intron is a functional mutation. Given the reported associations of the B2 allele with
increased CETP mass and/or activity, the most
plausi-ble explanation is that this polymorphism is in linkage disequilibrium with a still unknown functional muta-tion in the regulatory region of the CETP gene. In addition to the TaqIB polymorphism, some variations on the CETP gene have been associated with CETP activity and HDL-C levels [4,18,42]. The real impact of CETP activity on atherosclerosis is still unknown [43].
CETP is susceptible to play a proatherogenic role, since it mediates a redistribution of plasma cholesterol from
lipoproteins associated with a protection against
atherosclerosis into the proatherogenic apo B-contain-ing lipoproteins. This concept is also supported by the fact that animal species that are resistant to diet in-duced atherosclerosis have little CETP activity. How-ever, CETP mediates one of the steps in reverse cholesterol transport, an antiatherogenic process [44]. On the other hand, experiments carried out in trans-genic mice have showed that environmental factors play an important role in the modulation of the expression of the CETP gene [45]. Various studies in human populations have analyzed the possible interaction be-tween some environmental factors and CETP TaqIB polymorphism on plasma HDL-C levels. Kondo et al. [36] showed that practically the whole effect of the CETP gene on the HDL-C was due to the effect observable in non-smokers. In another study carried out in Finland [20], smoking men with the B2 allele tended to have HDL-C levels lower by 10% than smok-ing men with the B1 allele, but this effect was not observed in women, and the authors also concluded a regulation by sex hormones. Fumeron et al. [16] did not find an interaction with tobacco smoking, but they found an important interaction with alcohol consump-tion in the ECTIM study. Informaconsump-tion about other environmental factors such as exercise, education or marital status that have been important predictors of HDL-C related to lifestyle [46 – 49] was scarce. In the present study, when gene-environmental terms were tested in a multivariate regression model, no statisti-cally significant interactions of the TaqIB genotypes with alcohol consumption, tobacco smoking, physical activity or education level were found. This observation allowed us to conclude that the effect of this polymor-phism on plasma HDL-C in this healthy population seems statistically independent and uniform across sev-eral levels of these environmental factors. However, we observed a non significant trend suggesting that the TaqIB association with HDL-C levels was less promi-nent in smokers and in non-drinkers. Our hypothesis is that alcohol and smoking could be ‘markers’ for an-other unknown environmental variable(s) that may be differentially expressed in the populations studied. Al-ternatively, interactions between this CETP marker and alcohol or smoking could be obscured by other stronger modulators for which we have not accounted. However, for a more accurate estimation of the effect in the previously defined environmental strata, further research on a large number of subjects is needed.
married men are at lower risk of CHD, unless they were married to women of higher education. [47]. How-ever, at least one large population study [48] has exam-ined this issue in detail and it has shown that married men tend to have lower HDL-C levels and higher cholesterol levels. The factors responsible for these effects are not known, but one can speculate that this may be due in part to several behavioral factors that are different in married and never married people. Venters et al. [48] reported that married subjects have less consumption of alcoholic beverages and also less physical activity. There are other factors, such as so-cioeconomic status that may induce different type of stress in single versus married people. Also, in agree-ment with those observations, Woo et al. [49] have shown that single men had a better cardiovascular risk factor profile consisting of lower BMI, DBP, TG, and
TC/HDL-C. Therefore, the literature shows a
consis-tent pattern that could be explained by a cluster of behavioral factors that are different in single and mar-ried subjects and that may have a significant effect on plasma lipid levels.
The study of these gene-environmental interactions can provide an important basis for refining the predic-tive value of traditional epidemiological risk factors and for targeting intervention and prevention activities for high-risk individuals [26 – 28].
Acknowledgements
We gratefully acknowledge the contributions of Dr J. Folch and M.D. Pe´rez-Merelo for assistance in con-ducting fieldwork. We are grateful to O. Coltell, lec-turer of the Computer Sciences Department at the Jaume I University, Castello´n, Spain, for his assistance in computer and software support. We thank all the subjects participating in the study. This work was sup-ported by grants from the Universitat de Vale`ncia (UV98-2723) and the Conselleria de Cultura, Educacio´ i Cie`ncia de la Generalitat Valenciana (GV98-12-123) and by Grant HL54776 from the NHLBI, Bethesda, MD.
