THE EFFECT OF PHOSPHATE SUPPLY ON PHOSPHATASE ACTIVITY OF PHOSPHATE SOLUBILIZING MICROORGANISMS
BETTY N. FITRIATIN, BENNY JOY*, AND TOTO SUBROTO**
*Department of Soil Science Faculty of Agriculture Padjadjaran University ** Department of Chemistry Faculty of Mathematics and Natural Sciences
Padjadjaran University E-mail : [email protected]
ABSTRACT
The phosphate solubilizing microorganisms has capable to produce extracelluler enzyme, i.e. group of phosphatase enzyme which able to mineralized of organic P to inorganic P. The objective of this experiment was to examine of phosphatase enzyme activity from phosphate solubilizing microorganism (bacteria and fungi) i.e. Pseudomonas mallei, Bacillus subtilis, Aspergilus niger and Penicillium sp. affected by phosphate supply (organic phosphate/ phytic acid and inorganic phosphate/KH2PO4) in medium.
The result of experiment showed that increasing of organic P (phytic acid ) and inorganic P (KH2PO4) affect phosphatase enzyme activity of phosphate solubilizing
microorganisms and the amount of dissolve P. Furthermore, increasing of organic P substrate increase phosphatase activity; on the contrary, addition to inorganic P decrease phosphatase activity. Phosphatase activity of fungi (Aspergilus niger and Penicillium sp.) were higher than bacteria (Pseudomonas mallei, Bacillus subtilis).
Key words : phosphatase activity, phosphate supply, phospahate solubilizing
microorganisms
INTRODUCTION
of PSM are more important in rhizosphere than non-rhizosphere soil (Kucey et al., 1989). PSM occur in both fertile and P-deficient soils and the fastest initial rates of P incorporation were observed in P-deficient soils (Oehl, 2001).
Penicillium and Aspergillus niger. were isolated from soil that they are dominant
P solubilizing fungi found in rhizosphere. Pseudomonas mallei and Bacillus subtilis are phosphate solubilizing bacteria that were isolated from rhizosphere (Fitriatin, 2006). Furthermore, their ability to mineralize organic P need investigate more. They has capable to produce extracelluler enzyme, i.e. group of phosphatase enzyme which able to mineralized of organic P to inorganic P so that prepare high P for plant. Due to the low availability of inorganic P in soil, the organic P mainly contributes to plant nutrition and to microbial uptake through its mineralization and subsequent release of inorganic P. P mineralization rate depends on microbial activity and on the activity of phosphatases (Saparatka, 2003). Consequently, the release of inorganic P through the destruction of the organic matter is usually divided in biological mineralization and biochemical mineralization. Biological mineralization involves the release of inorganic P as a consequence of the carbon oxidation and the microbial growth and turnover, while in biochemical mineralization the release of inorganic P, independent of microbial respiration, is controlled by the supply and need for P and involves the hydrolysis of ester-phosphates by extra-cellular hydrolytic enzymes (phosphatases) both free in solution and stabilised by sorption to the colloidal fraction (McGill and Cole, 1981).
There are several soil phosphatases (Saparatka, 2003; Cookson, 2002) and the most commonly determined are: phosphomonoesterases, phosphodiesterases and phytases. Phosphomonoesterases act on phosphate monoesters and according to their optimum pH are divided in acid and alkaline phosphomonoesterases. Both are adaptive enzymes: acid phosphomonoesterase predominates in acid soils while alkaline phosphomonoesterase predominates in neutral and basic soils (George, et al., 2002).
content, total N content, C/N ratio and total P content (Djordjevic et.al. 2003). The kind of organic phosphorous of medium and pH affect phosphatase activity of soil microorganisms (Fitriatin, et al.,2008).
The objective of this experiment was to examine of phosphatase enzyme activity from phosphate solubilizing microorganism (bacteria and fungi) i.e. Pseudomonas mallei, Bacillus subtilis, Aspergilus niger and Penicillium sp. affected by
phosphate supply (organic phosphate/ phytic acid and inorganic phosphate/KH2PO4) in
medium.
MATERIALS AND METHODS
Some concentration of inorganic P and organic P were used to find out effect phosphate supply on activity of phosphatase enzyme and phosphate solvent in medium which cultured of phosphate solubilizing microorganisms (bacteria and fungi) i.e. Pseudomonas mallei., Bacillus subtilis, Aspergillus niger and Penicillium sp.
