Expression of parathyroid hormone-related protein in human and
experimental atherosclerotic lesions: functional role in arterial
intimal thickening
Michiro Ishikawa
a,b, Masahiro Akishita
a, Koichi Kozaki
a, Kenji Toba
a,
Atsushi Namiki
b, Tetsu Yamaguchi
b, Hajime Orimo
a, Yasuyoshi Ouchi
a,*
aDepartment of Geriatric Medicine,Graduate School of Medicine,The Uni6ersity of Tokyo,7-3-1 Hongo,Bunkyo-ku,
Tokyo 113-8655, Japan
bThird Department of Internal Medicine,Toho Uni6ersity School of Medicine,Ohashi Hospital,2-17-6 Ohashi,Meguro-ku,
Tokyo 153, Japan
Received 16 February 1999; received in revised form 14 September 1999; accepted 3 November 1999
Abstract
We investigated the expression of parathyroid hormone-related protein (PTHrP) in atherosclerotic lesions and the role of PTHrP in the development of arterial neointima formation. Immunohistochemical staining of PTHrP in the neointima of rat aorta produced by balloon injury and of rat femoral artery produced by non-obstructive polyethylene cuff placement, and in the atherosclerotic lesion of human coronary artery was performed using anti-human PTHrP-(1-34) antibody. Anti-muscle actin antibody, HHF-35, and anti-macrophage antibody, HAM-56, were used to identify smooth muscle cells and macrophages, respectively. Immunoreactivity of PTHrP was detected in the thickened intima of rat and human lesions where the predominant cell types were smooth muscle cells or macrophages dependently on the lesion type. In the next series of experiments, we examined the effect of PTHrP on the development of cuff-induced intimal thickening of rat femoral artery. Either PTHrP-(1-34) or PTHrP-(7-34), a PTH/PTHrP receptor antagonist, suspended in pluronic F-127 gel was locally applied around the rat femoral artery. Intimal thickening induced by cuff placement was evaluated 2 weeks later. PTHrP-(1-34) dose-dependently inhibited intimal thickening determined as intima/media ratio and % stenosis whereas PTHrP-(7-34) dose-dependently enhanced that. These results suggest that PTHrP, which is expressed in atherosclerotic lesions, inhibits the development of neointimal formation. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Parathyroid hormone-related protein; Vascular smooth muscle cell; Immunohistochemistry; Atherosclerosis; Neointima
www.elsevier.com/locate/atherosclerosis
1. Introduction
Atherosclerosis is characterized by arterial luminal narrowing associated with thickening of the arterial intima. Similar pathological changes have been demon-strated at the restenotic site of coronary arteries after angioplasty or atherectomy [1,2]. A large number of biologically active substances such as growth factors, cytokines and vasoactive peptides have been considered to play an important role in the atherogenic and restenotic processes [3,4]. Since the discovery of
platelet-derived growth factor [5], various substances with atherogenic properties have been identified and their relation with the development of atherosclerosis or restenosis has been extensively investigated. However, the information concerning the role of vasoactive sub-stances with anti-atherogenic property in this process is scanty.
Parathyroid hormone-related protein (PTHrP) was originally identified in human tumor tissues as a causative factor of the syndrome of humoral hypercal-cemia of malignancy [6 – 11]. The presence of PTHrP has been demonstrated not only in tumor tissues but also in a variety of tissues [12 – 14]. Some recent reports have shown that PTHrP is expressed in vascular smooth muscle cells (VSMCs) [15] and vascular en-* Corresponding author. Tel.:+81-3-5800-8830; fax:+
81-3-5800-6529.
E-mail address:[email protected] (Y. Ouchi).
dothelial cells [16,17], and a common receptor for parathyroid hormone (PTH) and PTHrP exists in vas-cular smooth muscle [18]. However, there have been few reports demonstrating the expression of PTHrP in the thickened intima of atherosclerotic and restenotic lesions [19,20]. Moreover, the pathophysiological role of PTHrP in the vascular wall, either in the normal or diseased state, has not been elucidated.
