www.elsevier.com / locate / bres
Research report
Antagonist of nicotinic acetylcholine receptors (nAChR) enhances
formalin-induced nociception in rats: tonic role of nAChRs in the
control of pain following injury
1
*
´ ´
Aldric Hama , Frederique Menzaghi
Merck Research Laboratories, San Diego, 505 Coast Blvd. South, La Jolla, CA 92037, USA Accepted 19 September 2000
Abstract
Following tissue injury, spinal neurons increase in spontaneous activity and in responsiveness to peripheral stimulation. These changes in spinal neurons may underlie abnormal pain behavior. Nicotinic acetylcholine receptor (nAChR) agonists are analgesic when evaluated in animal models of pain, but it is not known if the nAChRs differentially modulate acute and tonic pain. To test this, mecamylamine, a non-subtype selective nAChR antagonist, was systemically injected into rats prior or after hind paw injection of formalin. Formalin injection results in biphasic pain-related behaviors, characterized by a first phase (i.e. acute pain) immediately following formalin injection, then by a second phase (i.e. tonic pain) 15–60 min after formalin injection. Either pre- or post-formalin treatment with mecamylamine decreased phase 1 behaviors and significantly increased phase 2 pain behaviors in a dose-dependent manner. These results suggest that nAChRs may exert opposing effects on acute versus tonic pain and, as such, may have implications for the potential development of nAChR ligands for the treatment of pain. 2001 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Pain modulation: pharmacology
Keywords: Acute pain; Chronic pain; Mecamylamine; Nociception
1. Introduction activation of the cholinergic system is mediated, at least in
part, through activation of nAChRs.
Recent studies have shown that the nicotinic acetyl- Tissue injury is known to result in persistent or abnor-choline receptor (nAChR) ligands nicotine and epibatidine mal pain perception. For instance, formalin injection into have analgesic effects in animal models of pain, which is the rat hind paw results in biphasic pain-related behaviors. reduced with the ion channel blocker mecamylamine The first phase (i.e. acute pain) is observed immediately [1,3,4,14]. Binding sites for nAChR ligands and mRNA for following formalin injection and lasts only a few minutes, nAChRs have been found in regions that modulate pain whereas the second phase (i.e. tonic pain) appears about 15 perception including dorsal root ganglia, spinal cord dorsal min after formalin injection and lasts at least 60 min horn and medullary nuclei [11,17]. Elevating cerebrospinal post-formalin. Both phases are characterized by an in-fluid (CSF) levels of acetylcholine with the acetylcholine crease in pain-related behaviors (such as licking, flinching esterase inhibitor neostigmine also leads to analgesia, and biting of the injured paw) but seem to involve two which is partially attenuated by mecamylamine [3,7]. distinct mechanisms. The initial pain behaviors are These observations suggest that the analgesia following believed to be driven by primary afferent nociceptor activity, as opposed to the pain behaviors in phase 2 which are thought to arise from nociceptive spinal neuron
hy-*Corresponding author. Tel.: 11-858-452-5892; fax:
11-858-452-peractivity, a consequence of the injury-induced excitatory
9279.
barrage from primary afferents [5]. It has been
hypoth-E-mail address: aldric [email protected] (A. Hama). ]
1
Present address: Arena Pharmaceuticals, Inc., San Diego, CA, USA. esized that a decrease of inhibitory input to spinal neurons
also underlies spinal neuron hyperactivity. Activation of 2, converted to a percent of maximal effect (%MPE) GABA , serotonergic andA a-adrenergic receptors leads to according to the following:
analgesia. Conversely, blockade of each of these receptors
(Total pain behaviors) with antagonists prior to hind paw formalin injection %MPE5]]]]]]3100,
Emax
results in increased pain behaviors [9,13]. The elevation of
pain behaviors following blockade of endogenous inhib- where E was the observed maximum total pain
be-max
itory systems suggest that these systems tonically modulate haviors. The E in phase 1 and 2 were 48 and 277,
max
pain following tissue injury. respectively. Acute noxious stimulation increases CSF levels of
acetylcholine, but it is not known if the cholinergic system
2.2. Mecamylamine post-treatment is activated during prolonged pain states [6]. It is
hypoth-esized that the cholinergic system, particularly via
activa-Robust enhancement by mecamylamine of formalin-tion of nAChRs, may play an inhibitory role in tonic pain.
