www.elsevier.com / locate / bres
Interactive report
Evidence that neurotensin mediates the central effect of leptin on food
qintake in rat
a ,
*
b aAbhiram Sahu
, Robert E. Carraway , Yi-Peng Wang
a
Department of Cell Biology and Physiology, University of Pittsburgh School of Medicine, S-829 Scaife Hall, 3550 Terrace Street Pittsburgh,
PA15261, USA b
Department of Physiology, University of Massachusetts Medical School, Worcester, MA 06510, USA Accepted 27 October 2000
Abstract
Recent evidence suggests that leptin’s action on food intake and body weight regulation is mediated by a number of orexigenic and anorectic neuronal systems in the hypothalamus. Our previous demonstration that central injections of leptin induce hypothalamic neurotensin (NT) gene expression in association with a reduced food intake and decreased body weight in rats indicates that NT, an anorectic peptide, is involved in mediating leptin’s action on feeding and body weight regulation. To begin to examine the relative role of NT in this regard we evaluated the effects of NT antiserum (NT-AS) or NT receptor antagonist, SR 48692, on the satiety action of leptin in rats. In the first experiment, 3rd cerebroventricular (i.c.v.) administration of either 1 or 5ml of NT-AS, 30 min prior to leptin (4mg) injection, completely blocked the effects of leptin on food deprivation (FD)-induced feeding. In the second experiment, intraperitoneal (i.p.) administration of SR 48692 (40mg / kg) also completely prevented leptin’s satiety action on FD-induced feeding. These results showing the reversal of leptin’s satiety action by either NT immunoneutralization or NT-receptor antagonism support our hypothesis that NT is involved in mediating leptin’s action on feeding and further suggest that this neuropeptide is a quantitatively important component of the leptin sensitive neural circuitry. 2001 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Neuropeptides and behavior
Keywords: Neurotensin; Leptin; SR 48692; Feeding
1. Introduction hypothalamus [7,15,25,28,30]. In addition, our recent study suggests that leptin not only alters neuropeptide gene Leptin, a product of the ob gene, has been implicated to expression but it may also modulate neuropeptide release play an important role in regulation of a variety of and action [26].
physiological functions, including food intake and body We have recently demonstrated that central leptin ad-weight maintenance [5,10,37]. Although the hypothalamus ministration increased gene expression of neurotensin (NT) has been implicated as the major site of leptin action, the in the hypothalamus in association with decreased food hypothalamic mechanism(s) by which leptin exerts its intake and body weight in the rat [25]. This observation satiety action is not clear. However, several orexigenic and along with the findings that (1) central injection of NT, a anorectic neuropeptides and neurotransmitters have been 13 amino acid peptide [3], decreases food intake in a identified to be potential targets of leptin action in the variety of experimental paradigms [16,17,29]; (2) NT neurons and terminals are present in those sites of the hypothalamus that have been implicated in feeding
be-q
A part of this work has been presented at the 4th International havior and body weight regulation [8,13,14]; (3) NT gene Congress of Neuroendocrinology, Kitakyushu, Japan, October 11–16, expression is decreased in ob /ob mice lacking leptin 1998. Published on the World Wide Web on 1 December 2000.
[34,35]; and (4) NT neurons in the hypothalamus express *Corresponding author. Tel.: 11-412-648-9445; fax: 1
1-412-383-leptin receptor [12] suggest that NT is involved in mediat-7159.
E-mail address: [email protected] (A. Sahu). ing leptin’s action on feeding and body weight regulation. 0006-8993 / 01 / $ – see front matter 2001 Elsevier Science B.V. All rights reserved.
344 A. Sahu et al. / Brain Research 888 (2001) 343 –347
While our previous study has identified NT as a target of (1 ml) on leptin’s (4 mg) satiety action was examined. leptin action, it is not clear whether an increased NT gene Experimental protocol was similar to that of the experi-expression [25], and thereby a possible increase in NT ment 1a.
