Effect of cyclodextrin-encapsulated
b
-carotene on
progesterone production by bovine luteal cells
¸S. Arikan
∗, R.G. Rodway
Department of Animal Physiology and Nutrition, University of Leeds, Leeds LS2 9JT, UK
Received 16 March 2000; received in revised form 2 August 2000; accepted 22 August 2000
Abstract
Experiments were conducted to examine the effect of cyclodextrin-encapsulated b-carotene on basal or cholesterol (cyclodextrin-encapsulated), LH and dibutyryl cyclic AMP (dbcAMP)-stimulated progesterone production by bovine corpus luteum cells isolated from mid-luteal heifer ovaries by collagenase digestion. Cells were cultured with serum-free DMEM/Ham’s F12 medium in serum pre-treated plastic culture dishes for periods of up to 11 days. Medium was replaced after 24 h and thereafter every 48 h.b-Carotene was added to cultures in a carrier molecule, dimethyl-b-cyclodextrin, to facilitate dissolution. All treatments were started on day 3 of cul-ture. Treatment of cells with 1 or 2mmol/lb-carotene resulted in sharp inhibition of progesterone production. On the contrary, treatment of cells with 0.1mmol/lb-carotene resulted in significant stimulation (P <0.05) of both basal and cholesterol-stimulated progesterone secretion. The ef-fect ofb-carotene on LH or dbcAMP-stimulated progesterone production was also examined. Treatment of cells with LH or dbcAMP always resulted in stimulation of progesterone secretion (P <0.001). However, cells treated with LH plusb-carotene or dbcAMP plusb-carotene both produced significantly (P < 0.01) less progesterone relative to those cells treated with LH or dbcAMP alone on days 7, 9 and 11 of culture. These results indicate thatb-carotene can enhance luteal steroidogenesis when present at low concentrations but is inhibitory at higher concentrations and that encapsulation ofb-carotene in cyclodextrin is an effective method of supplying it to cells in culture. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:b-Carotene; Cattle-endocrinology; Luteal cells; Cyclodextrin; Progesterone
1. Introduction
b-Carotene is present in extremely high concentrations in the bovine corpus luteum (CL) (O’Fallon and Chew, 1984; Holt et al., 1995), giving the CL its characteristic bright
∗Corresponding author. Present address: Faculty of Veterinary Medicine, University of Kirikkale, Kirikkale 71100, Turkey. Tel.:+90-318-3573301; fax:+90-318-3573304.
E-mail address: [email protected] ( ¸S. Arikan).
yellow colour. As well as acting as a precursor for vitamin A there is increasing evidence that b-carotene may be necessary for optimal steroid production, possibly acting as an anti-oxidant (Young et al., 1995). It has been reported previously thatb-carotene may effect luteal cell steroid production in vitro (Pethes et al., 1985; O’Shaughnessy and Wathes, 1988) and in vivo (Dembinski and Bronicki, 1994). Also, Graves-Hoagland et al. (1988) found that a positive relationship existed between in vitro bovine luteal cell progesterone production and plasmab-carotene during the winter when plasmab-carotene concentrations are low in dairy herds. They showed that during the summer when plasmab-carotene is increased, this relationship is lost. However, some studies in dairy cattle have failed to observe an effect of b-carotene supplementation on plasma steroid hormone levels (Folman et al., 1979; Wang et al., 1982).
Previous work in this laboratory has demonstrated thatb-carotene will stimulate pro-gesterone production in bovine luteal cells maintained in tissue culture and also that the b-carotene concentration in the culture medium becomes depleted during incubation and that this occurs more rapidly when the cells are stimulated with either LH or cAMP (Arikan and Rodway, 1997, 1998), suggesting thatb-carotene is metabolised during the process of steroidogenesis.