induced pain behaviors was observed in rats treated with Thus, prevention of acetylcholine activity by blockade of
0.5% formalin. In a separate group of rats, mecamylamine nAChRs with mecamylamine, either pre- or post-formalin,
(1.5, 3, 6 mg / kg) was injected s.c. 6 min following hind should increase formalin-induced pain behaviors. The role
paw injection of 0.5% formalin. Pain behaviors were of nAChRs in acute pain is also not well explored and this
recorded as described above. Upon termination of the study will compare the effect of blockade of nAChRs on
experiment, rats were promptly euthanized. acute versus tonic pain.
2.3. Statistics
2. Materials and methods Calculations and comparisons of the mecamylamine
pre-treatment and vehicle pre-pre-treatment A50 values were All experiments followed guidelines of the National performed using computer software based on Tallarida and Institutes of Health and were approved by the institutional Murray [16] and statistical significance was evaluated by animal care committee. Male Sprague–Dawley rats (275– t-test. Comparisons of the total pain behaviors in each 325 g; Sasco–Harlan) were group-housed, on a 12 h phase were made between mecamylamine- and saline-light–dark cycle and had free access to food and water. treated rats using one-way analysis of variance, with post-hoc analysis using Dunnett’s test when appropriate. P values less than 0.05 were considered significant.
2.1. Mecamylamine pre-treatment
Individual rats were acclimated to Plexiglas observation
chambers for 30–60 min prior to behavioral evaluation. 3. Results
Rats were then treated with either mecamylamine
hydro-chloride (1.5, 3, 6 mg / kg; Research Biochemicals, Inc.) or 3.1. Mecamylamine pre-treatment saline vehicle, injected subcutaneously (s.c.) in a volume of
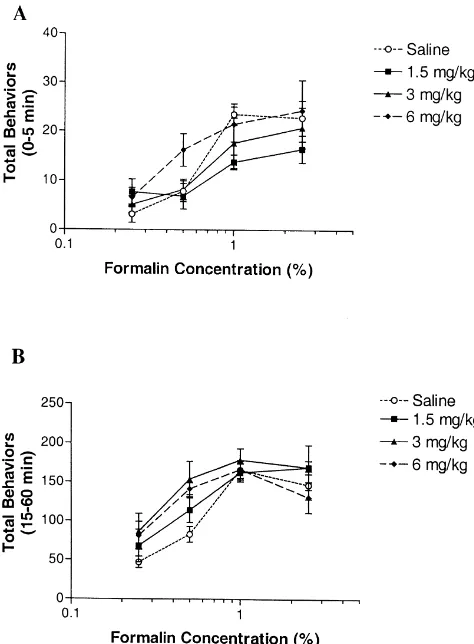
1 ml / kg. Pain behaviors as a function of formalin concentration in Five minutes following injection of either mecamylamine and saline-treated rats are illustrated in Fig. mecamylamine or vehicle, 50ml of formalin (0.25, 0.5, 1, 1. Following formalin injection, the saline-treated group 2.5%) was injected s.c. into the left plantar hind paw. Low exhibited significant biphasic pain behaviors over time, concentrations of formalin (0.25–2.5%) were tested in suggesting that the concentrations of formalin that were order to better observe increases in pain behaviors. used for this study were adequate to induce painful tissue The number of pain behaviors (flinches and licking of injury. Regardless of the pre-treatment (i.e. saline or the hind paw) were counted for 1 min, every 5 min for the mecamylamine), formalin increased total pain behaviors in duration of the 60 min observation period. The phases a dose-dependent manner (Fig. 1).
mecamylamine, the A50 was shifted from 1.4 to 0.6%, which is a 1.6-fold leftward shift compared to saline-treated rats (P,0.05; Table 1. Fig. 1B).