synthesis and release, may play a major role in mediating
the action of leptin on feeding. Thus to examine further the 2.2.3. Experiment 2
relative role of NT in mediating leptin action, we examined To assess further the role of endogenous NT in mediat-the effects of NT antiserum (NT-AS [4,18,33]) and an NT ing leptin action in the hypothalamus, the effects of a receptor antagonist, SR 48962 [11], on the satiety action of selective nonpeptide NT-receptor antagonist, SR 48692 leptin in the rat. [14], on leptin’s satiety action were tested. This experiment was essentially similar to that of experiment 1a, except that
´
SR 48692 (Sanofi Recherche, France) was injected i.p. at a 2. Materials and methods dose of 40mg / kg or vehicle (0.4 ml) alone prior to i.c.v. leptin administration. This dose of SR 48692 was selected 2.1. Animals on the basis of a previous report [11]. SR 48692 was dissolved in a drop of Tween 80 and then diluted in sterile Adult male Sprague–Dawley rats (Taconic, German- distilled water according to the manufacturer’s instruction. town, NY), weighing 275–300 g, were housed individually In a separate study, the effects of SR 48692 on feeding in a temperature (228C) and light (14 h light and 10 h dark, were compared to that of vehicle-treated groups.
lights on 05:00 h) controlled room with free access to food
(rodent chow) and water. After 1 week of acclimatization, 2.3. Statistical analysis the rats were permanently implanted stereotaxically with
22-gauge stainless steel cannulae (Plastic One, Roanoke, Food intake was expressed as the mean6S.E.M. Statisti-VA) into the third cerebroventricle under pentobarbital cal analyses were performed with analysis of variance anesthesia. After 2 weeks of recovery, the animals were (ANOVA) followed by Student–Newman–Keuls multiple used in the following experiments. Separate groups of rats range test (GB-STAT Software for the Macintosh, Dy-were used for different experiments. The rats Dy-were handled namic Microsystems, Inc., Silver Spring, MD). Compari-daily for 7 days before the experiment in order to minimize sons with P,0.05 were considered to be significantly
nonspecific stress. different.
2.2. The effects of NT antiserum (NT-AS) and NT
receptor antagonist(SR 48692) on the satiety action of 3. Results
leptin in rats
3.1. Response to anti-NT antisera 2.2.1. Experiment 1a
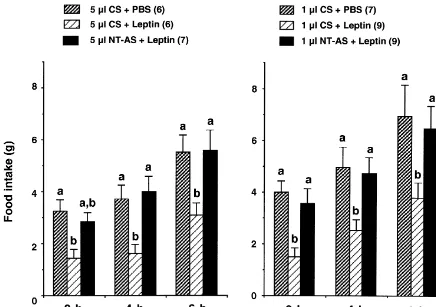
Rats were injected intracerebroventricularly (i.c.v.) with Administration of 4mg of leptin resulted in a decrease 5ml of NT-AS or control rabbit serum (CS) in the middle in cumulative food intake that were 44610% (P50.007), of the light period (at |13:00 h when the rats do not eat) 4469% (P50.007) and 5668% (P50.013) of CS1PBS followed 30 min later by 4mg of rat leptin (rLeptin, R&D control at 2-, 4- and 6-h after injection, respectively (Fig. Systems Inc., Minneapolis) in phosphate-buffered saline 1). Prior administration of NT-AS at a dose of 5 ml (PBS) or PBS alone. Thereafter, food was withdrawn from completely blocked the effects of leptin on food intake at 4 all rats. Twenty-two hours later similar injections were h (F(1,11)50.11, P50.74 vs. CS1PBS) and 6 h made and 30 min after the last injection preweighed rat (F(1,11)50.007, P50.93 vs. CS1PBS). At a 2-h period, chow was provided to the animals. Cumulative food intake leptin-induced decrease in cumulative food intake was was monitored for 2-, 4- and 6-h periods. The fasting partially blocked by NT-AS treatment because the food paradigm was used to induce feeding during the day time intake values in leptin1NT-AS group was not significantly when the rats do not eat, so that the effects of the drugs different from either control vehicle (F(1,11)50.27, P5 could be easily monitored at certain intervals for a 0.61) or leptin1CS (F(1,11)53.66, P50.08) group. considerable period. In a separate but similar experiment, Administration of NT-AS (5 ml) alone had no effect on the effect of NT-AS (NT-AS1PBS) on food intake was food intake as compared to that of the CS group compared to that of CS treated animals (CS1PBS). (mean6S.E.M. for CS1PBS vs. NT-AS1PBS; 2 h: Concentrations of NT-AS and CS IgG were 4.5 mg /ml. 4.9660.24 g vs. 4.7660.49 g; 4 h: 5.860.6 g vs. Preparations of NT-AS and CS, and the specificity of this 7.6360.68 g; 6 h: 8.5762.14 g vs. 10.8360.88 g; n53 in antisera have been described previously [4,18,33]. each group).