References
[1] Miller GJ, Miller NE. Plasma high-density lipoprotein concen-tration and development of ischaemic heart disease. Lancet 1975;1:16 – 9.
[2] Gordon DJ, Probstfield JL, Garrison RJ. High density lipo-protein cholesterol and cardiovascular disease. Circulation 1989;79:8 – 15.
[3] Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb J, Tall AR. Increased coronary heart disease in Japanese – American men with mutations in the cholesteryl ester transfer protein despite increased HDL levels. J Clin Invest 1996;97:2917 – 23.
[4] Moriyama Y, Okamura T, Inazu A, Doi M, Iso H, Mouri Y, Ishikawa Y, Suzuki H, Iida M, Koizumi J, Mabuchi H, Ko-machi Y. A low prevalence of coronary heart disease among subjects with increased high-density lipoprotein cholesterol lev-els, including those with plasma cholesterol ester transfer protein deficiency. Prev Med 1998;27:659 – 67.
[5] Kiechl S, Willeit J, Rungger G, Egger G, Oberhollenzer F, Bonora E. Alcohol consumption and atherosclerosis: what is the relation? Prospective results from the Bruneck Study. Stroke 1998;29:900 – 7.
[6] Lloyd T, Chinchilli VM, Rollings N, Kieselhorts K, Tregea DF, Henderson NA, Sinoway LI. Fruit consumption, fitness, and cardiovascular health in female adolescents: the Penn State Young Women’s Health Study. Am J Clin Nutr 1998;67:624 – 30. [7] Austin MA, King MC, Bawol RD, Hulley SB, Friedman GD. Risk factors for coronary heart disease in adult female twins: genetic heritability and shared environmental influences. Am J Epidemiol 1987;125:308 – 18.
[8] Tall AR. Plasma cholesterol ester transfer protein. J Lipid Res 1993;34:1255 – 74.
[9] Drayna D, Jarnagin AS, McLean J, Henzel W, Kohr W, Field-ing C, Lawn R. ClonField-ing and sequencField-ing of human cholesteryl ester transfer protein cDNA. Nature (London) 1987;327:632 – 4. [10] Bu X, Warden YR, Xia CD, Meester CD, Puppione DL, Teruya S, Lokensgard B, Daneshmand S, Brown J, Gray R, Rotter J, Lusis AJ. Linkage analysis of the genetic determinants of high-density lipoprotein concentrations and composition: evidence for involvement of the apolipoprotein A-II and cholesteryl ester transfer protein (CETP) loci. Hum Genet 1994;93:639 – 48. [11] Agellon LB, Quinet EM, Gillette TG, Drayna DT, Brown ML,
Tall AR. Organization of the human cholesteryl ester transfer protein gene. Biochemistry 1990;29:1372 – 6.
[12] Tall AR. Plasma cholesteryl ester transfer protein and high-den-sity lipoproteins: new insight from molecular genetic studies. J Internal Med 1995;237:5 – 12.
[13] Inazu A, Jiang XC, Haraki T, Yagi K, Kamon N, Koizumi J, Mabuchi H, Takeda R, Takata K, Moriyama Y, Doi M, Tall A. Genetic cholesteryl ester transfer protein deficiency caused by two prevalent mutations as a major determinant of increased levels of high density lipoprotein cholesterol. J Clin Invest 1994;94:1872 – 82.
[14] Mitchell RJ, Earl L, Williams J, Bisucci T, Gasiamis H. Poly-morphisms of the gene coding for the cholesteryl ester transfer protein and plasma lipid levels in Italian and Greek migrants to Australia. Hum Biol 1994;66:13 – 25.
[15] Drayna D, Lawn R. Multiple RFLPs at the human cholesteryl ester transfer protein (CETP) locus. Nucleic Acids Res 1987;11:4698.