The effect of mecamylamine was dependent on the concentration of formalin tested. Thus the effect of mecamylamine on phase 1 was more pronounced at a concentration of 1% formalin whereas the effect of mecamylamine on phase 2 was more pronounced at a concentration of 0.5% of formalin (Fig. 2).
3.2. Mecamylamine post-treatment
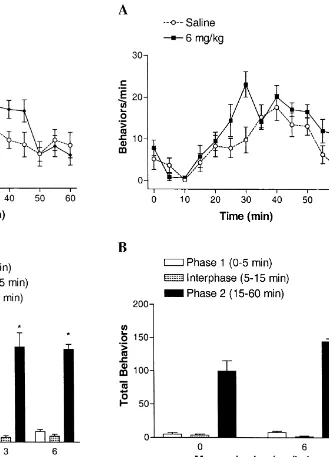
To evaluate the effect of blocking the cholinergic ion channel following tissue injury, the highest dose of mecamylamine was injected during the interphase (i.e. 6 min after injection of 0.5% formalin). Rats treated with mecamylamine after formalin displayed a slight, but significant, increase of total pain behaviors in phase 2 (P,0.05 versus vehicle; Fig. 3).
4. Discussion
The present study demonstrates that blockade of nAChRs by mecamylamine, before formalin injection in the rat hind paw, leads to an enhancement of phase 2 pain behaviors, whereas phase 1 pain behaviors tended to be reduced. The increase in pain behaviors was also observed
Fig. 1. Effect of mecamylamine pre-treatment on pain behaviors induced
by varying concentrations of formalin. Concentrations of formalin, in when mecamylamine was administered after phase 1. This percent, are plotted on a log scale. Mecamylamine (j1.5 mg / kg; m3 suggests that in the formalin test, nAChRs may have mg / kg; ♦ 6 mg / kg) or saline was injected 5 min prior to hind paw
opposing roles in modulating the initial and long-term
injection of formalin. (A) Effect of mecamylamine pre-treatment on phase
response to tissue injury.
1 total pain behaviors (0–5 min post-formalin). (B) Effect of
The current study utilized different concentrations of
mecamylamine on phase 2 total pain behaviors (15–60 min
post-for-malin). Data are expressed as mean6S.E.M.; n56–8 / group. formalin to evaluate the effect of antagonizing nAChRs on
acute and tonic pain. Effects on pain behaviors, par-shifted from 2.1 to 14%, which is a 6.6-fold rightward shift ticularly in phase 2, may not have been apparent if a compared to saline-treated rats (P,0.05; Table 1, Fig. common stimulus, a high concentration of formalin (e.g. 1A). This shift was not observed at doses greater than 1.5 5%), was utilized. A weaker stimulus, 0.5% formalin,
mg / kg. which was enough to evoke long lasting pain behaviors,
On the other hand, mecamylamine dose-dependently did reveal an enhancement by mecamylamine. Increases in increased pain behaviors induced by formalin in phase 2, pain behavior with mecamylamine were not evident at the with the most effective doses being the highest doses higher concentrations (1–2.5%) of formalin, likely due to a tested (3 and 6 mg / kg). At the 3 mg / kg dose of ceiling effect. On the other hand, a decrease in pain
Table 1
a
A50values (95% confidence interval) of formalin for phase 1 and phase 2 total pain behaviors in vehicle- and mecamylamine-treated rats
Mecamylamine Phase 1 Potency Phase 2 Potency
b
(mg / kg) ratio ratio
0 2.1 (1.6–2.9) 1.4 (1.0–1.9)
1.5 14.0 (6.4–30.8)* 0.2 0.9 (0.6–1.3) 1.6
3 3.4 (2.1–5.5) 0.6 0.6 (0.3–1.2)* 2.3
6 1.8 (1.1–3.1) 1.2 1.1 (0.5–2.5) 1.3
a
The A50is the formalin concentration that will elicit 50% maximal pain behaviors.
