www.elsevier.com / locate / bres
Research report
GABAergic neurons of the cat dorsal raphe nucleus express c-fos
during carbachol-induced active sleep
*
Pablo Torterolo, Jack Yamuy, Sharon Sampogna, Francisco R. Morales, Michael H. Chase
Department of Physiology and the Brain Research Institute, UCLA School of Medicine, Los Angeles, CA 90095, USA
Accepted 22 August 2000
Abstract
Serotonergic neurons of the dorsal raphe nucleus (DRN) cease firing during active sleep (AS, also called rapid-eye-movement sleep). This cessation of electrical activity is believed to play a ‘permissive’ role in the generation of AS. In the present study we explored the possibility that GABAergic cells in the DRN are involved in the suppression of serotonergic activity during AS. Accordingly, we examined whether immunocytochemically identified GABAergic neurons in the DRN were activated, as indicated by their expression of
c-fos, during carbachol-induced AS (AS-carbachol). Three chronically-prepared cats were euthanized after prolonged episodes of AS that
was induced by microinjections of carbachol into the nucleus pontis oralis. Another four cats (controls) were maintained 2 h in quiet wakefulness before being euthanized. Thereafter, immunocytochemical studies were performed on brainstem sections utilizing antibodies against Fos, GABA and serotonin. When compared with identically prepared tissue from awake cats, the number of Fos1neurons was larger in the DRN during AS-carbachol (35.965.6 vs. 13.964.4, P,0.05). Furthermore, a larger number of GABA1Fos1neurons were observed during AS-carbachol than during wakefulness (24.863.3 vs. 4.061.0, P,0.001). These GABA1 Fos1 neurons were distributed asymmetrically with a larger number located ipsilaterally to the site of injection. There was no significant difference between control and experimental animals in the number of non-GABAergic neurons that expressed c-fos in the DRN. We therefore suggest that activated GABAergic neurons of the DRN are responsible for the inhibition of serotonergic neurons that occurs during natural AS.
2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Biological rhythms and sleep
Keywords: Active sleep; REM sleep; Dorsal raphe; Fos; GABA; Serotonin
1. Introduction DRN neurons project to brainstem nuclei implicated in the
genesis and modulation of this state, such as the laterodor-Serotonergic (5-HT) neurons comprise one of the most sal and pedunculo-pontine tegmental nuclei (LDT-PPT), widely distributed neurochemical systems in the vertebrate the nucleus pontis oralis (NPO) and the locus coeruleus central nervous system [19]. The dorsal raphe nucleus [17,45,46,51,56]. Furthermore, recordings from presumed (DRN) contains the largest number of serotonin containing serotonergic neurons in the DRN of chronic unanesthetized neurons in the entire brain [49,60]. This nucleus has been animals have revealed that the activity of these cells varies implicated in a number of physiological processes, e.g. during the states of sleep and wakefulness. They exhibit a nociception, analgesia, thermoregulation, as well as being slow tonic rate of discharge during wakefulness (W) and involved in motor and autonomic functions [19,57]. their activity decreases and loses its typical regularity Numerous studies have demonstrated that serotonergic during quiet sleep (QS); these neurons cease firing during neurons of the DRN are also involved in the regulation of AS [7,16,31,47,54]. Decreases in serotonin levels in sever-sleep and wakefulness [19,21,40]. With respect to AS, al brain regions during AS are correlated with the decrease in firing rate that also occurs during this state (reviewed by [40]). It has been postulated that this decrease in activity
*Corresponding author. Tel.: 11-310-206-3499; fax: 1
1-310-206-has a ‘permissive’ effect on neuronal systems that generate
3499.
E-mail address: [email protected] (M.H. Chase). the behavioral and physiological indices of AS [30,41,43].
This suppression of cellular discharge was reversed follow- maintained with a gas mixture of halothane (1–3%) in ing the iontophoretic application of GABAA antagonists oxygen. Thereafter, the head was positioned in a stereo-into the DRN during AS [25]. In addition, microdialysis taxic frame and the calvarium was exposed. Stainless steel experiments have demonstrated that GABA release in the screw electrodes were placed in the frontal and parietal DRN increases during AS, and that microperfusion of the bones for recording the electroencephalogram (EEG) and GABA antagonist picrotoxin decreases the time spent in into the orbital portion of the frontal bone to record eye AS while GABA agonist muscimol increases the duration movements (EOG). Deep bipolar electrodes were im-of this state [37]. These facts suggest that GABAergic planted in both lateral geniculate nuclei in order to monitor inhibition may be responsible for the cessation of activity ponto-geniculo-occipital waves (PGO). A Winchester plug in serotonergic neurons that accompanies this state. How- (connected to these electrodes) and a chronic head-restrain-ever, the origin of the GABAergic control of DRN neurons ing device were bonded to the calvarium with acrylic during AS is not clear. cement. A small hole, 5 mm in diameter, which was made We and others have used the expression of c-fos during in the calvarium overlying the cerebellar cortex, was then AS induced by microinjection of carbachol into the NPO filled with bone-wax. This hole was subsequently used to of the cat to examine neural activity during this state provide access for drug administration. At the end of these
[9,33,34,48,62–64]. The AS-carbachol state resembles surgical procedures, an analgesic (Buprenex , 0.01 mg / naturally occurring AS polygraphically, electrophysiologi- kg, i.m.) was administered. Incision margins were kept cally and behaviorally [3,12,32,55]. Furthermore, in a clean and a topical antibiotic was administered on a daily similar model, carbachol microinjection into the NPO was basis. After the animals had recovered from these surgical found to produce a cessation of firing of serotonergic procedures, they were adapted to the head-restraining neurons in the DRN, which is similar to that which occurs device for 2 weeks.