Fig. 1. Effects of neurotensin antiserum (NT-AS) or control serum (CS) on leptin’s effect on food intake induced by food deprivation. Values are presented as mean6S.E.M. Numbers in parentheses in this and subsequent figures indicate the number of animals. Values with dissimilar superscripts are significantly different from each other.
F(1,14)50.30, P50.58 vs. CS1PBS; 4 h: F(1,14)50.05, 4. Discussion
P50.81 vs. CS1PBS; 6 h: F(1,14)50.09, P50.76 vs.
CS1PBS). Injection of NT-AS (1ml) alone had no effect The present studies demonstrated that intracerebroven-on food intake (mean6S.E.M. for CS1PBS vs. NT-AS1 tricular administration of leptin decreased food intake in PBS; 2 h: 3.9860.45 g vs. 3.7460.44 g; 4 h: 4.9560.8 g rats previously food deprived for 22 h and that prior vs. 5.2260.89 g; 6 h: 6.9261.23 g vs. 6.9861.02 g; n57 injection of either NT-antibody or NT receptor antagonist, and 5, respectively). SR 48692, completely reversed the effects of leptin on
feeding.
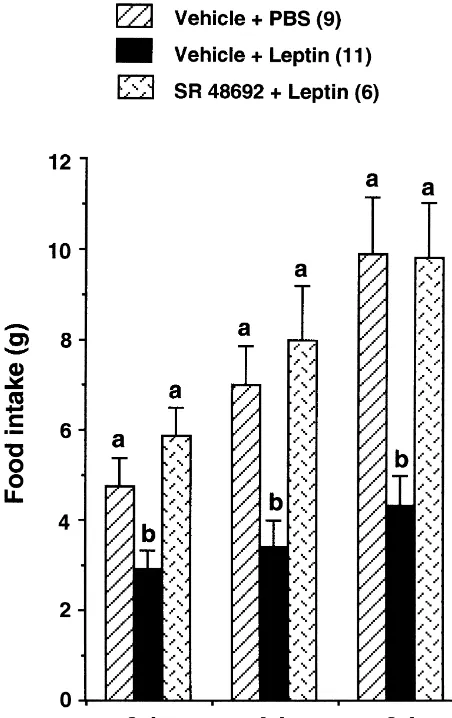
Understanding the mechanism by which leptin inhibits 3.2. Response to SR 48692 feeding is the subject of intense research. In this regard the hypothalamus has been implicated as the major site of Similar to the experiment 1, i.c.v. leptin administration leptin action [7,10,25,28]. Initially it was thought that decreased cumulative food intake to 6168% (P50.02), leptin acts by inhibiting activity of the hypothalamic 4968% (P50.002), and 4367% (P50.0006) of the neurons producing NPY [30], a potent orexigenic signal vehicle1PBS control at 2-, 4- and 6-h after injection, [19,27]. Further studies revealed that sensitivity to leptin respectively (Fig. 2). The effect of leptin on food intake on feeding increased in NPY-knock-out mice [9] sug-was completely abolished by prior i.p. administration of gesting that non-NPY neurons are also involved in mediat-SR 48692 at 2-h (F(1,13)51.549, P50.23 vs. vehicle1 ing leptin action. Subsequently several other hypothalamic PBS), 4-h (F(1,13)50.468, P50.50 vs. vehicle1PBS), orexigenic neurons have been identified as potential targets and 6-h (F(1,13)50.003, P50.95 vs. vehicle1PBS) of leptin signaling [25]. Although, amongst the anorectic periods (Fig. 2). Also the administration of SR 48692 signals, melanocortin neuronal system has been identified alone had no effect on food intake (values are as one of the major pathways mediating leptin action mean6S.E.M. for vehicle1PBS vs. SR 486921PBS; 2 h: [28,36], accumulating evidence suggests that several other 4.760.53 g vs. 5.3260.90 g; 4 h: 7.1761.03 g vs. anorectic signals, including NT, are also the targets of 6.8760.47 g; 6 h: 9.661.85 g vs. 8.7261.15 g, n54 in leptin signaling [5,7,15,25].