Asb-carotene is almost completely insoluble in water and most other polar solvents, several methods have previously been used to deliverb-carotene to cells in culture. These include the use of water miscible organic solvents such as ethanol (Gross et al., 1997), dimethylsulphoxide (Young et al., 1995) and tetrahydrofuran (Bertram et al., 1991, Cooney et al., 1993). However, the use of such organic solvents may cause cytotoxicity, as well as having effects on cell metabolism, proliferation and membrane permeability (Yu and Quinn, 1994) and solvents such as DMSO have been demonstrated to stimulate progesterone synthesis in incubated luteal cells (Arikan and Rodway, 1997). b-Carotene is normally transported to the ovary incorporated in the lipid component of high density lipoprotein (HDL) in the bovine (O’Shaughnessy and Wathes, 1988). However, HDL also supplies other fat soluble substances such as cholesterol, vitamin A and vitamin E to the corpus luteum (Ribaya-Mercado et al., 1993; Aten et al., 1994). Although HDL has been used previously as a method of deliveringb-carotene to luteal cells (O’Shaughnessy and Wathes, 1988; Arikan and Rodway, 1997), the presence of these other substances in the lipoprotein preparations (Ribaya-Mercado et al., 1993) and the variable content ofb-carotene in the control preparations leads to difficulties in the interpretation of the results. In view of these problems it was decided to investigate the use of cyclodextrins for the delivery ofb-carotene to luteal cells in vitro.
b-Cyclodextrins are cage-like molecules consisting of seven glucose units and have the appropriate size to form inclusion complexes with many hormones, vitamins and drugs having a size compatible with the dimensions of the cavity (Pitha, 1981; Uekama and Irie, 1987; Albers and Muller, 1992). Hydrophobic guest molecules such asb-carotene can be incorporated into the cavity of the cyclodextrin by displacing water. The resulting complex is water-soluble, although the guest molecule can be released relatively easily and taken up by cells in culture.
cyclodextrin on LH, dibutyryl cAMP and cholesterol-stimulated luteal progesterone production.
2. Materials and methods
Unless otherwise indicated all chemicals used in the experiments were obtained from Sigma. All solvents used in theb-carotene analysis were of HPLC grade.
2.1. Preparation of cyclodextrin-encapsulatedβ-carotene
Preparation of water soluble b-carotene was carried out using following procedures. Dimethyl-b-cyclodextrin (500 mg, Sigma) was dissolved in water (1 ml). Crystalline b -carotene (40 mg) was added, the mixture was gassed with argon and sonicated for 2 h with frequent cooling. The mixture was then stirred rapidly for 24 h at room temperature and finally sonicated for another 2 h. The free carotene separated from the encapsulated one by filtering through 0.22 membrane filters. Concentration ofb-carotene in complex was 2.7 mg/gb-cyclodextrin. Cholesterol in cyclodextrin preparation was obtained from Sigma.
2.2. β-Carotene analysis
The quantitative determination ofb-carotene in the cyclodextrin complex was performed by HPLC using a C-18 reverse-phase column (Resolve, Millipore) and elution with a mo-bile phase consisting of 50:45:5 (v/v/v) methanol–acetonitrile–tetrahydrofuran (Oliver and Kafwembe, 1992).
2.3. Preparation and incubation of luteal cells
The mean extraction efficiency was 94.2%, the limit of sensitivity was 6.1 pg/tube and the intra- and inter-assay coefficients of variation were 7.31 and 8.87%, respectively.
2.4. Statistical analysis
Different treatments were assessed by analysis of variance (ANOVA) and Duncan’s multiple range test. Significance was defined asP < 0.05. All statistical analysis were carried out using the Statistical Package for the Social Sciences (SPSS). All results are reported as means±S.E.M.
3. Results
3.1. Effect of cyclodextrin-encapsulatedβ-carotene on basal or cholesterol-stimulated progesterone production by luteal cells
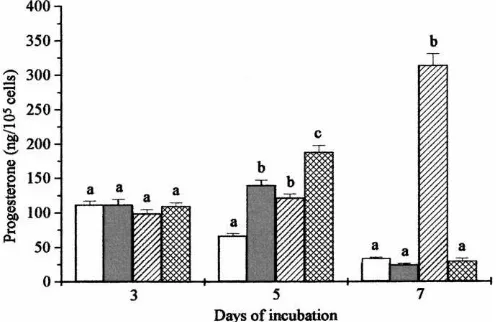
The first experiment investigated the influence of cyclodextrin-encapsulatedb-carotene alone (2mmol/l) or in combination with cholesterol (25mg/ml) on progesterone production by bovine luteal cells. At the start of treatment on day 3 of culture, as expected, there was no significant difference between the treatment groups in terms of progesterone production on day 3 (Fig. 1). On day 5 treatment of cells with eitherb-carotene alone, cholesterol alone or with cholesterol plusb-carotene resulted in significant stimulation (P < 0.01). However, the combined treatment caused significantly more stimulation (P <0.01) than the individual treatments. However, by day 7 cells treated withb-carotene alone orb-carotene plus cholesterol were producing no more progesterone than the control. Treatment with
Fig. 2. Effect of cyclodextrin-encapsulatedb-carotene on basal progesterone secretion by bovine luteal cells. Control (h), 0.1mmol/l ( ), 1mmol/l ( )b-carotene. Treatment was started on day 3. Within each day different letters indicate significant differences (P <0.01); ND: not detectable.
cholesterol alone reversed the decline in progesterone production seen in the control and other experimental groups and resulted in progesterone production being approximately 10-fold greater than controls.