PTHrP has a potent vasodilator action [21 – 24] which is associated with increased production of cyclic AMP and decreased intracellular free calcium concentration in vascular smooth muscle [24]. Also, PTHrP exhibits an inhibitory effect on VSMC migration and prolifera-tion [25]. These acprolifera-tions of PTHrP and its expression in a variety of tissues have led us to examine the expres-sion of PTHrP and its pathophysiological role in atherosclerotic lesions. Here we report that PTHrP is expressed in human atherosclerotic lesions as well as experimentally produced neointimal lesions. Further-more, we demonstrate that locally administered PTHrP inhibits and PTHrP antagonist enhances intimal thick-ening induced by non-obstructive polyethylene cuff in rats.
2. Materials and methods
2.1. Materials
Human (h) PTHrP-(1-34) and hPTHrP-(7-34) were purchased from Peptide Institute Inc., Osaka, Japan. Rabbit anti-hPTHrP-(1-34) polyclonal antibody was purchased from Peninsula Laboratories Inc. (Belmont, CA, USA) [16]. This antibody showed no cross-reactiv-ity with hPTH-(1-34), rat PTH-(1-34) or
Asp6-hPTH-(1-84). Anti-muscle actin monoclonal antibody,
HHF-35 [26] (Enzo Diagnostics Inc., New York, NY, USA), and anti-macrophage monoclonal antibody, HAM-56 [27] (Enzo Diagnostics Inc.), were also used.
2.2. Rat neointimal lesion
Male Wistar rats (12 weeks old), purchased from Nippon Bio-Supply Center (Tokyo, Japan), were anes-thetized with ether. A 2F embolectomy balloon catheter (Baxter Healthcare Co., Santa Ana, CA, USA) was passed into the aorta via the left femoral artery and positioned at the distal end of the aortic arch. The balloon was then inflated with 1.0 ml saline and was withdrawn slowly to the aortic bifurcation. This proce-dure was repeated twice. Thoracic aorta was removed 2 weeks after injury, fixed in 10% formalin neutral buffer solution, and embedded in paraffin.
Male Wistar rats (12 weeks old) were anesthetized with ether. Through a left inguinal incision, the left femoral artery was cleared of surrounding connective
tissue and loosely sheathed with a PE-160 polyethylene cuff (10 mm in length, 1.14 mm in inner diameter, 1.57 mm in outer diameter, Becton Dickinson and Com-pany, NJ, USA) and the incision was then closed [28,29]. The femoral artery was removed 2 weeks later, fixed in 10% formalin neutral buffer solution, and embedded in paraffin.
2.3. Human atherosclerotic lesion
The atherosclerotic lesion of a human coronary artery was obtained at directional coronary atherec-tomy (DCA) performed at Toho University Ohashi Hospital, Tokyo, Japan. The patient was a 55-year-old male in whom restenosis occurred 3 months after percu-taneous transluminal coronary angioplasty (PTCA) of the left anterior descending coronary artery. Another human coronary artery with a primary atherosclerotic lesion was obtained at autopsy at The Social Health Insurance Medical Center, Tokyo, Japan. The patient was a 72-year-old male. The specimens were fixed in 10% formalin neutral buffer solution (Wako Pure Chemical Industries Ltd., Tokyo, Japan) and were em-bedded in paraffin blocks.
2.4. Immunohistochemical staining of PTHrP
The formalin-fixed and paraffin-embedded tissues were sliced. The tissue sections were deparaffinized with xylene three times (3 min for each xylene treatment) and rehydrated with 100% ethanol twice and 95% etha-nol twice (3 min for each ethaetha-nol treatment). The sections were then washed with phosphate-buffered sa-line (PBS, pH 7.4) for 5 min. After blocking endoge-nous peroxidase activity with 0.03% hydrogen peroxide in methanol, the sections were incubated with 10% normal goat serum in PBS for 30 min. After these procedures, the sections were incubated overnight at 4°C with rabbit polyclonal antibody raised against hPTHrP-(1-34) diluted with PBS containing 10% fetal bovine serum. The dilution of this antibody was opti-mized, and 1:500 dilution was used in this study. The streptavidin-biotin-peroxidase (SAB) method was per-formed using Histofine SAB kit (Nichirei Co., Tokyo, Japan). Briefly, the tissue sections were incubated with biotinylated secondary antibody for 45 min at room temperature, and were subsequently incubated with streptavidin conjugated with horseradish peroxidase for 30 min at room temperature. The reaction was termi-nated by washing three times with PBS (5 min for each washing). Immunoreactive PTHrP was visualized by
3,3%-diaminobenzidine substrate kit (Vector
serum. HHF-35 (1:50 dilution) or HAM-56 (1:50
dilu-tion) was also used to identify VSMCs and
macrophages, respectively.