346 A. Sahu et al. / Brain Research 888 (2001) 343 –347
leptin exerts a direct action in stimulating NT release. Recent work suggests that NT and leptin may act syner-gistically in regulating food intake [2]. In addition, daily leptin injections for 7 days have been shown to alter hypothalamic NT content [22]. In total, these results are consistent with the notion that NT is an important mediator for leptin and that it may also modulate leptin’s effects on other neurons involved in feeding behavior.
Finally, it is worth noting that in addition to its own action on feeding, NT has been shown to regulate some of the hypothalamic signals that are involved in feeding and body weight regulation [23]. In this respect, NT and corticotropin-releasing hormone (CRH) connection is worth mentioning. The evidence such as NT stimulates CRH release [24] and NT antagonist, SR 48692, decreases CRH mRNA levels in the paraventricular nucleus [20], suggest that NT may have an important role in regulating CRH neuronal activity. In addition, CRH is a potent anorectic signal [19,36], and it has also been identified as a potential target of leptin signaling because (a) leptin stimulates CRH gene expression [32] and CRH release [6], and (b) CRH antagonist attenuates leptin’s satiety effect [32]. Taken together, it is possible that leptin’s effect on feeding may be mediated through the NT-CRH axis. In addition, it has been shown recently that NT antagonizes feeding induced by melanin concentrating hormone (MCH), an important orexigenic signal of lateral hypo-thalamic origin [21,31]. We have demonstrated that leptin inhibits hypothalamic MCH gene expression [25] as well as MCH-induced food intake in the rat [26]. Thus it is likely that leptin’s action on CRH and MCH neurons may Fig. 2. Effects of neurotensin antagonist, SR 48692, on leptin’s effect on also involve the NT neuronal system.
food intake induced by food deprivation. Values with dissimilar
In summary, using immunoneutralization and receptor superscripts are significantly different from each other.
antagonism techniques, we have provided new evidence that reinforces our hypothesis that NT is involved in body weight regulation is evident from the findings that (a) mediating leptin action on feeding and further suggests that central injection of NT inhibits food intake [16,17,29], (b) this neuropeptide is a quantitatively important component NT gene expression and peptide levels are decreased in of the leptin sensitive neural circuitry.
ob /ob mice [34,35], and (c) NT levels in several
hypo-thalamic nuclei are decreased in Zucker obese rats [1]. The
possibility that leptin signaling might involve an effect on Acknowledgements NT neurons was tested in our previous study in which
leptin was shown to increase hypothalamic NT gene This work was supported by Competitive Medical expression [25]. This observation with other supporting Research Fund of the University of Pittsburgh Medical data [34,35] led us to postulate that NT may be one of the Center and NIH DK52844 to AS. Thanks are due to Ms. important mediators of leptin action in the hypothalamus. Carolyn M. Phalin for excellent technical assistance and to In the present study, immunoneutralization and receptor Dr. Danielle Gully, Sanofi Recherche, Toulouse Cedex, antagonism strategies have been used to address the role of France, for supplying SR 48692.
endogenous NT in mediating leptin’s satiety action in the hypothalamus. Our finding that prior administration of either anti-NT antibody or NT-receptor antagonist
com-References pletely blocked the anorectic action of leptin in FD rats
suggests that NT, released perhaps from hypothalamic
[1] B. Beck, A. Burlet, J.-P. Nicolas, C. Burlet, Neurotensin in neurons [14], is an essential mediator of leptin’s inhibitory microdissected brain nuclei and in the pituitary of the lean and obese effect on feeding in rats. Since hypothalamic NT neurons Zucker rats, Neuropeptides 13 (1988) 1–7.
hypothalamic neurotensin signals leptin effects on feeding behavior [21] D. Qu, D.S. Ludwig, S. Gammeltoft, M. Piper, M.A. Pelleymounter, in normal and fat preferring rats, Biochem. Biophys. Res. Commun. M.J. Cullen, W.F. Mathes, J. Przypek, R. Kanarek, E. Maratos-Flier, 252 (1998) 634–638. A role for melanin-concentrating hormone in the central regulation [3] R.E. Carraway, S.E. Leeman, The amino acid sequence of a of feeding behaviour, Nature 380 (1996) 243–247.