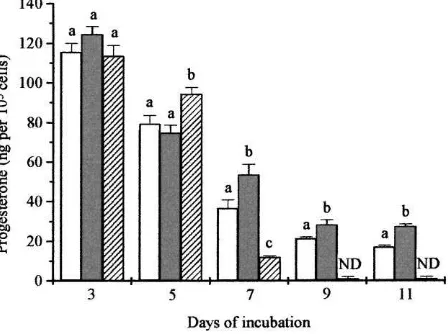
The effects of incubation with two lower concentrations ofb-carotene encapsulated in cyclodextrin are shown in Fig. 2. As found previously, the production of progesterone in control incubations fell steadily with time and by day 11 was less than 20% of the day 3 value. By day 5 treatment with 0.1mmol/lb-carotene had not affected progesterone production, however, the same concentration resulted in significant stimulation (P <0.05) on days 7, 9 and 11. Addition of a higher concentration ofb-carotene (1mmol/l) resulted in a significant stimulation on day 5, but caused a rapid decline in progesterone production by day 7 and on days 9 and 11 progesterone production was undetectable.
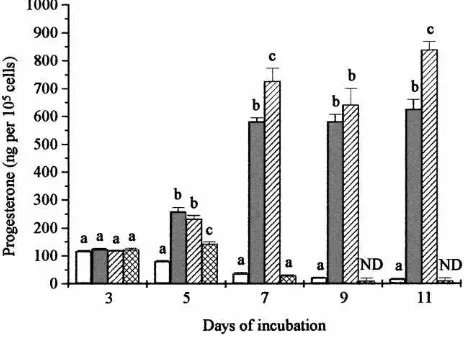
Fig. 3 illustrates the effects of 0.1 and 1mmol/lb-carotene in the presence of cyclodextrin-encapsulated cholesterol on progesterone production. Again all treatments, including choles-terol, were started on day 3 of incubation. Treatment of cells with cholesterol (25mg/ml) alone resulted in a significant stimulation on all treatment days (P < 0.001). Combined treatment with cholesterol plusb-carotene (0.1mmol/l) produced significantly more stimu-lation than cholesterol alone. However, the higher concentration ofb-carotene (1mmol/l) in combination with cholesterol resulted in significant (P <0.001) inhibition of progesterone production which eventually fell to undetectable levels by day 9 of incubation.
3.2. Effect of cyclodextrin-encapsulatedβ-carotene on LH or dbcAMP stimulated progesterone production
Fig. 3. Effects of cyclodextrin-encapsulatedb-carotene on cholesterol-stimulated progesterone production by bovine luteal cells. After the initial incubation for 3 days without treatment, cells were then incubated for a further 8 days with the following treatments: control (h), 25mg/ml cholesterol ( ), 0.1mmol/lb-carotene plus with 25mg ml−1cholesterol ( ) or 1mmol/lb-carotene plus with 25mg/ml cholesterol ( ). Within each day, different
letters indicate significant differences (P <0.05).
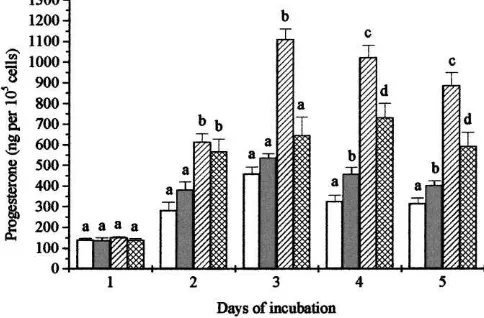
investigated the effects ofb-carotene when used in combination with either LH or dibutyryl cAMP to maintain steroidogenesis. Again treatment was started on day 3. As shown in Fig. 4 both dbcAMP (1mmol/l) and LH (100 ng/ml) as expected significantly stimulated progesterone production throughout the incubation period (P <0.01). Unfortunately, due to
Fig. 5. Effect of cyclodextrin-encapsulatedb-carotene on LH stimulated progesterone production by cultured luteal cells. Treatment was started on day 3, on which cells were cultured with medium under basal conditions (h), with 0.1mmol/lb-carotene ( ), 100 ng/ml LH ( ) or with 0.1mmol/lb-carotene plus 100 ng/ml LH ( ). Every groups including basal contained 25mg/ml cholesterol. Within each day, groups with different letters are significantly different (P <0.05).
