Biochemical Systematics and Ecology 29 (2001) 77}104
Chemical variability of peel and leaf essential oils
of 15 species of mandarins
Marie-Laure Lota, Dominique de Rocca Serra, Fe
H
lix Tomi,
Joseph Casanova
*
Universite&de Corse - Equipe Chimie et Biomasse, URA CNRS 2053, Route des Sanguinaires, 20000 Ajaccio, France
Received 1 December 1999; received in revised form 24 February 2000; accepted 28 February 2000
Abstract
Peel and leaf oils of 58 mandarin cultivars, belonging to 15 di!erent species were obtained from fruits and leaves collected on mandarin-trees submitted to the same pedoclimatic and cultural conditions. Their chemical composition was investigated by capillary GC, GC/MS and 13C NMR and the results were submitted to a cluster analysis and a discriminant analysis. Three major chemotypes, limonene, limonene/c-terpinene and linalyl acetate/limonene, were distinguished for peel oils while three other chemotypes, sabinene/linalool,c-terpinene/linalool and methylN-methylanthranilate, were observed for leaf oils. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Rutaceae; Citrus; Mandarin; Peel oil; Leaf oil; Essential oil composition; GC; GC/MS;
13C NMR; Statistical analysis
1. Introduction
Mandarins (Rutaceaefamily,Citrusgenus) predominate with oranges the fresh fruit market. According to Tanaka (1961), they are classi"ed into more than 30 species, comprising from one to several tens of varieties. Cultivars of mandarin present a great diversity of morphological and horticultural characters.
*Corresponding author. Tel.: 33-4-95-52-41-21; fax: 33-4-95-52-41-42.
E-mail address:[email protected] (J. Casanova).
As for most of theCitrus, mandarin peel oil and leaf or `petit graina oil can be obtained, respectively, by cold pressing and hydrodistillation of the fresh material. Studies concerning the chemical composition of peel and leaf oils of mandarins have been reviewed (Shaw, 1979; Lawrence, 1995a, b, 1996; Mondello et al., 1997). Never-theless, in most cases, the species and/or the varieties remained unspeci"ed and the results are not useful from the taxonomic point of view.
The aim of our work was to study the inter- and intraspeci"c chemical variability of the large mandarin group. We divided our work into two parts:
f the"rst concerned 41 cultivars fromC. reticulata Blanco, (Lota et al., 2000); f the second dealt with 15 other species of mandarin, includingC. clementinaHort. ex
Tan.
We report our results on the chemical variability of these 15 species represented by 58 cultivars of mandarin. We will compare the chemical composition of peel oils on the one hand and that of leaf oils on the other. We will discuss chemical variability including our previous results concerning cultivars fromC. reticulata Blanco (Lota et al., 2000).
2. Materials and methods
2.1. Plant materials
Clonal propagated trees, grafted onTroyer citrange rootstock, were 12 years old and grown in the same pedoclimatic and cultural conditions in the germplasm collection orchard of the`Station de Recherches Agronomiquesaof INRA-CIRAD, located at San Ghjulianu (Corsica, France). The Citrus varieties collection of INRA-CIRAD in Corsica is one of the FAO recognized Citrus collection in the world. In this arboretum, each tree has a computerized identi"cation number. Geo-graphic and climatic characteristics were: average per year: rainfall 840 mm and temperature 15.23C, soil derived from alluvial deposits and classi"ed as fersiallitic, pH range 5.0}5.6. Trees were in good vigor, disease-free and without visible insect infestation.
2.2. Sampling,peel and leaf essential oil
For each cultivar of mandarin, about 500 g of leaves from the last autumn leaf#ush and at least 30 ripe fruits were collected from many parts round the same tree, early in the morning and only by dry weather during the period November 1996 to April 1997. The peel of fresh fruits was cold-pressed and then the essential oil was separated from the crude-extract by centrifugation (10 min at 15000 rpm). Fresh leaves were subjected to hydrodistillation for 3 h using a Clevenger-type apparatus. Yield ranged between 0.05 and 0.60%.