hypothalamic peptide, neurotensin, J. Biol. Chem. 250 (1975) 1907– [22] S. Richy, A. Burlet, J.-P. Max, C. Burlet, B. Beck, Effect of chronic
1911. intraperitoneal injections of leptin on hypothalamic neurotensin
[4] R.E. Carraway, S.E. Leeman, Radioimmunoassay for neurotensin, a content and food intake, Brain Res. 862 (2000) 276–279. hypothalamic peptide, J. Biol. Chem. 251 (1976) 7035–7044. [23] W.H. Rostene, M.J. Alexander, Neurotensin and neuroendocrine [5] F.F. Casanueva, C. Dieguez, Neuroendocrine regulation and actions regulation, Front. Neuroendocrinol. 18 (1997) 115–173.
of leptin, Front. Neuroendocrinol. 20 (1999) 317–363. [24] W. Rowe, V. Viau, M.J. Meaney, R. Quirion, Stimulation of CRH-[6] A. Costa, A. Poma, E. Martignoni, G. Nappi, E. Ur, A. Grossman, mediated ACTH secretion by central administration of neurotensin: Stimulation of corticotropin-releasing hormone release by the obese evidence for the participation of the paraventricular nucleus, J. (ob) gene product, leptin, from hypothalamic explants, Neuroreport Neuroendocrinol. 7 (1995) 109–117.
8 (1997) 1131–1134. [25] A. Sahu, Evidence suggesting that galanin (GAL), melanin-concen-[7] J. Elmquist, E. Maratos-Flier, C. Saper, J. Flier, Unraveling the trating hormone (MCH), neurotensin (NT), proopiomelanocortin central nervous system pathways underlying responses to leptin, (POMC) and neuropeptide Y (NPY) are targets of leptin signaling in Nature Neurosci. 1 (1998) 445–450. the hypothalamus, Endocrinology 139 (1998) 795–798.
[8] P.C. Emson, M. Goedert, P.W. Mantyh, Neurotensin-containing [26] A. Sahu, Leptin decreases food intake induced by melanin-concen-¨
neurons, in: A. Bjorklund, T. Hokfelt (Eds.), Handbook of chemical trating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) neuroanatomy, Vol. 4, Elsevier Science, New York, 1985, pp. 355– in the rat, Endocrinology 139 (1998) 4739–4742.
402. [27] A. Sahu, S.P. Kalra, Neuropeptidergic regulation of feeding be-[9] J.C. Erickson, K.E. Clegg, R.D. Palmiter, Sensitivity to leptin and havior: neuropeptide Y, Trends Endocrinol. Metab. 4 (1993) 217–
susceptibility to seizures of mice lacking neuropeptide Y, Nature 381 224.
(1996) 415–418. [28] M.W. Schwartz, S.C. Woods, D. Porte Jr., R.J. Seeley, D.G. Baskin, [10] J.M. Friedman, J.L. Halaas, Leptin and the regulation of body Central nervous system control of food intake, Nature 404 (2000)
weight in mammals, Nature 395 (1998) 763–770. 661–671.
[11] D. Gully, M. Canton, R. Boigegrain, F. Jeanjean, J.C. Molimard, M. [29] B.G. Stanley, S.F. Leibowitz, N. Eppel, S. St-Pierre, B.G. Hoebel, Poncelet, C. Gueudet, M. Heaulme, R. Leyris, A. Brouard, D. Suppression of norepinephrine-elicited feeding by neurotensin: Pelaprat, C. Labbe-Jullie, J. Mazella, J.P. Maffrand, W. Rostene, P. evidence for behavioral, anatomical and pharmacological specificity, Kitabgi, G. Le Fur, Biochemical and pharmacological profile of a Brain Res. 343 (1985) 297–304.
potent and selective nonpeptide antagonist of the neurotensin [30] T.W. Stephens, M. Baslnski, P.K. Bristow, J.M. Bue-Valleskey, S.G. receptor, Proc. Natl. Acad. Sci. USA 90 (1993) 65–69. Burgett, L. Craft, J. Hale, J. Hoffman, H.M. Halung, A. Kriauclunas, [12] M.-L. Hakansson, H. Brown, N. Ghilardi, R.C. Skoda, B. Meister, W. MacKellar, P.R. Rostack Jr., B. Schoner, D. Smith, F.C. Tinsley, Leptin receptor immunoreactivity in chemically defined target X. Zhang, M. Helman, The role of neuropeptide Y in the antiobesity neurons of the hypothalamus, J. Neurosci. 18 (1998) 559–572. action of the obese gene product, Nature 377 (1995) 530–532. [13] Y. Ibata, F. Kawakami, K. Fukof, H.L. Obata-Tsuto, T. Kubo, H. [31] N.A. Tritos, D. Vicent, J. Gillette, D.S. Ludwig, E.S. Flier, E.