during natural AS [50]. Using the AS-carbachol model, we
previously demonstrated that the DRN has a larger number 2.3. Experimental procedures of c-fos-expressing neurons during AS-carbachol than
during wakefulness and that the majority of these neurons In the experimental animals, carbachol (0.8mg in 0.2ml are not serotonergic [63]. of saline) was microinjected into the NPO (AP22 to 23, In the present study, using a double-labeling procedure L 1 to 2, H23.5 to25, according to Berman’s atlas [6]) for GABA and Fos, we found that the number of activated using a 2-ml Hamilton syringe. After the animals had spent GABAergic neurons in the DRN increases during AS- approximately 2 h in the AS-carbachol state, they were carbachol. We therefore hypothesize that these cells may be deeply anesthetized with sodium pentobarbital i.p. (60 GABAergic neurons that inhibit serotonergic neurons of mg / kg) and perfused for immunocytochemistry (see the DRN during this state. Preliminary results of this study below). Within this time period, the c-fos protein product have been presented in abstract form [53]. (Fos) is known to reach optimal concentration [9,34,62].
The control animals underwent the same surgical and habituation procedures as the experimental animals. All of
2. Material and methods them remained for 2 h in a state of quiet wakefulness before they were euthanized. In one control cat (C4), 2.1. Animals saline (0.2ml) was microinjected into the NPO 2 h before the animal was euthanized. The EEG, EOG, PGO and neck Seven male adult cats (three experimental and four electromyogram (EMG, obtained using acutely placed controls) were used in this study. The animals were bipolar electrodes) were recorded and stored in a Power
obtained from and determined to be in good health by the Macintosh G3 microcomputer usingSUPERSCOPE software.
UCLA Division of Laboratory Animal Medicine. All of
the experimental procedures were conducted in accord 2.4. Histological procedures with the Guide for the Care and Use of Laboratory
Animals (7th edition, National Academy Press, Washington The animals were perfused under deep anesthesia with 1 DC, 1996) and approved by the Chancellor’s Animal l of heparinized saline followed by 1.5 l of a solution of Research Committee of the UCLA Office for Protection of 4% paraformaldehyde, 15% saturated picric acid and 0.5% Research Subjects. of glutaraldehyde in phosphate buffer (PB, 0.1 M, pH 7.4). Subsequently, perfusion with 500 ml of the same solution 2.2. Surgical procedures with 10% sucrose was carried out. The brainstem was removed and immersed in a postfixative solution for 24 h; Before anesthesia, the animals were premedicated with the solution consisted of 2% paraformaldehyde, 15%
Xylazine (2.2 mg / kg, i.m.), atropine (0.04 mg / kg, i.m.) saturated picric acid and 10% of sucrose in PB. Following
the brainstem was frozen and cut into 14mm-thick sections out in seven sections for each animal (one section every using a Reichert–Jung cryostat. The sections were stored 500 mm). The mean number of immunostained neurons in a solution of 0.1% sodium azide, 0.25% in phosphate (Fos1, GABA1 Fos1 and GABA2 Fos1) on each side buffered saline (PBS, 0.1 M). of the brain and for each animal were then calculated. Brainstem sections were first immunostained for Fos. These means were used to compare stained neurons during For this purpose, free-floating sections from selected brain AS-carbachol and wakefulness (n53 and n54, respective-stem sections were incubated overnight in a rabbit poly- ly).
clonal Fos antiserum (Fos Ab5; Oncogene Research Prod- The serotonergic area extends beyond the cytoarchitec-ucts / Calbiochem, La Jolla, CA, USA) at a dilution of tonic limits of the DRN [42,60]. In order to standardize the 1:40 000 in PBS. The sections were rinsed four times in area of analysis, the lateral borders were defined by a PBS for a total duration of 30 min and then incubated for parasagittal plane traced from the lateral border of the 90 min in a biotinylated donkey anti-rabbit immuno- main fascicle of the medial longitudinal fascicle (mlf, see globulin G (1:700). Subsequently, the sections were incu- Fig. 3B). The dorsal and ventral borders of the DRN were bated with the ABC complex (Vector ABC Elite kit, determined according to standard criteria [52]. Serotoner-1:500) for 60 min. After rinsing again, the tissue was gic neurons were observed throughout the defined area of reacted for 10–20 min with 0.6% nickel ammonium analysis. In caudal sections, special care was taken to sulfate, 0.02% diaminobenzidine tetrahydrochloride avoid including neurons of the dorsal tegmental nucleus of (Sigma) and 0.015% hydrogen peroxide in 50 ml of 50 Gudden in this analysis.