limitations on the number of cells available, no dose-response data for LH or dbcAMP could be obtained. However, treatment of cells with a combination of cholesterol and either LH or dbcAMP resulted in a more than three-fold increase in progesterone production compared with LH or dbcAMP alone. Surprisingly, cells treated withb-carotene (0.1mmol/l) plus LH or dbcAMP produced significantly (P <0.001) less progesterone from day 7 until the end of culture period than did those treated with LH or dbcAMP alone.
The final experiment was performed to confirm the previous observation thatb-carotene when given alone (plus cholesterol) caused a stimulation of progesterone production, but when given in combination with LH (plus cholesterol) its effect was inhibitory. Fig. 5 indicates thatb-carotene (0.1mmol/l) plus cholesterol caused an increase in progesterone production which was significant from day 9 onwards, while when given together with LH it substantially inhibited the stimulation given by LH alone.
4. Discussion
to form a cage-like structure that holds substances such asb-carotene in aqueous solution (Pszczola, 1988).
Two distinct mechanisms for uptake ofb-carotene by the cells have been reported: active transport (endocytosis) and passive transport, dependent on which preparation method is used for supplementation ofb-carotene (Wei et al., 1998). For instance, when DMSO, THF or water-dispersible beadlets were used in deliveringb-carotene to several different cell lines passive transport ofb-carotene may predominate (Scita et al., 1992; Wei et al., 1998). However, supplementation of cells with liposome-encapsulatedb-carotene resulted in active transport being predominant (Schwartz et al., 1991; Grolier et al., 1992). As theb-carotene becomes decapsulated when the complex is dissolved in aqueous culture medium, it seems reasonable to assume that passive transport predominates under the conditions chosen for this study. Bioavailability of the guest molecule is increased when cyclodextrins are used to facilitate dissolution (Uekama and Irie, 1987) and comparisons with concentrations of b-carotene used in other studies are, therefore, difficult.
The results from the present study also indicate that the selection of an optimum dose for the treatment of luteal cells is critical. This is evident from the earlier studies in this work in which treatment of cells with either 1 or 2mmol/lb-carotene resulted in an initial stimulation of progesterone secretion but rapidly became inhibitory. The possible reasons whyb-carotene exhibits this inhibitory effect at higher concentrations or with longer in-cubation times are discussed later, but the increased bioavailability ofb-carotene when complexed with cyclodextrin may mean that concentrations lower than normal biological concentrations may be toxic when cyclodextrin is used to deliverb-carotene to luteal cells. The concentration of 2mmol/l used initially was selected as being half the concentration of that found in bovine follicular fluid (Schweigert and Zucker, 1988). That present in follicular fluid is, however, predominantly bound to HDL and as mentioned above, the bioavailability may be different from the cyclodextrin-encapsulatedb-carotene used here.
Both theb-carotene and the cholesterol preparations used were encapsulated in cyclodex-trin. Although it is unlikely that such a glucose seven-mer would have effects on P4 pro-duction, ‘empty’ cyclodextrin was included in cultures where necessary to ensure that all contained the same cyclodextrin concentration.
The effect of cholesterol encapsulated in cyclodextrin was, as expected, in all cases stimulatory. As cholesterol is the precursor of progesterone, cholesterol supply is obviously a factor in the control of the rate of steroidogenesis (Pate and Condon, 1982). O’Shaughnessy and Wathes (1985) reported that progesterone production by cultured luteal cells dissociated from mid-luteal bovine ovary fell rapidly in serum free culture even in the presence of LH and insulin. A similar decline in progesterone production has also been reported in culture of luteal cells of sheep (Grazul-Bilska et al., 1991) and monkey (Gulyas et al., 1979). In the present study the same effect was found, but the inclusion of cholesterol not only prevented this decline but by day 7 of culture had increased the P4 production about threefold even in the absence of any other stimulatory factor.