2.5. Effect of PTHrP-(1-34)and PTHrP-(7-34) on cuff-induced intimal thickening
Male Wistar rats (12 weeks old) were anesthetized with ether. Through a left inguinal incision, the left femoral artery was cleared of the surrounding connec-tive tissue and loosely sheathed with a non-obstrucconnec-tive polyethylene cuff as above described. PTHrP-(1-34)
and/or PTHrP-(7-34), a PTH/PTHrP receptor
antago-nist [30] was dissolved in 25% (w/v) pluronic F-127 gel
solution [31,32] (BASF Wyandotte Co., Wyandotte,
MI, USA). Immediately after this procedure, 200ml of
the gel solution containing PTHrP-(1-34) and/or
PTHrP-(7-34) was applied to the femoral artery, and the wound was closed. The unique characteristic of the gel is reverse thermal gelation. Briefly, 25% (w/v) solu-tion of the gel is fluid at refrigerator temperature (4 – 5°C), but is soft gel at body temperature. Preliminary experiments showed that PTHrP molecule was slowly and constantly released from the gel solution. The artery was treated with the gel solution alone in the control study. Two weeks later, the rats were perfusion-fixed with 10% formalin neutral buffer solution. Then the femoral artery was removed, postfixed in 10% for-malin neutral buffer solution, and embedded in paraffin. The middle segment of the artery was cut into
cross-sectional pieces with 5 mm thickness and stained
by Elastica van Gieson staining. Cross-sectional area of the intima and of the media of the femoral arteries were measured with NIH Image software using a Macintosh computer, and the ratio of intimal area to medial area
(I/M ratio) and the ratio of intimal area to the area
within the internal elastic lamina (% stenosis) were calculated.
2.6. Statistics
Data were analyzed by one-way analysis of variance. When statistically significant effects were found, Bon-ferroni test was performed to isolate the differences
between the groups. A P value of less than 0.05 was
considered significant. All data are presented in the text
and figures as mean9S.E.M.
3. Results
3.1. Immunohistochemical detection of PTHrP in experimentally produced neointima and human atherosclerotic lesion
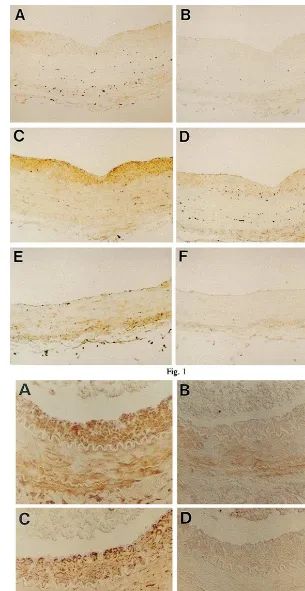
As shown in Fig. 1, a marked intimal thickening
was observed in the rat thoracic aorta 2 weeks after balloon injury. Immunoreactivity of PTHrP in the thickened intima of the balloon-injured rat thoracic aorta was detected by immunohistochemical staining with anti-hPTHrP-(1-34) polyclonal antibody (Fig. 1A). Negative control prepared with non-immune rab-bit serum showed no significant staining (Fig. 1B). In non-injured rat aorta, weak immunoreactivity of PTHrP was detected at medial smooth muscle layer (Fig. 1E).
As shown in Fig. 2, non-obstructive cuff-induced intimal thickening was observed after 2 weeks in the rat femoral artery [29]. Immunoreactivity of PTHrP was also detected in the cuff-induced thickened intima of the rat femoral artery as well as in the media (Fig. 2A).