mM tris buffer, pH 7.5. Photomicrographs were obtained using a Spot digital In order to identify GABAergic neurons, polyclonal camera attached to an Olympus B360 microscope. Images
guinea pig antibodies raised against GABA-keyhole limpet were analyzed using Adobe PHOTOSHOP software with a
hemocyanin conjugated with glutaraldehyde (NT-108, Power Macintosh G3 computer. Conventional and Protos) were employed. After the sections were treated for Nomarsky optics were employed. A 1003 oil immersion Fos immunostaining, they were incubated overnight with objective lens was used to measure the diameter of soma GABA antibody (1:3500) and normal donkey serum profiles. The distribution of labeled neurons was deter-(NDS; 3%). Then, after the sections were rinsed, they were mined from drawings using a camera lucida attachment. incubated for 60 min with biotinylated donkey anti-guinea The level of statistical significance of the difference pig antibody (1:300) plus NDS. After another rinse, the between the mean number of immunoreactive neurons in tissue was incubated in the ABC complex (1:200) for 60 control and AS-carbachol cats was evaluated using the min. Finally, the tissue was exposed to diaminobenzidine Student’s t-test. The criterion chosen to discard the null (without nickel enhancement). hypothesis was P,0.05.
Serotonin immunocytochemistry was performed on brainstem sections of both an AS-carbachol cat and an
awake cat (which were also both employed for GABA and 3. Results
Fos analysis), in order to determine the distribution of
serotonergic neurons. Following Fos immunocytoch- Following the administration of carbachol, all the ex-emistry, the sections were incubated overnight with poly- perimental animals exhibited the AS-carbachol state. The clonal goat serotonin antiserum (Incstar, 1:10 000) and latency to the onset of AS-carbachol was 2.061.3 min NDS. Subsequently, the tissue was incubated with (mean6S.E.M.) and the duration of this induced state was biotinylated donkey anti-goat immunoglobulin (1:1000) 107.864.8 min. Table 1 presents these data for each followed by incubation with ABC complex (1:400). Final- animal.
ly, the tissue was reacted with diaminobenzidine (without
nickel enhancement). 3.1. Characteristic of immunostained neurons In all procedures, four final rinses with 0.01 M PBS
preceded the mounting of sections on slides. Control As illustrated in Fig. 1, Fos immunoreactivity was omission experiments were performed without the expo- restricted to the neuronal nuclei. Fos1 neurons were sure of the tissue to the primary antibody. In representative identified by their dark-stained nuclei.
sections, Fos immunocytochemistry was followed by a Serotonergic immunoreactive neurons exhibited brown-Pyronin-Y counterstain.
(which corresponds to the rostral DRN) to approximately P
AS-C 2 99.3 1.0
22.5 (which corresponds to the caudal DRN) were
uti-AS-C 3 116.0 0.5
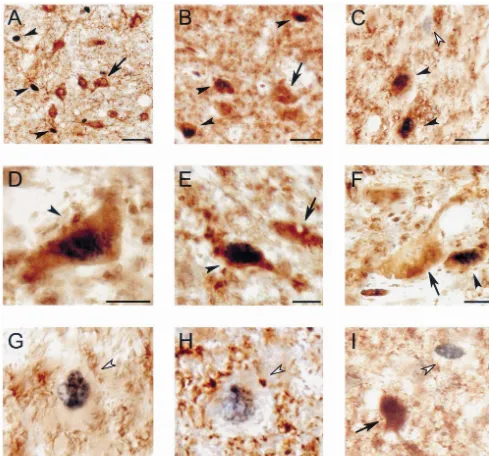
Fig. 1. Photomicrographs of the DRN illustrating serotonin, GABA and Fos immunoreactive neurons in AS-carbachol (A–H) and awake cats (I). (A) Serotonin and Fos immunoreactive neurons in the DRN during AS-carbachol. Serotonergic neurons are stained in brown. The nuclei of the serotonergic cells do not exhibit Fos immunostaining. Non-serotonergic Fos1neurons, which are identified by their black-stained nucleus and empty cytoplasm (arrowhead) are located near serotonergic neurons. (B) GABAergic neurons that express c-fos (GABA1Fos1) intermingled with GABA1Fos2cells (arrow). Oval-shaped GABA1Fos1neurons are indicated by arrowheads (Fos is immunostained black). GABA1Fos1neurons of this type were the most commonly observed within the DRN. (C) Oval-shaped GABA1Fos1neurons (filled arrowheads) near a GABA2Fos1neuron (open arrowhead). (D) Multipolar-shaped GABA1Fos1neuron (arrowhead). This type of GABA1Fos1neuron was scarce in the DRN. (E) Fusiform GABA1Fos1 neuron (arrowhead). These neurons were mainly located in the ventromedial region of the DRN. The arrow indicates an adjacent GABA1Fos2neuron. (F) Small, oval-shaped GABA1 Fos1 neuron (arrowhead) in close proximity to a larger GABA1 Fos2 neuron (arrow). (G and H) Examples of non-GABAergic Fos-immunoreactive neurons (GABA2 Fos1, open arrowheads) during AS-carbachol. In (G) the neuronal body is surrounded by GABAergic immunoreactive profiles. (I) GABA2 Fos1 neuron (open arrowhead) in the vicinity of a GABAergic Fos2 neuron (arrow) during wakefulness. All photomicrographs were taken from 14 mm-thick sections and processed with the diaminobenzidine method enhanced with nickel. Calibration bars: (A) 50mm; (B and C) 20mm; (D–I) 10mm.
stained soma and dendrites (Fig. 1A). The distribution of non-serotonergic neurons. We have previously analyzed serotonergic neurons observed in the present study was c-fos expression in serotonergic neurons [63], therefore
Table 2
1). Other structures that are known to contain GABAergic a
Mean cell counts from individual cats
neurons, e.g. cerebellum, globus pallidus, dorsal nucleus of
Cat GABA1Fos1 GABA2Fos1 Total Fos
the lateral lemniscus, etc, also exhibited strong
immuno-staining [35]. No GABA immunoreactivity was observed Controls
in control omission experiments. C1 6.2 9.5 16.7
C2 3.4 1.6 5.0
In brainstem sections immunostained for GABA and
C3 3.3 15.3 18.9
Fos, double-labeled neurons (GABA1 Fos1 neurons)
C4 3.1 11.0 15.1
displayed a brown cytoplasm and a black nucleus.