of the cultured luteal cells withb-carotene reduced the amount of cross-linking between the cholesterol side-chain cleavage cytochrome P450 enzyme and its electron donor, an-drenodoxin (Young et al., 1995). Their result was later confirmed by Rapoport et al. (1998), who determined the levels of some antioxidants includingb-carotene and the steroidogenic cytochrome P450scc at different developmental stages of bovine corpora lutea to examine their correlation with steroidogenic status. They found a significant correlation between the levels of luteal P450scc andb-carotene and levels of plasma progesterone. P450 enzymes produce oxygen free radicals that can cause cross-linking between proteins in close proxim-ity (Rapoport et al., 1995). The present findings that the presence of low concentrations of b-carotene in cultured luteal cells will maintain progesterone secretion agree with this idea. As expected, both LH and cAMP increased P4 production between two and threefold during the incubation period. The roles of LH and cAMP in progesterone synthesis are well-known and this subject has been reviewed by Milvae et al. (1996). Production of progesterone appears to be highly correlated to the number of LH receptors (Harrison et al., 1987; Jones et al., 1992). Binding of LH to its specific receptor in the cell membrane activates adenylate cyclase, resulting in increased cellular concentrations of cAMP and activation of protein kinase A (PKA). The activated PKA stimulates two steps involved in the biosynthesis of progesterone, which are (1) cholesterol ester hydrolase, which converts cholesterol esters to cholesterol, (2) the transport of cholesterol into mitochondria where P-450 cholesterol side-chain cleavage enzyme is located (Milvae et al., 1996). The mechanism by which b-carotene interacts with this system in not clear.
The experiments with LH and dibutyryl cAMP gave somewhat unexpected results. When given alone, both stimulated progesterone secretion as expected. Whenb-carotene was given in combination with LH or dibutyryl cAMP, stimulation given by theb-carotene plus LH or dibutyryl cAMP was significantly less than that given by the LH or dibutyryl cAMP alone. LH has been shown to increase gap junction formation between luteal cells and this is thought that this gap junctional communication may be important in the regulation not only of steroidogenesis but also of luteolysis (Khan-Dawood et al., 1996). Asb-carotene has been shown in other cell types to increase connexin 43 and gap junction formation, it is therefore possible that the combination of high LH andb-carotene concentration may act to stimulate gap junction formation to such an extent that apoptosis and luteolysis may occur. This may explain why in the presence of both these factors progesterone synthesis was reduced whenb-carotene was in its highest concentration.
There is some evidence for the involvement of free radicals, especially the superoxide radical in the control of steroidogenesis (Schrek and Baeuerle, 1991). The phospholipase C protein kinase C second messenger system may stimulate production of superoxide radicals which have been shown to inhibit progesterone synthesis in the rat ovary (Gatzuli et al., 1991). Superoxide radicals are converted to hydrogen peroxide by the enzyme superoxide dismutase and the hydrogen peroxide so formed may result in the formation of hydroxyl rad-icals which, being very lipid soluble, accumulate in cellular membranes. Sinceb-carotene, together witha-tocopherol, acts within lipid membranes to quench free radicals, this may be involved in the ability ofb-carotene to maintain progesterone synthesis as demonstrated here.
present. The reason for this inhibitory effect is unclear, but may be related to the finding that at high concentration in some circumstancesb-carotene appears to act as a pro-oxidant and to increase oxidative damage (Truscott, 1996; Lankin et al., 1999). If this was the case, then the increase cellular damage may inhibit steroidogenesis, however there appears to be no decrease in cell number or viability under these circumstances.
In conclusion, encapsulation of both cholesterol andb-carotene in cyclodextrin appears to be an efficient method of administration of these substances to luteal cells in culture, and has confirmed earlier work thatb-carotene can stimulate progesterone production. It appears likely that this effect may be due to its action as a lipid soluble antioxidant.
Acknowledgements
We would like to thank Dr. A.F. Parlow, Pituitary Hormones and Antisera Centre, for providing bovine LH hormone.
References
Albers, E., Muller, B.W., 1992. Complexation of steroid hormones with cyclodextrin derivatives: substituent effects of the guest molecule on solubility and stability in aqueous solution. J. Pharm. Sci. 81, 756–761.
Arikan, S., Rodway, R.G., 1997. The effect of beta-carotene either in organic solvent or as HDL on progesterone production by bovine luteal cells. J. Reprod. Fertil., Abst. Ser. No. 19, 64.