2.3. GC,GC/MS and13CNMR analyses
Identi"cation of components and data processing were carried out as previously reported for peel and leaf oils fromC. reticulata(Lota et al., 2000). All peel and leaf oils were investigated by GC. Five peel oils and seven leaf oils were analysed by GC/MS while 23 peel oils and 25 leaf oils were analysed by13C NMR, following a methodo-logy"rst reported by FormaHcek and Kubeczka (1982), developed in our laboratory (Tomi et al., 1995) and well-suited for chemical polymorphism studies (Salgueiro et al., 1997; Corticchiato et al., 1998; Castola et al., 2000). Samples submitted to GC/MS and/or13C NMR analysis were selected on the basis of their chromatographic pro"le. Note that 56 peel oils instead of 58 were analysed because the mandarin-trees of
`ougonaand`shekwashaacultivars do not produce fruits in the climatic conditions of San Ghjulianu.
2.4. Data analyses
The data (components*1% for peel and leaf oils, respectively) were processed by cluster analysis using hierarchical clustering (Ward`s technique and Euclidean dis-tance measure) and were submitted to discriminant analysis. These processing were performed with thexlStat-pro software.
3. Results
The 58 following cultivars, belonging to 15 species, were investigated. For the convenience of comparison of the present results with the previous ones for 41 varieties fromC. reticulata(Lota et al., 2000), we numbered the samples of this study from 42 to 99.
Citrus clementinaHort. ex Tan. species : MA3 (no. 42), Nules (no. 43), MA2 (no. 44), Hernandina (no. 45), Tardia Villareal (no. 46), Reina (no. 47), Ca$n (no. 48), MacBean (no. 49), Oroval (no. 50), Monreal (no. 51), Bruno (no. 52), Tomatera (no. 53), Commune (no. 54), Marisol (no. 55), Ragheb (no. 56), Guillermina (no. 57),
C. deliciosaTen. species : Late Emperor (no. 58), Empress (no. 59), Emperor (no. 62), Peau rugueuse (no. 63), Peau lisse (no. 76), Commune (no. 79), de Chios (no. 92), Avana Apireno (no. 93), Willow leaf (no. 90), Tardivo di Ciaculli (no. 94),
C. nobilisLour. species : Geleking (no. 74), Yellowking (no. 75), King of Siam (no. 80), Du Japon (no. 85), King (no. 86), Rode king (no. 87), Kunembo (no. 89),
C. tangerinaHort. ex Tan. species : Vohangisahy (no. 60), Beauty of Glen Retreat (no. 61), Brickaville (no. 64), Dancy (no. 66), Redskin (no. 68), Swatow (no. 78),
C. unshiuMac. Mark. species : Wase (no. 65), Clausellina (no. 70), URSS (no. 71), Owari (no. 72),
C. suhuiensisHort. ex Tan. species : Sihue Gan (no. 73), Szibat (no. 91), Szinkom (no. 96);
C. templeHort. ex Y. Tan. species : Temple Sue Linda (no. 81), Temple]Temple (no. 83), Temple (no. 84),
C. paratangerinaHort. ex Tan. species : Ladu (no. 69), Ladu Ordinary (no. 77),
C. amblycarpaHassk. Ochse. species : Nasnaran (no. 88),
C. depressaHay. species : Shekwasha (no. 98),
C. erythrosaHort. ex Tan. species : Fuzhu (no. 67),
C. reshniHort. ex Tan. species : Cleopatra (no. 82),
C. suavissimaHort. ex Tan. species : Ougon (no. 99),
C. sunkiHort. ex Tan. : Sunki (no. 95),
C. yatsushiroHort. ex Tan. : Yatsushiro (no. 97).
In order to simplify the discussion, we will"rst describe our results forC. clementina
Hort. ex Tan. All other taxa will be reported in Section 3.2.