Non-GABAergic neurons that expressed c-fos (GABA2 Fos1 AS-carbachol
neurons) were also observed. In about 4% of the Fos1 AS-C1 30.7 14.0 46.6
AS-C2 24.3 7.1 33.1
neurons it was not possible to determine whether or not
AS-C3 19.4 7.3 27.9
they were GABAergic because of their small size and the
a
Each value represents an average of neurons in seven sections (see
intensity of background staining. These neurons were
Material and methods). The ‘Total Fos’ group included Fos1 neurons
counted as Fos1, but were not classified as either GABA1
that were impossible to identify either as GABA1or as GABA2(see
Fos1 or GABA2 Fos1. Representative examples of text). GABA1 Fos1, GABA2 Fos1 and GABA1 Fos2
neurons are presented in Fig. 1.
3.3. GABAergic neurons (GABA1) 3.2. Fos immunoreactivity during AS-carbachol
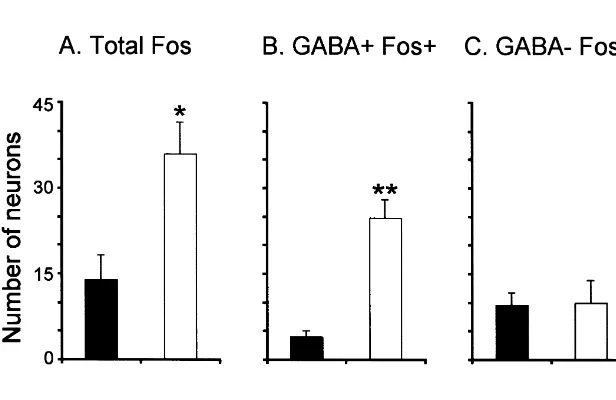
The camera lucida drawing in Fig. 3B depicts the The mean number of Fos1 neurons (GABA1 Fos1 distribution of GABAergic neurons in the DRN. These and GABA2 Fos1) was larger during AS-carbachol neurons were mainly located in the lateral regions of the compared to wakefulness (35.965.6 vs. 13.964.4, P, DRN; very few were found in the central region. As shown 0.05, see bar chart in Fig. 2A). Table 2 presents the mean in Fig. 3A and B, the distribution of Fos1 neurons was cell counts from individual cats. The photomicrograph in similar to the distribution of GABAergic neurons. Fig. 3A, illustrates the distribution of Fos1neurons in the There was a 620% increase in the number of GABA1
dorsal raphe in a section counterstained with Pyronin-Y. Fos1 neurons during AS-carbachol compared to control Fos1 neurons were located adjacent to the mlf and in the (24.863.3 vs. 4.061.0, P,0.001), as illustrated in Fig. 2B lateral portions of the raphe nucleus. They were scarce in (see also Table 2). Of the total number of Fos1 neurons, the central raphe, where the largest concentration of 69% were GABAergic in the AS-carbachol animals and serotonergic neurons is located. only 29% in awake animals (24.8 GABA1 Fos1 out of
of 8.760.3 mm. GABA1 Fos1 neurons were also heterogeneous in shape. The majority (51%) of these neurons were oval-shaped (Fig. 1B,C and F), 39% were fusiform (Fig. 3E) and 10% were multipolar (Fig. 3D). Fig. 4A consists of camera lucida drawings from waking and AS-carbachol cats. Note the larger number of GABA1
Fos1neurons that are present in the section obtained from the AS-carbachol cat.