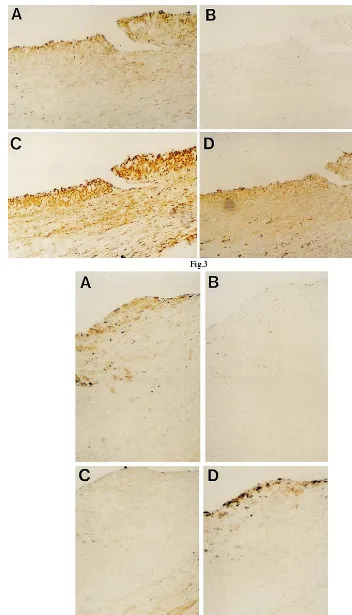
In human coronary arteries, immunoreactivity of PTHrP was detected in the restenotic lesion after PTCA obtained at DCA and a primary atheroscle-rotic lesion obtained at autopsy (Fig. 3A and Fig. 4A). Negative control prepared with non-immune rab-bit serum showed no significant staining in these spec-imens (Fig. 3B and Fig. 4B).
In the thickened intimal lesion of balloon-injured rat aorta, in the cuff-induced intimal thickening of rat femoral artery and in the restenotic lesion of human coronary artery after PTCA obtained at DCA, the immunoreactivity of PTHrP was observed in the re-gions where smooth muscle cells were predominant (Fig. 1C and Fig. 2C and Fig. 3C). In contrast, in the primary atherosclerotic lesion of human coronary artery obtained at autopsy, the immunoreactivity of
PTHrP was observed in the regions where
macrophages were predominant (Fig. 4D) although weak staining for muscle actin was also detected in the same regions (Fig. 4C).
3.2. Effect of PTHrP-(1-34) and PTHrP-(7-34) on cuff-induced intimal thickening
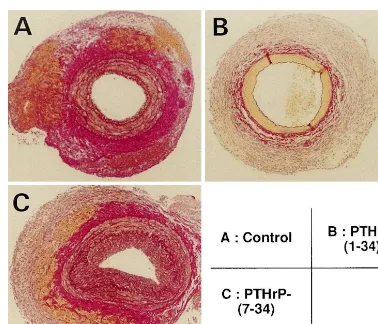
As shown in Fig. 5, moderate intimal thickening was induced by a non-obstructive cuff in the rat femoral artery. Quantitative analysis of intimal
thick-ening revealed that the I/M ratio was 0.2790.04 and
% stenosis was 19.693.3% in the control group.
Lo-cally applied PTHrP-(1-34) dissolved in pluronic
F-127 gel decreased intimal thickening whereas
PTHrP-(7-34) increased that (Fig. 5). Morphometric
analysis revealed that the decreases in the I/M
ratio and % stenosis by PTHrP-(1-34) were pendent (Fig. 6). In contrast, PTHrP-(7-34)
dose-de-pendently enhanced % stenosis. Furthermore,
Fig. 1. Photomicrographs showing immunohistochemical staining of balloon-injured rat thoracic aorta (A, B, C and D) and uninjured rat thoracic aorta (E and F). Rabbit anti-human parathyroid hormone-related protein (PTHrP)-(1-34) polyclonal antibody was used as the first antibody (A and E). Control study was performed using non-immune rabbit serum (B and F). HHF-35, a mouse anti-muscle actin monoclonal antibody (C), or HAM-56, a mouse anti-macrophage monoclonal antibody (D), was used as the first antibody. Original magnification×100.
Fig. 3. Photomicrographs showing immunohistochemical staining of a restenotic lesion of human coronary artery after percutaneous transluminal coronary angioplasty obtained at directional coronary atherectomy. Rabbit anti-human parathyroid hormone-related protein (PTHrP)-(1-34) polyclonal antibody was used as the first antibody (A); The control study was performed using non-immune rabbit serum (B); HHF-35, a mouse anti-muscle actin monoclonal antibody (C); or HAM-56, a mouse anti-macrophage monoclonal antibody (D), was used as the first antibody. Original magnification×400.