Okamura, N. Morimoto, C. Yanaihara, N. Yanaihara, Light and Maratos-Flier, Functional interactions between melanin-concentrat-electron microscopic immunocytochemistry of neurotensin-like im- ing hormone, neuropeptide Y, and anorectic neuropeptides in the rat munoreactive neurons in the rat hypothalamus, Brain Res. 302 hypothalamus, Diabetes 47 (1998) 1687–1692.
(1984) 221–230. [32] Y. Uehara, H. Shimizu, K. Ohtani, N. Sato, M. Mori, Hypothalamic [14] D. Kahn, G.M. Abrams, E.A. Zimmerman, R. Carraway, S.E. corticotropin-releasing hormone is a mediator of the anorexigenic
Leeman, Neurotensin neurons in the rat hypothalamus: an immuno- effect of leptin, Diabetes 47 (1998) 890–893.
cytochemical study, Endocrinology 107 (1980) 47–54. [33] E. Vijayan, R. Carraway, S.E. Leeman, S.M. McCann, Use of [15] P. Kristensen, M.E. Judge, U. Ribel, K.N. Christjansen, B.S. Wulff, antiserum to neurotensin reveals a physiological role for the peptide J.T. Calusen, P.B. Jensen, O.D. Madsen, N. Vrang, J.P. Larsen, S. in rat prolactin release, Proc. Natl. Acad. Sci. USA 85 (1988) Hastrup, Hypothalamic CART is a new anorectic peptide regulated 9866–9869.
by leptin, Nature 393 (1998) 72–76. [34] J.P.H. Wilding, S.G. Gilbey, C.J. Bailey, R.A.L. Batt, G. Williams, [16] A.S. Levine, J. Kneip, M. Grace, J.E. Morely, Effect of centrally M.A. Ghatei, S.R. Bloom, Increased neuropeptide Y messenger administered neurotensin on multiple feeding paradigms, Pharmacol. ribonucleic acid (mRNA) and decreased neurotensin mRNA in the Biochem. Behav. 18 (1983) 19–24. hypothalamus of the obese (ob /ob) mouse, Endocrinology 132 [17] D. Luttinger, R.A. King, D. Sheppard, J. Strupp, C.B. Nemeroff, (1993) 1939–1944.
A.J. Prange, The effect of neurotensin on food consumption in the [35] G. Williams, H. Cardoso, Y.C. Lee, M.A. Ghatei, P.R. Flatt, C.J. rat, Eur. J. Pharmacol. 81 (1982) 499–503. Bailey, S.R. Bloom, Reduced hypothalamic neurotensin concen-[18] D.W. Marshak, R.E. Carraway, C.F. Ferris, Characterization of trations in the genetically obese diabetic (ob /ob) mouse: possible
immunoreactive substance P and neurotensin in the goldfish retina, relationship to obesity, Metabolism 40 (1991) 1112–1116. Exp. Eye Res. 44 (1987) 839–848. [36] S. Woods, R.J. Seeley, D.J. Porte, M.W. Schwartz, Signals that [19] J.E. Morley, Neuropeptide regulation of appetite and weight, regulate food intake and energy homeostasis, Science 280 (1998)
Endocrinol. Rev. 8 (1987) 256–287. 1378–1383.
[20] A. Nicot, W.B. Rowe, E.R. De Kloet, C. Betancur, D.S. Jessop, S.L. [37] Y. Zhang, R. Proenca, M. Maffei, M. Barone, L. Leopold, J.M. Lightman, R. Quirion, W. Rostene, A. Berod, Endogenous neuro- Friedman, Positional cloning of the mouse obese gene and its human tensin regulates hypothalamus–pituitary–adrenal axis activity and homologue, Nature 372 (1994) 425–432.