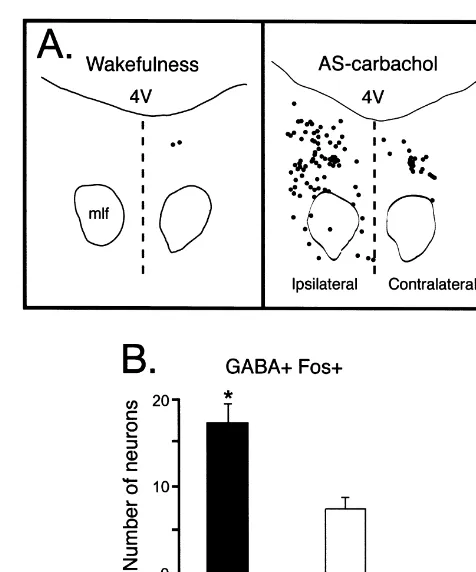
The number of GABA1 Fos1 neurons in the AS-carbachol cats was larger ipsilateral to the injection site than on the contralateral side (17.362.2 vs. 7.561.2,
P,0.05; Fig. 4B). No significant differences were ob-served between the right and left side of the sections obtained from cats that were awake. In the saline-injected animal, the number of GABA1Fos1neurons was similar between the ipsilateral (1.460.7, average of seven
sec-Fig. 3. Distribution of Fos1and GABA1neurons in the DRN. (A) This photomicrograph illustrates Fos immunostaining with Pyronin-Y counters-taining in the ventromedial region of the DRN during AS-carbachol. c-fos-expressing neurons were mainly distributed in the periphery close to the medial longitudinal fasciculus (examples are indicated by ar-rowheads). Fos immunoreactivity was not observed in the large, centrally located neurons. The photomicrograph was obtained from a 14-mm thick section and processed with the diaminobenzidine method enhanced with
nickel. Calibration bar5100mm. (B) Camera lucida drawing that depicts Fig. 4. (A) Camera lucida drawings illustrating the distribution of the distribution of neurons immunostained for GABA in a representative GABA
1 Fos1 in representative brainstem sections of awake and AS-section (P21.5) of the DRN. Each dot represents one immunolabeled carbachol cats (P
21.5). Compared to wakefulness, the increase in the neuron. Note that GABAergic neurons were concentrated mainly in the number of GABA
1 Fos1 neurons in the section obtained from AS-lateral regions of the DRN. Dashed lines indicate the borders of the area carbachol cat is readily apparent. On the side ipsilateral to the carbachol that was included in the present analysis. (A) and (B) were obtained from microinjection, GABA
1Fos1neurons were more abundant than in the adjacent sections of the same cat. 4V, fourth ventricle; mlf, medial contralateral side. Each mark indicates one labeled neuron. 4V, fourth longitudinal fasciculus. ventricle; mlf, medial longitudinal fasciculus. The dashed line designates the midline. (B) Regional differences in GABA1Fos1neurons during AS-carbachol. This plot presents the number of c-fos-expressing neurons in brainstem sections from AS-carbachol cats both ipsilateral and contralateral to the injection site. The mean number of GABA1Fos1
35.9 Fos1 neurons, and 4.0 out of 13.9, respectively). neurons was larger ipsilaterally (filled bars) than contralaterally (empty GABA1Fos1neurons were small, with an average major bars) (*, P,0.05, paired two-tailed Student’s t-test, n53). The error bars
tions) and the contralateral sides (1.760.8, average of AS also support the previously reported increase in meta-seven sections). bolic activity that has been described in the DRN during During AS-carbachol, the number of GABA1 Fos1 this state [27]. In addition, Maloney et al. [28] have shown neurons was significantly larger on both in the ipsilateral that the number of GABAergic neurons that express c-fos (17.362.2, P,0.001) and contralateral sides (7.561.2, in the DRN of the rat was positively correlated with the
P,0.005) compared to sections from the animals that were percent time spent in AS during recovery from AS awake (1.960.6, right side; 2.160.5, left side). deprivation.
In contrast to the GABAergic neurons, there was no 3.4. Non-GABAergic neurons (GABA2) significant difference in the number of non-GABAergic Fos1 (GABA2 Fos1) neurons in AS-carbachol and Compared to control conditions, no statistically signifi- awake cats.
cant changes in the number of GABA2Fos1 neurons An important question involves the source of the were observed in AS-carbachol cats (9.562.3 vs. 9.364.1; activation of GABAergic neurons of the DRN during AS. Fig. 2C, see also Table 2). The photomicrographs of Fig. 1 We suggest that neurons in the NPO may be involved in are examples of GABA2 Fos1 neurons in brainstem this process for the following reasons. Electrical stimula-sections from AS-carbachol cats (Fig. 1C, G and H) and tion of the NPO produces an inhibitory response in DRN from a cat during wakefulness (Fig. 1I). These neurons neurons that is blocked by GABA antagonists, but not byA
were intermingled with GABA1 Fos1 (Fig. 1C) as well glycine antagonists [59]. Neurons in the NPO are also as GABA1 Fos2 (Fig. 1I) neurons. activated during natural AS and directly by carbachol [13,14,18,29]. There are also fiber projections to the DRN from the NPO and surrounding areas [22,42,44].
There-4. Discussion fore, it is also possible that GABAergic DRN neurons are
activated by cholinoceptive AS-on neurons in the NPO. In The present results indicate that compared to wakeful- addition, a stronger ipsilateral NPO-DRN projection may ness, GABAergic neurons of the DRN are activated during explain why, in the present study, a larger number of AS-carbachol, whereas the activity of non-GABAergic GABA1 Fos1 neurons were located ipsilateral to the neurons was unchanged. injection site. It is possible that GABAergic DRN neurons GABAergic terminals establish synaptic contacts on are also driven by direct cholinergic input from the LDT-serotonergic DRN neurons [15,58]; GABAA receptors are PPT during naturally occurring AS; the LDT-PPT nuclei also located in these cells [11]. An inhibitory effect of are implicated in the generation of AS and projections GABA on serotonergic neurons has been demonstrated from these nuclei to the DRN have been described both in vivo and in vitro [24,38]. During AS, microdialysis [8,21,61].
studies have shown that GABA levels in the DRN increase A population of GABAergic neurons that did not compared to the levels present during other behavioral express c-fos during AS-carbachol was also observed. states [37]. Recently, Luppi et al. reported that the These neurons may be activated in natural AS, however, it iontophoretic application of the GABAA antagonist is also possible that there is a population of GABAergic bicuculline abolishes the suppression of activity of neurons within the DRN with a function unrelated to the serotonergic neurons during AS, confirming the impor- inhibition of serotonergic neurons during AS.