3.1. Citrus clementina Hort. ex Tan.
3.1.1. Peel oils
The chemical composition of the 16 investigated samples are presented in Table 1. The 30 identi"ed components accounted for 97.3}99.5% of the total amount of oil. Peel oils consisted almost exclusively of hydrocarbons with limonene as the major component (89.1}95.5%) with sabinene (0.3}4.0%) and myrcene (1.4}2.0%).a-Pinene,
b-phellandrene,b-pinene, (E)-b-ocimene, 3-carene andc-terpinene were identi"ed in almost all samples at low amounts (tr-0.6%). The oxygenated fraction was made up of linalool (0.6}2.3%), octanal, decanal, citronellal,a-terpineol,a-sinensal andb-sinensal ()0.7% for each one).
The homogeneous composition of our 16 samples is similar to that reported in the literature (percentage of limonene: 92}97%) for `communea and `nulesa cultivars from Uruguay (Verzera et al., 1998), for`orovala,`monrealaand`communeacultivars from Italy (Calabria) (Verzera et al., 1997) and for unspeci"ed cultivars from Italy (Calabria, Sicily) (Mondello et al., 1995), Algeria (Baaliouamer et al., 1992) and unspeci"ed cultivars from unspeci"ed origin (Calvarano et al., 1974; Huet, 1991; Gazea et al., 1998; Ruberto et al., 1993, 1994, 1997).
3.1.2. Leaf oils
The 45 identi"ed components accounted for 96.1 to 99.8% of the total amount of oil (Table 2). All samples exhibited a high sabinene/linalool composition (33.1}49.8%/16.6}24.7%). The other main components of the ole"nic fraction (20.9}28.2%) were limonene, 3-carene, (E)-b-ocimene, myrcene,b-pinene,c-terpinene,
a-pinene, terpinolene,a-terpinene andb-phellandrene (0.8}6.9% each). Terpinen-4-ol,
a-terpineol,trans-sabinene hydrate, citronellal, citronellol, geranyl acetate,a-sinensal andb-sinensal were also identi"ed in almost all samples (0.1}4.8%). The oxygenated fraction represented less than 40% of the whole oil.
The composition of a few leaf oils from clementin are reported in the literature. Three samples (unspeci"ed cultivar) from Italy were characterized by a b -pinene/linalool composition (approximately 45%/15%) (Di Giacomo et al., 1982). Spanish oils from 12 cultivars `rufatinaa, `clemennullesa, `clemenvillaa, `esbala,
Table 1
Chemical composition of clementin peel oils!
Constituents BP-20 BP-1 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57
Limonene 1199 1021 95.5 95.0 95.0 94.6 94.1 94.1 94.1 93.9 93.5 92.9 92.7 92.2 91.8 91.2 90.1 89.1 b-Phellandrene 1208 1021 0.3 0.3 0.3 0.2 0.3 0.3 0.3 0.3 0.3 0.3 0.3 0.3 0.3 0.3 0.3 0.3
Total 99.3 99.4 98.8 98.4 99.4 99.2 99.3 99.1 99.3 98.9 99.5 98.8 97.3 99.0 99.3 98.5
!Other compounds ( tr):p-cymene,a-phellandrene, terpinolene, nonanal,cis-limonene-1,2-oxide,trans-limonene-1,2-oxide, citronellol.
Cultivars : MA3 (42), Nules (43), MA2 (44), Hernandina (45), Tardia Villareal (46), Reina (47), Ca$n (48), Mac Bean (49), Oroval (50), Monreal (51), Bruno (52), Tomatera (53), Commune (54), Marisol (55), Ragheb (56), Guillerma (57).
Table 2
Chemical composition of clementin leaf oils!