4. Discussion
In the present study, we firstly demonstrated that immunoreactivity of PTHrP was detected in the
thick-ened intima induced by balloon injury of rat aorta and by placing a non-obstructive cuff around rat femoral artery. The immunoreactivity was also detected in the restenotic lesion of human coronary artery after PTCA
Fig. 5. Photomicrographs showing the effect of parathyroid hormone-related protein (PTHrP) on cuff-induced intimal thickening of rat femoral artery. (A) control; (B) locally applied 1mmol/l PTHrP-(1-34); (C) locally applied 1mmol/l PTHrP-(7-34). Elastica van Gieson staining. Original
magnification ×40.
and in the primary atherosclerotic lesion of another human coronary artery. These findings indicate that PTHrP is expressed both in experimentally induced neointima and in human coronary atherosclerotic le-sions. We secondly demonstrated that locally
adminis-tered PTHrP inhibited and PTHrP antagonist
augmented intimal thickening in a rat cuff injury model, suggesting that PTHrP, expressed in the atherosclerotic lesions, plays a critical role in preventing the lesions.
It has been reported that immunoreactivity of PTHrP is detected in the smooth muscle layer of the arterioles in the shell gland serosa of the chicken [33] and in cultured VSMCs [15,34]. Consistent with these reports, immunoreactive PTHrP was identified not only in the thickened intima but also in the medial smooth muscle layer of rat aorta, rat femoral artery and human coro-nary arteries in the present study. In the intimal lesion of balloon-injured rat aorta, the cuff-induced intimal thickening of rat femoral artery and the restenotic lesion of human coronary artery after PTCA, im-munoreactive PTHrP was stained in the regions where VSMCs were predominant. Similar findings have been reported also by other groups [19,20]. On the other hand, in the primary atherosclerotic lesion of human coronary artery obtained at autopsy, staining for PTHrP was observed in the regions where macrophages were predominant. These results suggest that PTHrP may be produced in macrophages as well as in VSMCs in the atherosclerotic lesion. However, there is no
re-port indicating that PTHrP is produced in
macrophages. Since the regions stained for a
macrophage marker were also stained for VSMC marker and cytokines produced by macrophages may stimulate PTHrP expression in VSMCs, we cannot conclude that macrophages produce PTHrP. Moreover, we could not clarify the lesion difference in the magni-tude of PTHrP expression. Further investigations in-cluding the double immunostaining of PTHrP and cell-specific markers and the quantitation of PTHrP expression using computer-aided analysis are needed to address these points.
It is known that arterial intimal thickening can be produced by placing a non-obstructive polyethylene
cuff around an artery in experimental animals
[28,29,35]. Histological observations in these studies elucidate that cuff placement causes polymorphonu-clear leukocyte infiltration and endothelial injury in the initial step by inflammatory responses. The next impor-tant processes for the development of intimal thicken-ing are the migration of medial VSMCs to the intima and the proliferation of migrated VSMCs with deposi-tion of extracellular matrix in the neointima. These steps are similar to those observed in the development of atherosclerosis in man [4]. We thus investigated the
effects of PTHrP on the development of intimal thick-ening induced by non-obstructive polyethylene cuff in rat femoral artery. In the present study, locally applied PTHrP-(1-34) was found to significantly inhibit the cuff-induced intimal thickening of rat femoral artery in a dose-dependent manner. This effect of PTHrP-(1-34) was completely inhibited by the simultaneous
adminis-tration of PTHrP-(7-34), a specific PTH/PTHrP
recep-tor antagonist. Therefore, the inhibirecep-tory effect of PTHrP-(1-34) on the arterial intimal thickening is
thought to be mediated through PTH/PTHrP receptors.