tance of GABAergic input in the cessation of serotonergic The data in this report suggest that the suppression of neuronal discharge during this state [25]. activity in serotonergic neurons during AS may be due to GABAergic neurons have been previously described in the activity of GABAergic neurons within the DRN. In the DRN [4,5,15,35,36]. A small proportion of these addition, other GABAergic inputs (from outside the DRN) GABAergic neurons project to the hypothalamus [10]; may also play a role in the suppression of serotonergic however, it remains to be determined if these neurons also activity during AS [26]. GABAergic projection to the innervate serotonergic DRN neurons. The GABAergic DRN from the lateral preoptic area, lateral hypothalamic neurons that were activated during AS-carbachol were area, periacueductal gray, substantia nigra, dorsal small in size and located mainly in the lateral areas of the paragigantocellular nucleus and the ventral tegmental area DRN. Electrophysiological studies in the DRN have have been described [26]. However, it is unlikely that the indicated the presence of neurons with characteristics source of the state-dependent, GABAergic inhibition of different from the typical wide action potentials and clock- serotonergic DRN neurons is rostral to the midbrain, like discharge patterns of serotonergic neurons [2]. Some because DRN units have been demonstrated to retain their of these (presumably) non-serotonergic neurons increase AS-off pattern of firing in cats that had been descerebrated their discharge during AS [1,7,23,47]. These neurons may at the precollicular level [39].
dis-[15] M. Harandi, M. Aguera, H. Gamrani, M. Didier, M. Maitre, A.
charge of serotonergic neurons within the DRN during this
Calas, M.F. Belin, g-Aminobutyric acid and 5-hydroxytryptamine
state.
interrelationship in the rat nucleus raphe dorsalis: combination of radioautographic and immunocytochemical techniques at light and electron microscopy levels, Neuroscience 21 (1987) 237–251. [16] J.A. Hobson, R.W. McCarley, J.P. Nelson, Location and spike-train
Acknowledgements
characteristics of cells in anterodorsal pons having selective de-creases in firing rate during desynchronized sleep, J. Neurophysiol.
We thank Dr. J.K. Engelhardt for critical comments 50 (1983) 770–783.
regarding the manuscript. National Institute of Health [17] T. Honda, K. Semba, Serotonergic synaptic input to cholinergic neurons in the rat mesopontine tegmentum, Brain Res. 647 (1994)
Grants NS-09999, NS-23426, and MH-43362 supported
299–306.
this work.
[18] K. Ito, R.W. McCarley, Alterations in membrane potential and excitability of cat medial pontine reticular formation neurons during changes in naturally occurring sleep–wake states, Brain Res. 292 (1984) 169–175.
References
[19] B.L. Jacobs, E.C. Azmitia, Structure and function of the brain serotonin system, Physiol. Rev. 72 (1992) 165–229.
[1] J. Adrien, L. Lanfumey, Ontogenesis of unit activity in the raphe [20] B.L. Jacobs, P.J. Gannon, E.C. Azmitia, Atlas of serotonergic cell dorsalis of the behaving kitten: its relationship with the states of bodies in the cat brainstem: an immunocytochemical analysis, Brain vigilance, Brain Res. 366 (1986) 10–21. Res. Bull. 13 (1984) 1–31.
[2] G.K. Aghajanian, R.Y. Wang, J. Baraban, Serotonergic and non- [21] B.E. Jones, Paradoxical sleep and its chemical / structural substrates serotonergic neurons of the dorsal raphe: reciprocal changes in firing in the brain, Neuroscience 40 (1991) 637–656.
induced by peripheral nerve stimulation, Brain Res. 153 (1978) [22] P. Kalen, M. Karlson, L. Wiklund, Possible excitatory amino acid 169–175. afferents to nucleus raphe dorsalis of the rat investigated with
3
[3] H.A. Baghdoyan, M.L. Rodrigo-Angulo, R.W. McCarley, J.A. retrograde wheat germ agglutinin andD-[ H]aspartate tracing, Brain
Hobson, Site-specific enhancement and suppression of desynchron- Res. 360 (1985) 285–297.
ized sleep signs following cholinergic stimulation of three brainstem [23] B. Kocsis, R.P. Vertes, Dorsal raphe neurons: synchronous discharge regions, Brain Res. 306 (1984) 39–52. with the theta rhythm of the hippocampus in the freely behaving rat, [4] M.F. Belin, M. Aguera, M. Tappaz, A. McRae-Degueurce, P. J. Neurophysiol. 68 (1992) 1463–1467.
Bobillier, J.F. Pujol, GABA-accumulating neurons in the nucleus [24] E.S. Levine, B.L. Jacobs, Neurochemical afferents controlling the raphe dorsalis and periaqueductal gray in the rat: a biochemical and activity of serotonergic neurons in the dorsal raphe nucleus: radioautographic study, Brain Res. 170 (1979) 279–297. microiontophoretic studies in the awake cat, J. Neurosci. 12 (1992) [5] M.F. Belin, D. Nanopoulos, M. Didier, M. Aguera, H. Steinbusch, 4037–4044.