The concentration of inorganic P (KH2PO4) which tested consisted of: :
P0 = 0 mg L-1
P1 = 10 mg L-1
P2 = 20 mg L-1
P3 = 30 mg L-1
The concentration of organic P (phytic acid) which tested consisted of : S1 = 5 mM
S2 = 10 mM
S3 = 15 mM
Phosphatase activity and dissolve P were observed on 5 days after incubation. Acid phosphatase activity was measured spectrophotometrically by monitoring the release of para-nitrophenol from para-nitrophenyl phosphate (PNPP) at 400 nm (Eivazi and Tabatabai, 1977 in Schinner et al., 1996).
RESULTS AND DISCUSSION
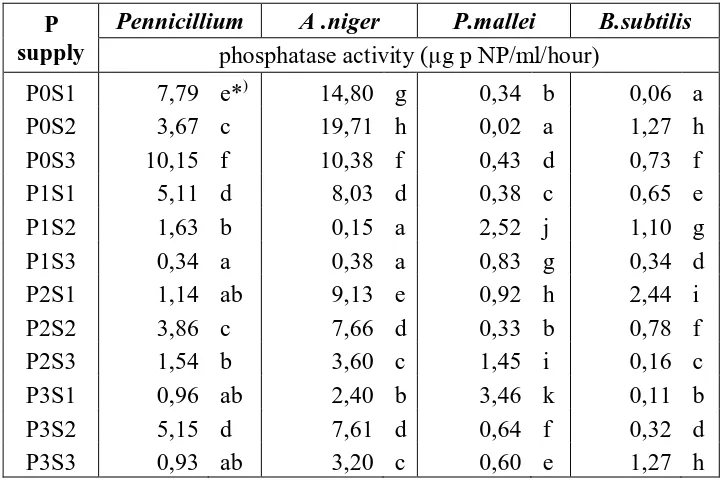
Table 1. The Effecf of phosphate (P) and phytic acid (S) on phosphatase activity of phosphate solubilizing microorganisms in medium
P supply
Pennicillium A .niger P.mallei B.subtilis phosphatase activity (µg p NP/ml/hour)
P0S1 7,79 e*) 14,80 g 0,34 b 0,06 a
P0S2 3,67 c 19,71 h 0,02 a 1,27 h
P0S3 10,15 f 10,38 f 0,43 d 0,73 f
P1S1 5,11 d 8,03 d 0,38 c 0,65 e
P1S2 1,63 b 0,15 a 2,52 j 1,10 g
P1S3 0,34 a 0,38 a 0,83 g 0,34 d
P2S1 1,14 ab 9,13 e 0,92 h 2,44 i
P2S2 3,86 c 7,66 d 0,33 b 0,78 f
P2S3 1,54 b 3,60 c 1,45 i 0,16 c
P3S1 0,96 ab 2,40 b 3,46 k 0,11 b
P3S2 5,15 d 7,61 d 0,64 f 0,32 d
P3S3 0,93 ab 3,20 c 0,60 e 1,27 h
Note : *) Figures in each column followed by the same letter(s) are not significantly different (P<0.05) according to Duncan’s multiple range test
Giving the treatment of various concentrations inorganic P (KH2PO4) and
phytic acid gave significant effect on phosphatase activity in each of the tested phosphate solubilizing microorganisms. The results of this experiment showed that supply P are affecting phosphatase activity. The results of this experiment consistent with the research Moura et al. (2001) who explains that the different concentration of organic P substrates affects bacterial phosphatase activity.
From Table 1, it can be seen that the treatment of 0 mg L-1 inorganic P (KH2PO4) and 10 mM phytic acid gave the highest phosphatase activity of Aspergilus
niger. Phosphatase activity of fungi (Aspergilus niger and Penicillium sp.) were higher
than bacteria (Pseudomonas mallei, Bacillus subtilis).
Table 2 . The Effecf of phosphate (P) and phytic acid (S) on dissolve P
Kode Penicilium A.niger B.subtilis P.mallei
Dissolve P (mgL-1)
P0S1 48,14 b*) 50,41 b 23,34 h 7,29 b
P0S2 39,93 b 48,12 b 22,94 g 7,30 b
P0S3 73,78 c 39,92 b 23,34 h 7,28 b
P1S1 10,51 a 73,75 c 22,94 g 7,29 b
P1S2 11,32 a 10,50 a 18,17 b 6,88 a
P1S3 12,12 a 11,30 a 18,17 b 6,88 a
P2S1 11,32 a 12,11 a 18,98 c 8,09 c
P2S2 68,54 c 11,00 a 19,38 d 8,50 d
P2S3 76,81 c 68,50 c 20,16 f 8,09 c
P3S1 57,26 b 76,50 c 19,78 e 8,09 c
P3S2 49,09 b 57,20 b 19,80 e 8,10 c
P3S3 54,84 b 49,01 b 7,29 a 8,50 d
Note : *) Figures in each column followed by the same letter(s) are not significantly different (P<0.05) according to Duncan’s multiple range test
CONCLUSION
The increasing of organic P (phytic acid ) and inorganic P (KH2PO4) affect
phosphatase enzyme activity of phosphate solubilizing microorganisms and the amount of dissolve P. Furthermore, increasing of organic P substrate increase phosphatase activity; on the contrary, addition to inorganic P decrease phosphatase activity. Phosphatase activity of fungi (Aspergilus niger and Penicillium sp.) were higher than bacteria (Pseudomonas mallei, Bacillus subtilis).