[16] Fumeron F, Betoulle D, Luc G, Behague I, Ricard S, Poirier O, et al. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J Clin Invest 1995;96:1664 – 71.
[17] Bernard S, Moulin P, Lagrost L, Picard S, Elchebly M, Ponsin G, Chapuis F, Berthezene F. Association between plasma HDL-Cholesterol concentration and Taq1B CETP gene polymorphism in non-insulin-dependent diabetes mellitus. J Lipid Res 1998;39:59 – 65.
[18] Gudnason V, Kakko S, Nicaud V, Savolainen MJ, Kesaniemi YA, Thavanainen E, Humphries S. Cholesteryl ester transfer protein gene effect on CETP activity and plasma high-density lipoprotein in European populations. Eur J Clin Invest 1999;29:116 – 28.
[20] Kauma H, Savolainen MJ, Heikkila E, Rantala AO, Lilja M, Reunanen A, et al. Sex difference in the regulation of plasma high density lipoprotein cholesterol by genetic and environmen-tal factors. Hum Genet 1996;97:156 – 62.
[21] Hannuksela ML, Liinamaa MJ, Kesaniemi YA, Savolainen MJ. Relation of polymorphisms in the cholesteryl ester transfer protein gene to transfer protein activity and plasma lipoprotein levels in alcohol drinkers. Atherosclerosis 1994;110:35 – 44. [22] Villar F, Banegas-Banegas JR, Rodriguez-Artalejo A,
Rey-Calero J. Mortalidad cardiovascular en Espan˜a y sus comu-nidades auto´nomas. Med Clin (Barc) 1998;110:321 – 7.
[23] Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr., Montoye HJ, Sallis JF, Paffenbarger RS. Compendium of physical activi-ties: Classification of energy cost of human physical activities. Med Sci Sport Exercise 1992;25:71 – 80.
[24] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499 – 502.
[25] Cannings C, Edwards AW. Expected genotypic frequencies in a small sample: deviation from Hardy – Weinberg equilibrium. Am J Hum Genet 1969;21:245 – 7.
[26] Khoury MJ. From genes to public health: The applications of genetic technology in disease prevention. Am J Public Health 1996;86:1717 – 22.
[27] Ambrosone CB, Kadlubar FF. Toward an integrated aproach to molecular epidemiology. Am J Epidemiol 1997;146:912 – 8. [28] Khoury MJ, Yang Q. The future of genetic studies of complex
human diseases: an epidemiologic perspective. Epidemiology 1998;9:350 – 4.
[29] Khoury MJ. Genetic epidemiology and the future of disease prevention and public health. Epidemiol Rev 1997;19:175 – 80. [30] Khoury MJ. Relationship between medical genetics and public
health: changing the paradigm of disease prevention and the definition of a genetic disease. Am J Med Genet 1997;71:289 – 91. [31] Freeman DJ, Griffin BA, Holmes AP, Lindsay GM, Gaffney D, Packard CJ, Shepherd J. Regulation of plasma HDL cholesterol and subfraction distribution by genetic and environmental fac-tors. Associations between the TaqIB RFLP in the CETP gene and smoking and obesity. Arterioscler Thromb 1994;14:336 – 44. [32] Kessling A, Oullette S, Bouffard O, Chamberland A, Be´tard C, Selinger E, Xhignesse M, Lussier-Cacan S, Davignon J. Patterns of association between genetic variability in apolipoprotein (Apo) B, Apo AI-CIII-AIV, and cholesterol ester transfer protein gene regions and quantitative variation in lipid and lipoprotein traits: influence of gender and exogenous hormone. Am J Hum Genet 1991;50:92 – 106.
[33] Song GJ, Han GH, Chae JJ, Namkoong Y, Lee HK, Park YB, Lee CC. The effects of the cholesterol ester transfer protein gene and environmental factors on the plasma high density lipo-protein cholesterol levels in the Korean population. Mol Cells 1997;31:615 – 9.