A. Verhofstad, M. Maitre, J.F. Pujol, Immunohistochemical evidence [25] P.H. Luppi, D. Gervasoni, P. Fort, Serotonergic neurons from the rat for the presence ofg-aminobutyric acid and serotonin in one nerve dorsal raphe nucleus are tonically inhibited during sleep, Sleep Res. cell. A study on the raphe nuclei of the rat using antibodies to Online 2 (Suppl 1) (1999) 57, http: / / www.sro.org / 1999 / Supple-glutamate decarboxylase and serotonin, Brain Res. 275 (1983) ment 1991 / Luppi P, Gervasoni D, Fort P/ 57.
329–339. [26] P.H. Luppi, D. Gervasoni, C. Peyron, C. Rampon, B. Barbagli, R. [6] A.L. Berman, The brain stem of the cat. A cytoarquitectonic atlas Boissard, P. Fort, GABAergic and glycinergic afferents to the dorsal with stereotaxic coordinates, University of Wisconsin Press, raphe and locus coeruleus nuclei, J. Sleep Res. 7 (Suppl. 2) (1998)
Madison, WI, 1968. 165.
[7] R. Cespuglio, H. Faradji, M.E. Gomez, M. Jouvet, Single unit [27] R. Lydic, H.A. Baghdoyan, L. Hibbard, E.V. Bonyak, M.R. De-recordings in the nuclei raphe dorsalis and magnus during the Joseph, R.A. Hawkins, Regional brain glucose metabolism is altered sleep-waking cycle of semi-chronic prepared cats, Neurosci. Lett. 24 during rapid eye movement sleep in the cat: a preliminary study, J.
(1981) 133–138. Comp. Neurol. 304 (1991) 517–529.
[8] J. Cornwall, J.D. Cooper, O.T. Phillipson, Afferent and efferent [28] K.J. Maloney, L. Mainville, B.E. Jones, Differential c-Fos expres-connections of the laterodorsal tegmental nucleus in the rat, Brain sion in cholinergic, monoaminergic, and GABAergic cell groups of Res. Bull. 25 (1990) 271–284. the pontomesencephalic tegmentum after paradoxical sleep depriva-[9] M. Dragunow, R. Faull, The use of c-fos as a metabolic marker in tion and recovery, J. Neurosci. 19 (1999) 3057–3072.
neuronal pathway tracing, J. Neurosci. Methods 29 (1989) 261–265. [29] R.W. McCarley, J.A. Hobson, Single neuron activity in cat giganto-[10] B. Ford, C.J. Holmes, L. Mainville, B.E. Jones, GABAergic neurons cellular tegmental field: selectivity of discharge in desynchronized
in the rat pontomesencephalic tegmentum: codistribution with sleep, Science 174 (1971) 1250–1252.
cholinergic and other tegmental neurons projecting to the posterior [30] R.W. McCarley, J.A. Hobson, Neuronal excitability modulation over lateral hypothalamus, J. Comp. Neurol. 363 (1995) 177–196. the sleep cycle: a structural and mathematical model, Science 189 [11] B. Gao, J.M. Fritschy, D. Benke, H. Mohler, Neuron-specific (1975) 58–60.
expression of GABAA-receptor subtypes: differential association of [31] D.J. McGinty, R.M. Harper, Dorsal raphe neurons: depression of thea1- anda3-subunits with serotonergic and GABAergic neurons, firing during sleep in cats, Brain Res. 101 (1976) 569–575. Neuroscience 54 (1993) 881–892. [32] F.R. Morales, J.K. Engelhardt, P.J. Soja, A.E. Pereda, M.H. Chase, [12] R. George, W. Haslett, D. Jenden, A cholinergic mechanism in the Motoneuron properties during motor inhibition produced by mi-brainstem reticular formation: induction of paradoxical sleep, Int. J. croinjection of carbachol into the pontine reticular formation of the Neuropharmacol. 3 (1964) 541–552. decerebrate cat, J. Neurophysiol. 57 (1987) 1118–1129.
[13] R.W. Greene, D.O. Carpenter, Actions of neurotransmitters on [33] F.R. Morales, S. Sampogna, J. Yamuy, M.H. Chase, c-Fos expres-pontine medical reticular formation neurons of the cat, J. Neuro- sion in brainstem premotor interneurons during cholinergically physiol. 54 (1985) 520–531. induced active sleep in the cat, J. Neurosci. 19 (1999) 9508–9518. [14] R.W. Greene, U. Gerber, R.W. McCarley, Cholinergic activation of [34] J.I. Morgan, T. Curran, Stimulus-transcription coupling in the medial pontine reticular formation neurons in vitro, Brain Res. 476 nervous system: involvement of the inducible proto-oncogenes fos
[35] E. Mugnaini, W.H. Oertel, An atlas of the distribution of gabaergic activity in freely moving cats is altered by manipulations of central neurons and terminals, in: A. Bjorklund, T. Hokfelt (Eds.), Hand- but not peripheral motor systems, Brain Res. 279 (1983) 77–84. book of chemical neuroanatomy, Vol. 4: GABA and neuropeptides in [51] T.L. Steininger, B.H. Wainer, R.D. Blakely, D.B. Rye, Serotonergic the CNS, Elsevier, Amsterdam, 1985, pp. 436–608. dorsal raphe nucleus projections to the cholinergic and noncholiner-[36] D. Nanopoulos, M.F. Belin, M. Maitre, G. Vincendon, J.F. Pujol, gic neurons of the pedunculopontine tegmental region: a light and Immunocytochemical evidence for the existence of GABAergic electron microscopic anterograde tracing and immunohistochemical neurons in the nucleus raphe dorsalis. Possible existence of neurons study, J. Comp. Neurol. 382 (1997) 302–322.
containing serotonin and GABA, Brain Res. 232 (1982) 375–389. [52] E. Taber, A. Brodal, F. Walberg, The raphe nuclei of the brainstem [37] D. Nitz, J. Siegel, GABA release in the dorsal raphe nucleus: role in in the cat. I. Normal topography, cytoarchitecture and general
the control of REM sleep, Am. J. Physiol. 273 (1997) R451–455. discussion, J. Comp. Neurol. 114 (1960) 161–188.