Constituents BP-20 BP-1 56 43 47 53 46 52 55 45 44 48 51 49 54 42 50 57
a-ThujeneH 1021 922 0.3 0.3 0.3 0.3 0.4 0.4 0.3 0.3 0.3 0.3 0.4 0.4 0.4 0.4 0.3 0.4
a-PineneH 1021 930 1.3 1.6 1.4 1.4 1.1 1.5 0.7 1.6 1.5 1.5 0.8 1.7 1.5 1.6 1.5 1.2
Camphene 1066 944 tr tr tr 0.1 tr tr tr tr tr tr tr tr tr tr tr tr
b-Pinene 1109 971 1.7 1.8 1.7 1.8 1.9 1.9 1.8 2.2 1.9 2.0 2.1 2.1 2.1 2.0 2.2 2.3
Sabinene 1119 964 33.1 35.1 34.3 35.2 38.1 37.9 36.9 40.1 39.9 41.8 43.5 42.5 42.0 41.9 47.0 49.8
3-Carene 1145 1005 4.5 6.5 6.5 6.2 4.8 4.4 5.1 3.8 3.8 2.6 3.3 3.8 4.1 3.8 2.4 3.0
(E)-Caryophyllene 1588 1420 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1 0.1 tr 0.1 0.1 0.2 0.1 tr
Total 99.2 96.1 98.9 99.8 99.5 99.3 98.5 98.9 98.6 99.7 99.1 98.8 99.8 99.5 99.6 98.6
!Other compounds (tr): octanal, nonanal, citronellyl acetate,a-humulene, (E,E)-a-farnesene,d-cadinene, thymol.
Cultivars : Ragheb (56), Nules (43), Reina (47), Tomatera (53), Tardia Villareal (46), Bruno (52), Marisol (55), Hernandina (45), MA2 (44), Ca$n (48), Monreal (51), Mac Bean (49), Commune (54), MA3 (42), Oroval (50), Guillerma (57).
Order of elution and percentages of components are given on BP-20 column, except compounds with an asterisk (percentages given on BP-1 column). All the components were identi"ed by GC-RI on polar and apolar columns. All the compounds of samples no. 47, 54 and 56 were also identi"ed by GC/MS. The major components (bold letters) of samples no. 50, 53, 54 and 56 were identi"ed by13C NMR.
(16.7}27.1%) (Ortiz Marcide et al., 1983). It is likely if not certain that the unidenti"ed major component reported by Ortiz Marcide is sabinene. The sabinene/linalool chemotype, found in our sampling, is con"rmed unambiguously for the"rst time for
C. clementinaleaf oils in this study.
3.2. The other species of mandarin
3.2.1. Peel oils
The total of the 44 identi"ed components accounted for 95.8}99.7% of the oil (Table 3). Even though the composition was dominated by limonene (55.8}96.7%) for 39 samples over 40, the content of the major components varied considerably from sample to sample. Several other monoterpene hydrocarbons were frequently identi-"ed at appreciable contents: c-terpinene (tr-19.9%), p-cymene (tr-12.0%), myrcene (0.7}24.0%), b-pinene (tr-14.2%), sabinene (0.1}8.7%), a-pinene (0.2}2.2%) and b -phellandrene (0.2}0.8%). Among the oxygenated compounds, linalool was present in all the samples (0.1}10.7%) whereas percentages of octanal,a-terpineol and decanal
were not over 0.5%. The contents of citronellal (9.9%) and of linalyl acetate (48.7%) were important, respectively, in two samples (no. 88,`Nasnarana cultivar fromC. amblycarpaspecies and no. 97,`Yatsushiroacultivar fromC. yatsushirospecies).
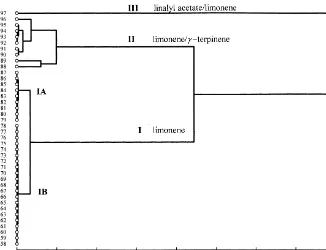
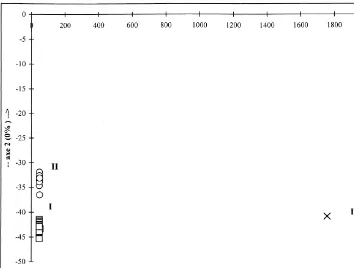
The dendrogram, obtained from cluster analysis and reported in Fig. 1, suggested the existence of three principal clusters (I, II and III) within the essential oil of the individuals of mandarin. Discriminant analysis (Fig. 2) con"rmed this clustering with respect to the contents of limonene,c-terpinene and linalyl acetate (Fig. 3).