ACKNOWLEDGEMENTS
The authors wish to thank the Riset Dasar Intensif Project Management of the Ministry of Research and Technology Republic of Indonesia for financial support.
REFERENCES CITED
Chabot, R., H. Antoun, and M.P. Cescas. 1993. Microbiological solubilization of inorganic P-fractions normally encountered in soils. p77-329 In Phosphorus, Sulfur and Silicon.
Cookson, P. 2002. Variation in phosphatase activity in soil : A case study. In : Agricultural Sciences (7) No. 1 : 65-72.
Djordjevic, S., Djukic, D., Govedarica, M., Milosevic, N. and Jarak, M. 2003. Effects of chemical and physical soil properties on activity phosphomonoesterase. Acta Agriculturae Serbica, Vol. VIII, 16 : 3 – 10.
Fitriatin, B,N. 2006. Analisis aktivitas fosfatase dari rhizosfir tanaman pangan dan jati pada Ultisols. Laporan Penelitian. Jurusan Ilmu Tanah Fakultas Pertanian Universitas Padjadjaran.
Fitriatin, B.N., T. Subroto, and B. Joy. 2008. The Influence of Organic Phosphorous Substrate on Phosphatase Activity of Soil Microbes. Proceeding International Seminar of Chemistry. 30-31 October, Indonesia.
George., T.S., P.J. Gregory, M. Wood, D. Read, R.J. Buresh. 2002. Phosphatase activity and organic acids in the rhizosphere of potential agroforestry species and maize. Soil Biology and Biochemystry 34 : 1487-1494.
Kucey, R.M.N., H.H. Janzen, and M.E. Leggett. 1989. Microbiologically mediated increases in plant-available-phosphorus. Adv. Agron. 42:199-228.
McGill, W.B. and C.V. Cole. 1981. Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 26:267-286.
Moura, R.S., F.J. Martin, A. Martin and P. Liras. 2001. Substrate analysis and molecular cloning of the extracellular alkaline phosphatase of Streptomyces griseus. J. Microbiol. 147: 1425-1533.
Oehl, F., M. Oberson, A. Probst, H. Fliessbach, R. Roth, and E. Frossard. 2001. Kinetics of microbial phosphorus uptake in cultivated soils. Biol. Fertil. Soil 34:31-41. Rodriguez, H. and R. Fraga. 1999. Phosphate solubilizing bacteria and their role in plant
growth promotion. Biotechnol. Adv. 17:319-339
Saparatka, N. 2003. Phosphatase activities (ACP, ALP) in Agroecosystem Soils. Doctoral thesis. Swedish University of Agricultural Sciences. Uppsala. diss-epsilon.slu.se/archive/ 00000286/01/Agraria_396_Docutech_Tryckfil [Diakses 15 Desember 2005]
Sarapatka, B., Dudova and M. Krskova. 2004. Effect of pH and phosphate supply on acid phosphatase activity in cereal roots. Biologia, Bratislava, 59: 127-131. Schinner, F., R. Oninger, E. Kandeler and R. Margesin. 1996. Methods in Soil Biology.
Springer-Verlag. Berlin Heidelberg-Jerman.
Whitelaw. 2000. Growth promotion of plants inoculated with phosphate solubilizing fungi. Adv. Agron. 69 : 99-151.
Wyss, M., Brugger, R., Kronenberger, A., Remy, R., Fimbel, R., Oesterhelt, G., Lehman, M., and Loon, A., 1999., Biochemical Characterization of Fungal Phytases (myo-Inositol hexakisphosphate phosphohidrolases : Catalytic properties. Applied and Environtmental Microbiology, Feb 1999. p 367 –373. Yadaf, R.S. and J.C. Tarafdar. 2003. Phytase and phosphatase producing fungi in arid