In contrast, PTHrP-(7-34) alone significantly enhanced the cuff-induced intimal thickening. VSMCs have been
reported to express the PTH/PTHrP receptor [18] and
the thickened intima was positively stained for a VSMC marker but not for a macrophage marker. Accordingly,
we speculate that the PTH/PTHrP receptor is expressed
predominantly in VSMCs in the cuffed femoral artery. The question remains to be defined whether locally applied PTHrP analogs affect systemic blood pressure or modulate vascular tone and thus influence intimal thickening. However, since PTHrP stimulates cyclic AMP production in VSMCs [24], our results are consis-tent with the study by Indolfi et al. [36] that
investi-gated the inhibitory effects of cyclic AMP on
neointimal formation in rats using topical drug applica-tion. The effects of exogenous PTH and PTHrP are indistinguishable in most tissues including VSMCs. Ac-tually, we have shown that the inhibitory effects of PTH on VSMC migration and proliferation are com-parable to those of PTHrP [25]. The recently identified PTH-specific receptor, PTH2 receptor [37], may have some role, however, its expression is low in VSMCs [38]. Therefore, it is conceivable that locally produced PTHrP plays more pathophysiological roles in VSMCs than circulating PTH. Taken together with the demon-stration of immunoreactive PTHrP in the cuff-induced thickened intima, these findings suggest that endoge-nous PTHrP produced in the lesion acts to inhibit cuff-induced neointimal formation in the rat femoral artery.
It has been reported that PTHrP is expressed in VSMCs [15] and vascular endothelial cells [16,17].
PTH/PTHrP receptor mRNA has been identified in a
expres-sion of PTHrP mRNA in VSMCs is stimulated by vasoconstrictive substances such as norepinephrine, en-dothelin-1, angiotensin II, and thrombin [15,34], which are known to be associated with the development of atherosclerosis [4]. Therefore, the expression of PTHrP in the neointima of the atherosclerotic lesion might be stimulated by these atherogenic substances. Further,
mechanical stimuli produced by the balloon and/or the
shear stress induced by blood flow alterations near the atherosclerotic plaque may induce PTHrP expression [41]. PTHrP produced in the atherosclerotic lesion might counteract the atherogenic stimuli of these fac-tors. The findings in the present study, thus, suggest that endogenous PTHrP produced in the atherosclerotic
lesion may act as an autocrine/paracrine
anti-athero-genic factor.
In conclusion, PTHrP is expressed both in the exper-imentally induced neointima and in the human coro-nary atherosclerotic lesion. Endogenous PTHrP can
inhibit neointimal formation through PTH/PTHrP
re-ceptors, and thus PTHrP may be an autocrine/
paracrine anti-atherogenic substance.
Acknowledgements
We thank Dr Jo Aikawa, Dr Masao Moroi and Dr Masayuki Fukazawa of the Third Department of Inter-nal Medicine, Toho University School of Medicine for their helpful comments and discussion. We also thank Ms Masae Watanabe and Ms Hitomi Yamaguchi for their excellent technical assistance. This work was sup-ported by a grant from Funds for Comprehensive Research on Aging and Health, Japan Foundation for Aging and Health.
References
[1] Waller BF, Gorfinkel HJ, Rogers FJ, Kent KM, Roberts WC. Early and late morphologic changes in major epicardial coronary arteries after percutaneous transluminal coronary angioplasty. Am J Cardiol 1984;53:42C – 7C.
[2] Waller BF, Johnson DE, Schnitt SJ, Pinkerton CA, Simpson JB, Baim DS. Histologic analysis of directional coronary atherec-tomy samples. A review of findings and their clinical relevance. Am J Cardiol 1993;72:80E – 7E.
[3] Ross R. The pathogenesis of atherosclerosis N0u¨ an update. N Engl J Med 1986;314:488 – 500.
[4] Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801 – 9.
[5] Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vivo. Proc Natl Acad Sci USA 1974;71:1207 – 10. [6] Burtis WJ, Wu T, Bunch C, Wysolmerski JJ, Insogna KL, Weir EC, Broadus AE, Stewart AF. Identification of a novel 17 000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem 1987;262:7151 – 6.
[7] Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall REH, Kemp BE, Suva LJ, Rodda CP, Ebeling PR, Hudson PJ, Zajac JD, Martin TJ. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci USA 1987;84:5048 – 52.
[8] Stewart AF, Wu T, Goumas D, Burtis WJ, Broadus AE. N-ter-minal amino acid sequence of two novel tumor-derived adenylate cyclase-stimulating proteins: identification of parathyroid hor-mone-like and parathyroid hormone-unlike domains. Biochem Biophys Res Commun 1987;146:672 – 8.
[9] Strewler GJ, Stern PH, Jacobs JW, Eveloff J, Klein RF, Leung SC, Rosenblatt M, Nissenson RA. Parathyroid hormone-like protein from human renal carcinoma cells. Structural and func-tional homology with parathyroid hormone. J Clin Invest 1987;80:1803 – 7.