[34] Lucotte G, Loirat F, Hazout S. Pattern of gradient of apolipo-protein E allele *4 frequencies in Western Europe. Hum Biol 1997;69:253 – 62.
[35] Stengard JH, Weiss KM, Sing CF. An ecological study of association between coronary heart disease mortality rates in men and the relative frequencies of common allelic variations in
the gene coding for apolipoprotein E. Hum Genet 1998;103:234 – 41.
[36] Kondo IK, Berg D, Drayna D. DNA polymorphism at the locus for human cholesteryl ester transfer protein (CETP) is associated with high density lipoprotein cholesterol and apolipoprotein levels. Clin Genet 1989;35:49 – 56.
[37] Kuivenhoven JA, de Knijff P, Boer JMA, Smalheer HA, Botma GJ, Seidell JC, Kastelein JJP, Pritchard PH. Heterogeneity at the CETP concentrations and HDL cholesterol levels. Arterioscler Thromb Vas Biol 1997;17:560 – 8.
[38] Freeman D, Packard J, Shepherd J, Gaffney D. Polymorphisms in the gene coding for cholesteryl ester transfer protein are related to plasma high-density lipoprotein cholesterol and trans-fer ester activity. Clin Sci 1990;79:575 – 81.
[39] Kuivenhoven JA, Jukema JW, Zwinderman AK, de Knijff P, McPherson R, Bruschke AVG, Lie KI, Kastelein JJP. The role of a common variant of the cholesteryl ester transfer protein gene in the progression of coronary atherosclerosis. N Engl J Med 1998;338:86 – 93.
[40] Sasai K, Okumura-Noji K, Hibino T, Ikeuchi R, Sakuma N, Fujinami T, Yokoyama S. Human cholesteryl ester transfer protein measured by enzyme-linked immonoabsorbent assay with two monoclonal antibodies against rabbit cholesteryl ester transfer protein: plasma cholesteryl ester transfer protein and lipoproteins among Japanese hypercholesterolemic patients. Clin Chem 1998;44:1466 – 73.
[41] Freeman DJ, Griffin BA, Holmes AP, Lindsay GM, Gaffney D, Packard CJ, Shepherd J. Regulation of plasma HDL cholesterol and subfraction distribution by genetic and environmental fac-tors: associations between the TaqI B RFLP in the CETP gene and smoking and obesity. Arterioscler Thromb 1994;14:336 – 44. [42] Kakko S, Tamminen M, Kesaniemi YA, Savolainen MJ. R451Q mutation in the cholesteryl ester transfer protein (CETP) gene is associated with high plasma CETP activity. Atherosclerosis 1998;136:233 – 40.
[43] Fielding CJ, Havel RJ. Cholesteryl ester transfer protein: Friend or foe? J Clin Invest 1996;97:2687 – 8.
[44] Stevenson CG. Cholesterol ester transfer protein: a molecule with three faces? Crit Rev Clin Lab Sci 1998;35:517 – 46. [45] Jiang XC, Agellon LB, Walsh A, Breslow JL, Tall AR. Dietary
cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice: dependence of natural flanking sequences. J Clin Invest 1992;90:1290 – 5.
[46] Mensink GBM, Loose N, Oomen CM. Physical activity and its association with other lifestyle factors. Eur J Epidemiol 1997;13:771 – 8.
[47] Kannel WB, Eaker ED. Psychosocial and other features of coronary heart disease: insights from the Framingham Study. Am Heart J 1986;112:1066 – 73.
[48] Venters M, Jacobs DR, Pirie P, Luepker RV, Folsom AR, Gillum RF. Marital status and cardiovascular risk: The Minne-sota Heart Survey and the MinneMinne-sota Heart Health Program. Prev Med 1986;15:591 – 605.
[49] Woo J, Leung SS, Ho SC, Sham A, Lam TH, Janus ED. Influence of educational level and marital status on dietary intake, obesity and other cardiovascular risk factors in a Hong Kong Chinese population. Eur J Clin Nutr 1999;53:461 – 7.