Limonene chemotype: most of the peel oils (no. 58}87) were characterized by a very high amount of limonene (87.1}96.7%). This chemotype (cluster I) could be divided into two subgroups on the basis of the content ofc-terpinene, which was very low ()0.9%) in the subgroup IA (samples no. 79}87) and higher (3.5}5.8%) in the subgroup IB (samples no. 58}78)
Limonene/c-terpinene chemotype: nine samples (no. 88}96) belonged to this chemo-type (cluster II). The composition was dominated by limonene (55.8}79.0%) asso-ciated withc-terpinene (0.1}19.9%) andp-cymene (0}12.0%). Three samples (no. 88, 89 and 96), which belonged to the same group, exhibited quantitative di!erences in their composition. Indeed, the sample no. 88 was discriminated by higher contents of
b-pinene (14.2% vs 0.2}1.6%), sabinene (8.7% vs 0.1}2.1%) and citronellal (9.9% vs tr-0.1%). Similarly, samples no. 89 and 96 were distinguished, respectively, by impor-tant amounts of myrcene (24.0% vs 1.2}1.7%) andp-cymene (12.0% vs 0}6.9%).
Linalyl acetate/limonene chemotype: only one sample (no. 97) belonged to the cluster III and was characterized by the predominance of linalyl acetate (48.7%) over limonene (22.8%),c-terpinene (5.6%) andb-pinene (5.4%).
Table 3
Chemical composition of peel mandarin oils!
Constituents BP-20 BP-1 IB
Limonene 1199 1021 91.4 90.8 90.9 90.2 90.4 90.5 90.2 90.3 87.1 88.4 89.2 87.6 88.0 88.0
b-Phellandrene 1208 1021 0.3 0.2 * 0.2 0.3 0.3 0.3 0.3 0.2 0.2 0.2 0.3 0.2 0.2
Total 99.3 99.5 99.6 99.3 99.5 99.2 99.5 99.1 98.7 99.3 99.4 98.0 99.2 99.1
Table 3*continued
Limonene 1199 1021 88.8 89.1 89.2 89.2 89.6 89.6 89.6 92.6 93.1 93.3 93.6 93.9 90.7 95.4
b-Phellandrene 1208 1021 0.3 0.2 0.3 0.3 0.3 0.2 0.3 0.3 0.8 0.2 0.3 0.3 0.2 0.3
Total 99.5 99.6 99.3 99.0 99.2 99.5 99.3 98.5 98.3 99.3 98.5 98.7 99.2 99.4
Constituents IA II III
Limonene 1199 1021 96.7 96.7 55.8 63.7 65.3 68.9 73.1 75.3 74.5 79.0 75.0 22.8
b-Phellandrene 1208 1021 0.3 0.3 0.5 0.3 0.2 0.2 0.2 0.2 0.3 0.2 0.4 0.2
c-Terpinene 1241 1048 tr tr 0.1 5.8 15.9 19.9 17.3 16.7 13.7 11.5 0.7 5.6
(E)-b-Ocimene 1245 1035 tr tr 0.2 * * * * tr tr * * 0.3
Total 99.6 99.7 97.9 98.4 99.0 99.3 99.3 99.3 98.2 97.7 95.8 99.1
Table 3*footnote continued
!Other compounds (tr-0.3%): camphene,a-phellandrene, (Z)-b-ocimene, nonanal,cis-limonene-1,2-oxide,trans-sabinene hydrate, octyl acetate,a-copaene, decanal,trans-a-bergamotene, (E)-caryophyllene, terpinen-4-ol,a-humulene, neral,a-terpinyl acetate, germacrene-D, bicyclogermacrene.