Arikan, S., Rodway, R.G., 1998. Uptake and depletion of beta-carotene by bovine corpus luteum cells. Proc. Br. Soc. Anim. Sci., 190.
Aten, R.F., Kolodecik, T.R., Behrman, H.R., 1994. Ovarian vitamin E accumulation: evidence a role of lipoproteins. Endocrinology 135, 533–539.
Bao, B., Thomas, M.G., Griffith, M.K., Burghardt, R.C., Williams, G.L., 1995. Steroidogenic activity, insulin-like growth factor-I production, and proliferation of granulosa and theca cells obtained from dominant preovulatory and nonovulatory follicles during the bovine oestrous cycle: effects of low-density and high density lipoproteins. Biol. Reprod. 53, 1271–1279.
Bertram, J.S., Pung, A., Churley, M., Kappock, T.J., Wilkins, L.R., Cooney, R.V., 1991. Diverse carotenoids protect against chemically induced neoplastic transformation. Carcinogenesis 12, 671–678.
Cooney, R.V., Kappock, T.J., Pung, A., Bertram, J.S., 1993. Solubilization, cellular uptake, and activity of
b-carotene and other carotenoids as inhibitors of neoplastic transformation in cultured cells. Methods Enzymol. 214, 55–69.
Dembinski, Z., Bronicki, M., 1994. Progesterone level in blood and the values of selected fertility indexes in cows fed various doses of carotenes. Bull. Vet. Instit., Pulawy. 38, 115–118.
Folman, Y., Ascarelli, L., Herz, Z., Rosenberg, M., Davidson, M., Halevi, A., 1979. Fertility of dairy heifers given a commercial diet free ofb-carotene. Br. J. Nutr. 41, 353–359.
Gatzuli, E., Aten, R.F., Behrman, H.R., 1991. Inhibition of gonadotrophin action and progesterone synthesis by xanthine oxidase in rat luteal cells. Endocrinology 128, 2253–2258.
Graves-Hoagland, R.L., Hoagland, T.A., Woody, C.O., 1988. Effect ofb-carotene and vitamin A on progesterone production by bovine luteal cells. J. Dairy Sci. 71, 1058–1062.
Grazul-Bilska, A.T., Redmer, D.A., Reynolds, L.P., 1991. Secretion of angiogenic activity and progesterone by ovine luteal cell types in vitro. J. Anim. Sci. 69, 2099–2107.
Grolier, P., Azais-Braesco, V., Zelmire, L., Fessi, H., 1992. Incorporation of carotenes in aqueous systems: uptake by cultured rat hepatocytes. Biochim. Biophys. Acta 1111, 135–138.
Gulyas, B.J., Stouffer, R.L., Hodgen, G.D., 1979. Progesterone synthesis and fine structure of dissociated monkey (Macaca mulatta) luteal cells maintained in culture. Biol. Reprod. 20, 779–792.
Harrison, L.M., Kenny, N., Niswender, G.D., 1987. Progesterone production, LH receptors, and oxytocin secretion by ovine luteal cell types on days 6, 10 and 15 of the oestrous cycle and day 25 of pregnancy. J. Reprod. Fertil. 79, 539–548.
Holt, A.J., Rodway, R.G., Findlay, J.B.C., Sands, H., Batchelder, D.N., 1995. Studies onb-carotene in bovine corpus luteum, J. Reprod. Fertil., Abst. Ser. No. 15, 46–47.
Ireland, J.J., Murphee, R.L., Coulson, P.B., 1980. Accuracy of predicting stages of bovine oestrus cycle by gross appearance of the corpus luteum. J. Dairy Sci. 63, 155–160.
Jones, L.S., Ottobre, J.S., Pate, J.L., 1992. Progesterone regulation of luteinizing hormone receptors on cultured bovine luteal cells. Mol. Cell. Endocrinol. 85, 33–39.
Khan-Dawood, F.S., Yang, J., Dawood, Y.M., 1996. Expression of gap junction protein connexin-43 in the human and baboon (papio anubis) corpus luteum. J. Clin. Endocrinol. Metab. 81, 835–842.
Lankin, V.Z., Tikhaze, A.K., Konovalova, G.G., Kozachenko, A.I., 1999. Concentration inversion of the antioxidant and pro-oxidant effects of beta-carotene in tissues in vivo. Biul. Exp. Biol. Med. 128, 216–314.