[10] Suva LJ, Winslow GA, Wettenhall REH, Hammonds RG, Moseley JM, Diefenbach-Jagger H, Rodda CP, Kemp BE, Ro-driguez H, Chen EY, Hudson PJ, Martin TJ, Wood WI. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science 1987;237:893 – 6. [11] Mangin M, Webb AC, Dreyer BE, Posillico JT, Ikeda K, Weir EC, Stewart AF, Bander NH, Milstone L, Barton DE, Francke U, Broadus AE. Identification of a cDNA encoding a parathy-roid hormone-like peptide from a human tumor associated with humoral hypercalcemia of malignancy. Proc Natl Acad Sci USA 1988;85:597 – 601.
[12] Thiede MA, Rodan GA. Expression of a calcium-mobilizing parathyroid hormone-like peptide in lactating mammary tissue. Science 1988;242:278 – 80.
[13] Thiede MA, Harm SC, McKee RL, Grasser WA, Duong LT, Leach RM. Expression of the parathyroid hormone-related protein gene in the avian oviduct: potential role as a local modulator of vascular smooth muscle tension and shell gland motility during the egg-laying cycle. Endocrinology 1991;129:1958 – 66.
[14] Selvanayagam P, Graves K, Cooper C, Rajaraman S. Expression of the parathyroid hormone-related peptide gene in rat tissues. Lab Invest 1991;64:713 – 7.
[15] Hongo T, Kupfer J, Enomoto H, Sharifi B, Giannella-Neto D, Forrester JS, Singer FR, Goltzman D, Hendy GN, Pirola C, Fagin JA, Clemens TL. Abundant expression of parathyroid hormone-related protein in primary rat aortic smooth muscle cells accompanies serum-induced proliferation. J Clin Invest 1991;88:1841 – 7.
[16] Ishikawa M, Ouchi Y, Akishita M, Kozaki K, Toba K, Namiki A, Yamaguchi T, Orimo H. Immunocytochemical detection of parathyroid hormone-related protein in vascular endothelial cells. Biochem Biophys Res Commun 1994;199:547 – 51. [17] Rian E, Jemtland R, Olstad OK, Endresen MJ, Grasser WA,
Thiede MA, Henriksen T, Bucht E, Gautvik KM. Parathyroid hormone-related protein is produced by cultured endothelial cells: a possible role in angiogenesis. Biochem Biophys Res Commun 1994;198:740 – 7.
[18] Urea P, Kong XF, Abou-Samra AB, Jppner H, Kronenberg HM, Potts JT, Segre GV. Parathyroid hormone (PTH)/PTH-re-lated peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology 1993;133:617 – 23. [19] Nakayama T, Ohtsuru A, Enomoto H, Namba H, Ozeki S,
Shibata Y, Yokota T, Nobuyoshi M, Ito M, Sekine I, et al. Coronary atherosclerotic smooth muscle cells overexpress human parathyroid hormone-related peptides. Biochem Biophys Res Commun 1994;200:1028 – 35.
[21] Nickols GA, Nana AD, Nickols MA, DiPette DJ, Asimakis GK. Hypotension and cardiac stimulation due to the parathyroid hormone-related protein, humoral hypercalcemia of malignancy factor. Endocrinology 1989;125:834 – 41.
[22] Winquist RJ, Baskin EP, Vlasuk GP. Synthetic tumor-derived human hypercalcemic factor exhibits parathyroid hormone-like vasorelaxation in renal arteries. Biochem Biophys Res Commun 1987;149:227 – 32.
[23] Trizna W, Edwards RM. Relaxation of renal arterioles by parathyroid hormone and parathyroid hormone-related protein. Pharmacology 1991;42:91 – 6.