Cultivars : Late Emperor (58,C. deliciosa), Empress (59,C. deliciosa), Vohangisahy (60,C. tangerina), Beauty of Glen Retreat (61,C. tangerina), Emperor (62, C. deliciosa), Peau rugueuse (63,C. deliciosa), Brickaville (64,C. tangerina), Wase (65,C. unshiu), Dancy (66,C. tangerina), Fuzhu (67,C. erythrosa), Redskin (68, C. tangerina), Ladu (69,C. paratangerina), Clauselina (70,C. unshiu), URSS (71,C. unshiu), Owari (72,C. unshiu), Sihue Gan (73,C. suhuiensis), Geleking (74,C. nobilis), Yellowking (75,C. nobilis), Peau lisse (76,C. deliciosa), Ladu Ordinary (77,C. paratangerina), Swatow (78,C. tangerina), Commune (79,C. deliciosa), King of Siam (80,C. nobilis), Temple Sue Linda (81,C. temple), Cleopatra (82,C. reshni), Temple x Temple (83,C. temple), Temple (84,C. temple), Du Japon (85, C. nobilis), King (86,C. nobilis), Rode king (87,C. nobilis), Nasnaran (88,C. amblycarpa), Kunembo (89,C. nobilis), Willow leaf (90,C. deliciosa), Szibat (91,C. suhuiensis), de Chios (92,C. deliciosa), Avana Apireno (93,C. deliciosa), Tardivo di Ciaculli (94,C. deliciosa), Sunki (95,C. sunki), Szinkom (96,C. suhuiensis), Yatsushiro (97,C. yatsushiro).
Order of elution and percentages of components are given on BP-20 column, except compounds with an asterisk (percentages given on BP-1 column). All the components were identi"ed by GC-RI on polar and apolar columns. All the compounds of samples no. 88, 96 and 97 were also identi"ed by GC/MS. The major components (bold letters) of samples no. 65, 66, 67, 69, 75, 77, 78, 80, 82, 83, 84, 88, 90, 92, 93, 94, 96 and 97 were identi"ed by13C NMR.
88
M.-L.
Lota
et
al.
/
Biochemical
Systematics
and
Ecology
29
(2001)
77
}
Fig. 1. Dendrogram obtained from the cluster analysis of 56 mandarin peel oils. Samples are clustered using Ward's technique with an Euclidean distance measure.
for four samples from an unspeci"ed japanese cultivar (Lawrence, 1989). To our knowledge, only one study concernsC. deliciosaspecies. Two samples (unspeci"ed variety) from Italy and Spain were characterized by the limonene/c-terpinene chemo-type (72}77%/14}19%) (Boelens and Jimenez, 1989). It should be pointed out that a very large number of commercial oils from Italy of certainly known but not reported species and variety (400 samples, Dugo, 1994), as well as oils from Argentina (Retamar, 1986) or from unspeci"ed origin (Lawrence, 1996) exhibited this last chemotype.
3.2.2. Leaf oils
The 58 identi"ed components accounted for 97.1}99.9% of the total amount of oil (Tables 4). We observed an important chemical variability with the occurrence of sabinene (0.1}57.3%), c-terpinene (0.1}67.4%), linalool (tr-59.3%) and methyl N-methylanthranilate (0}78.7%). For most samples (29 over 42), the content of monoter-pene hydrocarbons was important. The best represented components were sabinene,
c-terpinene, myrcene, limonene and (E)-b-ocimene. Conversely, for the 13 other samples, the oxygenated fraction was dominant with high contents of linalool, thymol or methylN-methylanthranilate.
The dendrogramm (Fig. 4) suggested the existence of three principal groups. The discriminant analysis con"rmed this repartition with respect to the contents of sabinene,c-terpinene, linalool and methylN-methylanthranilate (Figs. 5 and 6).
Fig. 2. Discriminant analysis scatterplott of 56 mandarin peel oils. (h) Cluster I; (L) Cluster II, (]) Cluster III.
Table 4
Chemical composition of leaf mandarin oils!