Milvae, R.A., Hinckley, S.T., Carlson, J.C., 1996. Luteotropic and luteolytic mechanism in the bovine corpus luteum. Theriogenology 45, 1327–1349.
O’Fallon, J.V., Chew, B.P., 1984. The subcellular distribution ofb-carotene in bovine corpus luteum. Proc. Soc. Exp. Biol. Med. 177, 406–411.
O’Shaughnessy, P.J., Wathes, D.C., 1988. Bovine luteal cell activity in culture. Maintenance of steroidogenesis by high density lipoprotein containing high or lowb-carotene concentrations. Anim. Reprod. Sci. 17, 165–176. O’Shaughnessy, P.J., Wathes, D.C., 1985. Characteristics of bovine luteal cells in culture: morphology, proliferation
and progesterone secretion in different media and effects of LH, dibutyryl cyclic AMP, antioxidants and insulin. J. Endocrinol. 104, 355–361.
Oliver, R.W.A., Kafwembe, E.M., 1992. A new spectrophotometric assay for the determination of vitamin A and related compounds in serum. Int. J. Vit. Nutr. Res. 62, 221–227.
Pate, J.L., Condon, W.A., 1982. Effect of serum and lipoproteins on steroidogenesis in cultured bovine luteal cells. Mol. Cell. Endocrinol. 28, 551–562.
Pethes, G., Horvath, E., Kulcsar, M., Huszenicza, G., Somorjai, G., Varga, B., Haraszti, J., 1985. In vitro progesterone production of corpus luteum cells of cows fed low and high levels ofb-carotene. Zentralbl. Veterinearmedzine. Rehia A 32, 289–296.
Pitha, J., 1981. Enhanced water solubility of vitamins A, D and K by substituted cycloamyloses. Life Sci. 29, 307–311.
Pszczola, D.E., 1988. Production and potential food applications of cyclodextrins. Food Technol. 42, 96–100. Rapoport, R., Sklan, D., Hanukogl, L., 1995. Electron leakage from the adrenal cortex mitochondrial, P450scc
and P450c11 systems: NADPH and steroid dependence. Arch. Biochem. Biophys. 317, 412–416.
Rapoport, R., Sklan, D., Wolfenson, D., Shaham-Albalancy, A., Hanukogl, L., 1998. Anti oxidant capacity is correlated with steroidogenic status of the corpus luteum during the bovine oestrous cycle. Biochim. Biophys. Acta 1380, 133–140.
Ribaya-Mercado, J.D., Lopez-Miranda, J., Ordovas, J.M., Blanco, M.C., Fox, J.G., Russell, R.M., 1993. Distribution ofb-carotene and vitamin A in lipoprotein fractions of ferret serum. Ann. N. Y. Acad. Sci. 691, 232–237.
Schrek, R., Baeuerle, R.A., 1991. A role for oxygen radicals for second messengers. Trends Cell Biol. 1, 39–42. Schwartz, J.L., Flynn, E., Trickler, D., Shklar, G., 1991. Directed lysis of experimental cancer byb-carotene in
liposomes. Nutr. Cancer 16, 107–124.
Schweigert, F.J., Zucker, H., 1988. Concentrations of vitamin A,b-carotene and vitamin E in individual bovine follicles of different quality. J. Reprod. Fertil. 82, 575–579.
Scita, G., Aponte, G.W., Wolf, G., 1992. Uptake and cleavage ofb-carotene by cultures of rat small intestinal-cells and human lung fibroblasts. J. Nutr. Biochem. 3, 118–123.
Truscott, T.G., 1996. Beta-carotene and disease: a suggested pro-oxidant and anti-oxidant mechanism and speculations concerning its role in cigarette smoking. J. Photochem. Photobiol. B 35, 233–235.
Wang, J.Y., Larson, L.L., Owen, F.G., 1982. Effect ofb-carotene supplementation on reproductive performance of dairy heifers. Theriogenology 18, 461–473.
Wei, R.R., Wamer, W.G., Lambert, L.A., Kornhauser, A., 1998.b-Carotene uptake and effects on intracellular levels of retinol in vitro. Nutr. Cancer 30, 53–58.
Young, F.M., Luderer, W.B., Rodgers, R.J., 1995. The antioxidantb-carotene prevents covalent cross-linking between cholesterol side-chain cleavage cytochrome P450 and its electron donor, adrenodoxin, in bovine luteal cells. Mol. Cell. Endocrinol. 109, 113–118.