b
Potency ratio: The ratio of the A ’s of vehicle to mecamylamine. A potency ratio of greater than 1 suggests a leftward shift of the formalin concentration50
curve and a potency ratio of less than 1 suggests a rightward shift. Thus, mecamylamine tended to increase the phase 1 A50and decrease phase 2 A .50 *
Fig. 3. Effect of post-formalin injection of mecamylamine on pain Fig. 2. Effect of pre-formalin injection of mecamylamine on pain
behaviors in rats. (A) Time course of the effect of mecamylamine or behaviors in rats. (A) Time course of the effect of mecamylamine or
saline injected 6 min after 0.5% formalin. (B) Effects of post-formalin saline injected 5 min prior to 0.5% formalin. (B) Effects of pre-formalin
injection of mecamylamine on total pain behaviors. Data are expressed as injection of mecamylamine on total pain behaviors. Data are expressed as
mean6S.E.M.; n56 / group. *P,0.05 versus Saline, one-way analysis of mean6S.E.M.; n58 / group. *P,0.05 versus Saline, one-way analysis of
variance (ANOVA) followed by Dunnett’s test. variance (ANOVA) followed by Dunnett’s test.
nAChRs will lead to increased pain behaviors. The dual References
effects observed in the current study may be due to
blockade of nAChRs at different sites. Also, the decrease / [1] A.W. Bannon, M.W. Decker, M.W. Holladay, P. Curzon, D. Don-nelly-Roberts, P.S. Puttfarcken, R.S. Bitner, A. Diaz, A.H.
Dicken-increase in pain behaviors following mecamylamine
treat-son, R.D. Porsolt, M. Williams, S.P. Arneric, Broad-spectrum,
non-ment may be due to altered properties of nAChRs in the
opioid analgesic activity by selective modulation of neuronal
acute versus the tonic pain state. It is possible that nicotinic acetylcholine receptors, Science 279 (1998) 77–81. following injury, hyperactivity of spinal or brain neurons [2] S.R. Chaplan, A.B. Malmberg, T.L. Yaksh, Efficacy of spinal
may induce alterations in the cholinergic system, such as NMDA recpetor antagonism in formalin hyperlagesia and nerve injury evoked allodynia in the rat, J. Pharmacol. Exp. Therap. 280
changing acetylcholine release or receptor function. To test
(1997) 829–838.
this hypothesis, mecamylamine was administered after
[3] A. Chiari, J. Tobin, H. Pan, D. Hood, J. Eisenach, Sex differences in
formalin injection. As with injection prior to formalin, cholinergic analgesia I: a supplemental nicotinic mechanism in mecamylamine injection after formalin resulted in an normal females, Anesthesiology 91 (1999) 1447–1454.
increase in phase 2 pain behaviors. This result suggested [4] P. Curzon, A. Nikkel, A. Bannon, S. Arneric, M. Decker, Differ-ences between the antinociceptive effects of the cholinergic channel
that activity of the cholinergic system is perceptibly
activators A-85380 and (6)-epibatidine in rats, J. Pharmacol. Exp.
modified, as assessed by pain behavior.
Ther. 287 (1998) 847–853.
Alternatively, as mecamylamine is a non-competitive [5] A. Dickenson, A. Sullivan, Peripheral origins and central modula-ion channel blocker, it may act at receptors other than the tion of subcutaneous formalin-induced activity of rat dorsal horn
nAChR, thus complicating any interpretation of neurones, Neurosci. Lett. 83 (1987) 207–211.