Constituents BP-20 BP-1 IB IA
74 75 86 87 80 83 82 63 84 62 59 78 81 58
a-ThujeneH 1021 922 0.6 0.8 0.5 0.5 0.5 0.5 0.5 0.6 0.4 0.5 0.5 1.2 0.3 0.5
a-PineneH 1021 930 1.7 2.1 0.9 0.9 1.3 2.2 1.9 1.0 1.9 1.9 2.0 1.4 1.4 1.8
b-Pinene 1109 971 2.6 2.9 3.4 3.3 3.5 2.7 2.7 2.8 2.5 2.3 2.4 2.3 1.9 2.2
Sabinene 1119 964 46.5 47.8 55.5 57.3 56.0 55.6 49.7 48.3 51.5 37.9 40.5 37.8 39.0 37.4
3-Carene 1145 1005 tr tr tr tr tr tr tr tr 1.5 tr tr tr 2.2 tr
Linalool 1539 1082 5.4 3.4 6.7 5.3 3.1 10.6 13.0 17.0 17.2 26.2 25.5 25.6 27.1 31.0
Table 4*continued
Constituents BP-20 BP-1 IB IA
74 75 86 87 80 83 82 63 84 62 59 78 81 58
trans-p-Menth-2-en-1-ol 1618 1123 0.3 0.3 0.2 0.2 0.2 0.2 * 0.1 0.1 0.1 0.1 0.2 0.1 0.1
Citronellyl acetate 1654 1332 * * * * * tr * * * * * * tr *
a-Humulene 1660 1453 0.1 0.1 0.1 0.1 0.1 tr 0.1 tr tr tr * tr tr tr
Neral 1674 1213 * tr tr * * * * * tr * 0.1 * 0.3 *
a-Terpineol 1688 1172 0.3 0.3 0.2 0.2 0.2 0.3 0.6 0.2 0.4 0.4 0.3 0.5 0.7 0.5
a-Bisabolene 1724 1496 * * * * * 0.1 * 0.4 * 0.5 0.4 0.4 * *
Neryl acetate 1725 1340 * * * * * * * * * * * * 0.3 *
Bicyclogermacrene 1727 1494 * 0.1 0.1 0.1 * * 0.1 * * * * * * 0.4
Geranyl acetate 1748 1358 * tr * * * * * * * * * * * *
Citronellol 1756 1207 * * * * * 0.1 * * 0.2 * * * 0.2 *
Nerol 1790 1207 0.1 0.1 * * * tr tr tr 0.6 tr * * 0.8 *
Geraniol 1837 1232 * * * * * tr tr * tr * * tr 0.1 *
Methyl N-methylanthranilate
2082 1385 * tr tr tr 0.1 * * * * * * * * *
Thymol 2189 1266 0.4 0.3 0.2 0.1 0.1 * * 0.1 * 0.1 * 0.1 * 0.2
b-Sinensal 2225 1673 0.5 0.5 tr tr tr 1.5 * 1.3 1.1 1.5 1.3 1.2 1.7 1.4
a-Sinensal 2323 1726 0.9 0.8 tr * tr 1.6 * 0.9 0.8 0.6 0.6 0.6 0.9 0.7
Total 99.3 97.8 98.7 98.7 98.3 99.1 97.2 98.4 99.5 99.1 98.3 98.2 98.7 99.4
92
M.-L.
Lota
et
al.