[24] Ishikawa M, Ouchi Y, Han S, Akishita M, Kozaki K, Toba K, Namiki A, Yamaguchi T, Orimo H. Parathyroid hormone-re-lated protein reduces cytosolic free Ca2+level and tension in rat
aortic smooth muscle. Eur J Pharmacol 1994;269:311 – 7. [25] Ishikawa M, Akishita M, Kozaki K, Toba K, Namiki A,
Ya-maguchi T, Orimo H, Ouchi Y. Amino-terminal fragment (1-34) of parathyroid hormone-related protein inhibits migration and proliferation of cultured vascular smooth muscle cells. Atherosclerosis 1998;136:59 – 66.
[26] Tsukada T, Rosenfeld M, Ross R, Gown AM. Immunocyto-chemical analysis of cellular components in atherosclerotic le-sions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Atherosclerosis 1986;6:601 – 13.
[27] Gown AM, Tsukada T, Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol 1986;125:191 – 207. [28] Hirosumi J, Nomoto A, Ohkubo Y, Sekiguchi C, Mutoh S,
Yamaguchi I, Aoki H. Inflammatory responses in cuff-induced atherosclerosis in rabbits. Atherosclerosis 1987;64:243 – 54. [29] Akishita M, Ouchi Y, Miyoshi H, Kozaki K, Inoue S, Ishikawa
M, Eto M, Toba K, Orimo H. Estorogen inhibits cuff-induced intimal thickening of rat femoral artery: effects on migration and proliferation of vascular smooth muscle cells. Atherosclerosis 1997;130:1 – 10.
[30] Nagasaki K, Yamaguchi K, Miyake Y, Hayashi C, Honda S, Urakami K, Miki K, Kimura S, Watanabe T, Abe K. In vitro and in vivo antagonists against parathyroid hormone-related protein. Biochem Biophys Res Commun 1989;158:1036 – 42. [31] Miyazaki S, Takeuchi S, Yokouchi C, Takada M. Pluronic
F-127 gels as a vehicle for topical administration of anticancer agents. Chem Pharm Bull 1984;32:4205 – 8.
[32] Simons M, Edelman ER, Dekeyser JL, Langer R, Rosenberg RD. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature 1992;359:67 – 70.
[33] Thiede MA, Harm SC, McKee RL, Grasser WA, Duong LT, Leach RM Jr. Expression of the parathyroid hormone-related protein gene in the avian oviduct: potential role as a local modulator of vascular smooth muscle tension and shell gland motility during the egg-laying cycle. Endocrinology 1991;129:1958 – 66.
[34] Pirola CJ, Wang H, Kamyar A, Wu S, Enomoto H, Sharifi B, Forrester JS, Clemens TL, Fagin JA. Angiotensin II regulates parathyroid hormone-related protein expression in cultured rat aortic smooth muscle cells through transcriptional and post-tran-scriptional mechanisms. J Biol Chem 1993;268:1987 – 94. [35] Gebrane J, Roland J, Orcel L. Experimental diffuse intimal
thickening of the femoral arteries in the rabbit. Virchows Arch Pathol Anat 1982;396:41 – 59.
[36] Indolfi C, Avvedimento EV, Di Lorenzo E, Esposito G, Rapac-ciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli G, Chiariello M. Activation of cAMP-PKA signal-ing in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med 1997;3:775 – 9.
[37] Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hor-mone, the PTH2 receptor. J Biol Chem 1995;270:15455 – 8. [38] Usdin TB, Bonner TI, Harta G, Mezey E. Distribution of
parathyroid hormone-2 receptor messenger ribonucleic acid in rat. Endocrinology 1996;137:4285 – 97.
[39] Roca-Cusachs A, Dipette DJ, Nickols GA. Regional and sys-temic hemodynamic effects of parathyroid hormone-related protein: preservation of cardiac function and coronary and renal flow with reduced blood pressure. J Pharmacol Exp Ther 1991;256:110 – 8.
[40] DiPette DJ, Christenson W, Nickols MA, Nickols GA. Cardio-vascular responsiveness to parathyroid hormone (PTH) and PTH-related protein in genetic hypertension. Endocrinology 1992;130:2045 – 51.
[41] Pirola CJ, Wang HM, Strgacich MI, Kamyar A, Cercek B, Forrester JS, Clemens TL, Fagin JA. Mechanical stimuli induce vascular parathyroid hormone-related protein gene expression in vivo and in vitro. Endocrinology 1994;134:2230 – 6.