[38] Z.Z. Pan, J.T. Williams, GABA- and glutamate-mediated synaptic [53] P. Torterolo, J. Yamuy, S. Sampogna, F. Morales, M.H. Chase, potentials in rat dorsal raphe neurons in vitro, J. Neurophysiol. 61 GABAergic neurons of the dorsal raphe nucleus express c-fos (1989) 719–726. during carbachol induced active sleep, Sleep Res. Online 2 (Suppl. [39] O. Pompeiano, K. Hoshino, Tonic inhibition of dorsal pontine 1) (1999) 94, http: / / www.sro.org / 1999 / Supplement 1991 /
Tor-neurons during the postural atonia produced by an anticholinesterase terolo P, Yamuy J, Sampogna S, Morales F, Chase MH / 94. in the decerebrate cat, Arch. Ital. Biol. 114 (1976) 310–340. [54] M.E. Trulson, B.L. Jacobs, Raphe unit activity in freely moving [40] C.M. Portas, B. Bjorvatn, R. Ursin, Serotonin and the sleep / wake cats: correlation with level of behavioral arousal, Brain Res. 163
cycle: special emphasis on microdialysis studies, Prog. Neurobiol. (1979) 135–150.
60 (2000) 13–35. [55] G. Vanni-Mercier, K. Sakai, J.S. Lin, M. Jouvet, Mapping of [41] C.M. Portas, M. Thakkar, D. Rainnie, R.W. McCarley, Microdialysis cholinoceptive brainstem structures responsible for the generation of perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH- DPAT) paradoxical sleep in the cat, Arch. Ital. Biol. 127 (1989) 133–164. in the dorsal raphe nucleus decreases serotonin release and increases [56] R.P. Vertes, B. Kocsis, Projections of the dorsal raphe nucleus to the rapid eye movement sleep in the freely moving cat, J. Neurosci. 16 brainstem: PHA-L analysis in the rat, J. Comp. Neurol. 340 (1994)
(1996) 2820–2828. 11–26.
[42] M.L. Rodrigo-Angulo, E. Rodriguez-Veiga, F. Reinoso-Suarez, [57] Q.P. Wang, Y. Nakai, The dorsal raphe: an important nucleus in pain Serotonergic connections to the ventral oral pontine reticular nu- modulation, Brain Res. Bull. 34 (1994) 575–585.
cleus: Implication in paradoxical sleep, J. Comp. Neurol. 418 (2000) [58] Q.P. Wang, H. Ochiai, Y. Nakai, GABAergic innervation of 93–105. serotonergic neurons in the dorsal raphe nucleus of the rat studied [43] K. Sakai, Executive mechanisms of paradoxical sleep, Arch. Ital. by electron microscopy double immunostaining, Brain Res. Bull. 29
Biol. 126 (1988) 239–257. (1992) 943–948.
[44] K. Sakai, D. Salvert, M. Touret, M. Jouvet, Afferent connections of [59] R.Y. Wang, D.W. Gallager, G.K. Aghajanian, Stimulation of pontine the nucleus raphe dorsalis in the cat as visualized by the horseradish reticular formation suppresses firing of serotonergic neurons in the peroxidase technique, Brain Res. 137 (1977) 11–35. dorsal raphe, Nature 264 (1976) 365–368.
[45] K. Semba, Aminergic and cholinergic afferents to REM sleep [60] L. Wiklund, L. Leger, M. Persson, Monoamine cell distribution in induction regions of the pontine reticular formation in the rat, J. the cat brain stem. A fluorescence histochemical study with quantifi-Comp. Neurol. 330 (1993) 543–556. cation of indolaminergic and locus coeruleus cell groups, J. Comp. [46] K. Semba, H.C. Fibiger, Afferent connections of the laterodorsal and Neurol. 203 (1981) 613–647.
the pedunculopontine tegmental nuclei in the rat: a retro- and [61] N.J. Woolf, J.B. Harrison, J.S. Buchwald, Cholinergic neurons of the antero-grade transport and immunohistochemical study, J. Comp. feline pontomesencephalon. II. Ascending anatomical projections, Neurol. 323 (1992) 387–410. Brain Res. 520 (1990) 55–72.
[47] K. Shima, H. Nakahama, M. Yamamoto, Firing properties of two [62] J. Yamuy, J.R. Mancillas, F.R. Morales, M.H. Chase, C-fos expres-types of nucleus raphe dorsalis neurons during the sleep–waking sion in the pons and medulla of the cat during carbachol- induced cycle and their responses to sensory stimuli, Brain Res. 399 (1986) active sleep, J. Neurosci. 13 (1993) 2703–2718.