[6] J.C. Eisenach, D.J. Detweiler, C. Tong, R. D’Angelo, D.D. Hood,
mecamylamine’s effects on pain behavior. As such,
Cerebrospinal fluid norepinephrine and acetylcholine concentrations
mecamylamine may inhibit the activity of NMDA
re-during acute pain, Anesth. Analg. 82 (1996) 621–626.
ceptors [12]. However, blockade of the NMDA receptor [7] J. Hwang, K. Hwang, J. Leem, P. Park, S. Han, D. Lee, The channel by NMDA receptor antagonists such as MK-801 antiallodynic effects of intrathecal cholinesterase inhibitors in a rat
has been shown to decrease, rather than to increase phase 2 model of neuropathic pain, Anesthesiology 90 (1999) 492–499. [8] S. Jinks, E. Carstens, Activation of spinal wide dynamic range
pain behaviors [2]. This rules out NMDA receptors but it
neurons by intracutaneous microinjection of nicotine, J.
Neuro-cannot be excluded that increased pain behaviors caused
physiol. 82 (1999) 3046–3055.
by mecamylamine may be due, at least in part, to blockade [9] M. Kaneko, D. Hammond, Role of spinal gamma-aminobutyric of other inhibitory ion channel receptors, such as the acidA receptors in formalin-induced nociception in the rat, J.
GABAA receptor [18]. This will have to be further Pharmacol. Exp. Ther. 282 (1997) 928–938.
[10] I.M. Khan, H. Buerkle, P. Taylor, T.L. Yaksh, Nociceptive and
investigated.
antinociceptive responses to intrathecally administered nicotinic
In conclusion, these data support the hypothesis that
agonists, Neuropharmacology 37 (1998) 1515–1525.
nAChRs play a tonic role in the control of tonic pain in [11] I.M. Khan, K.L. Youngblood, M.P. Printz, T.L. Yaksh, P. Taylor, rats. Inhibition of nAChRs with the antagonist Spinal nicotinic receptor expression in spontaneously hypertensive
mecamylamine, either before or after formalin injection, rats, Hypertension 28 (1996) 1093–1099.
[12] T. O’Dell, B. Christensen, Mecamylamine is a selective
non-com-results in an increase in pain behaviors and suggests that
petitive antagonist of N-methyl-D-aspartate- and aspartate-induced
the endogenous cholinergic system, mediated through
currents in horizontal cells dissociated from the catfish retina,
nAChRs, has, at least in part, an inhibitory role in tonic Neurosci. Lett. 94 (1988) 93–98.
pain as measured in the formalin test in rats. These data [13] K. Omote, T. Kawamata, M. Kawamata, A. Namiki,
Formalin-also indicate that nAChRs or certain nAChR subtypes may induced nociception activates a monoaminergic descending inhib-itory system, Brain Res. 814 (1998) 194–198.
have opposing effects on the modulation of acute and tonic
[14] T.S. Rao, L.D. Correa, R.T. Reid, G.K. Lloyd, Evaluation of
pain. Consequently, it is extrapolated that the treatment of
anti-nociceptive effects of neuronal nicotinic acetylcholine receptor
acute pain may be more amenable to nAChR antagonists, (NAChR) ligands in the rat tail-flick assay, Neuropharmacology 35 whereas the treatment of chronic pain may be more (1996) 393–405.
amenable to nAChR agonists or partial agonists. Although [15] K. Steen, P. Reeh, Actions of cholinergic agonists and antagonists on sensory nerve endings in rat skin, in vitro, Neurophysiology 70
the identity of the nAChR subtypes that regulate pain are
(1) (1993) 397–405.
currently unknown, modulating the function of these
[16] R.J. Tallarida, R.B. Murray, Manual of Pharmacologic Calculations
receptors with subtype selective ligands holds promise as a with Computer Programs, Springer-Verlag, New York, 1987, 297 pp. novel therapeutic approach for certain forms of pain. [17] E. Wada, K. Wada, J. Boulter, E. Deneris, S. Heinemann, J. Patrick, L.W. Swanson, Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat, J. Comp.
Acknowledgements
Neurol. 284 (1989) 314–335.
[18] V. Wotring, K. Yoon, The inhibitory effects of nicotinic antagonists
Expert statistical advice from Dr. Michael Ossipov was on currents elicited by GABA in rat hippocampal neurons,