/
Biochemical
Systematics
and
Ecology
29
(2001)
77
}
Table 4*continued
c-Terpinene 1241 1048 2.9 2.3 0.7 31.9 32.4 39.0 38.8 37.5 35.8 11.7 28.3 34.3 67.4 53.4
Table 4*continued
Constituents BP-20 BP-1 IA IIB
68 85 88 89 87 70 71 65 72 67 95 91 96 98
trans-p-Menth-2-en-1-ol 1618 1123 0.2 0.2 * * tr tr tr tr * * tr tr * tr
Citronellyl acetate 1654 1332 * * 0.1 0.3 0.5 * * * * * * * * *
a-Humulene 1660 1453 * * tr 0.1 0.1 0.2 0.1 0.3 0.2 * * tr tr *
Neral 1674 1213 0.1 * tr 0.4 1.5 * * 0.1 * * * 0.1 * *
a-Terpineol 1688 1172 0.3 0.6 0.3 0.1 0.2 0.1 0.1 0.3 0.5 0.3 0.4 0.5 0.1 0.6
a-Bisabolene 1724 1496 * * * * * 0.1 0.1 * 0.1 * * 0.1 0.3 0.1
Neryl acetate 1725 1340 0.3 0.3 * 1.9 2.8 * * * * * * * * *
Bicyclogermacrene 1727 1494 * * 0.1 * * * * * * 0.6 * * * *
Geranyl acetate 1748 1358 0.1 tr 0.1 1.4 3.2 * * * * * * * * *
Citronellol 1756 1207 * 0.3 7.7 0.1 0.2 * * * * * * * * *
Nerol 1790 1207 tr 0.5 * 0.3 0.6 * * tr * tr tr 0.1 tr *
Geraniol 1837 1232 tr 0.1 0.3 0.2 0.5 * * tr * 0.1 tr * * *
MethylN -methylanthranilate
2082 1385 0.1 tr * tr * * * * * * * * * *
Thymol 2189 1266 0.1 tr * * * 0.1 tr * 0.1 9.5 0.4 20.5 * 0.1
b-Sinensal 2225 1673 1.2 1.5 * * * * * * * 0.2 * * * *
a-Sinensal 2323 1726 0.5 * 0.2 * * tr * * * 0.8 * * 1.3 *
Total 98.6 97.6 97.2 97.5 99.3 98.6 99.1 97.6 97.1 97.7 98.4 98.8 98.7 99.6
94
M.-L.
Lota
et
al.
/
Biochemical
Systematics
and
Ecology
29
(2001)
77
}
Table 4*continued
c-Terpinene 1241 1048 59.7 15.2 14.5 15.7 12.0 13.4 10.7 3.0 0.1 21.4 16.5 28.6 22.6 24.1
(E)-b-Ocimene 1245 1035 2.4 9.4 9.3 10.3 11.0 10.1 0.5 4.1 1.7 0.6 0.5 0.5 0.5 0.5
linalool 1539 1082 13.7 37.6 42.9 42.4 41.4 40.9 47.0 59.3 tr 0.6 0.3 0.2 0.9 0.4
Table 4*continued
Total 99.1 98.6 98.9 99.2 99.4 97.4 98.9 98.1 98.8 99.6 99.9 99.8 98.2 99.6
!Other compounds ( tr-0.3%): camphene,a-phellandrene, octanal, 6-methylhept-5-en-2-one, nonanal, cis-limonene-1,2-oxide, trans-limonene-1,2-oxide, (E)-b-farnesene, germacrene-D, (E,E)-a-farnesene,d-cadinene, caryophyllene oxide, (E)-nerolidol, elemol, spathulenol,q-cadinol,a-cadinol, manoyl oxide.
Cultivars : Geleking (74,C. nobilis), Yellow King (75,C. nobilis), King (86,C. nobilis), Rode king (87,C. nobilis), King of Siam (80,C. nobilis), Temple ]Temple (83,C. temple), Cleopatra (82,C. reshni), Peau rugueuse (63,C. deliciosa), Temple (84,C. temple), Emperor (62,C. deliciosa), Empress (59,C. deliciosa), Swatow (78,C. tangerina), Temple Sue Linda (81,C. temple), Late Emperor (58,C. deliciosa), Redskin (68,C. tangerina), Du Japon (85,C. nobilis), Nasnaran (88, C. amblycarpa), Kunembo (89,C. nobilis), Yatsushiro (97,C. yatsushiro), Clausellina (70,C. unshiu), URSS (71,C. unshiu), Wase (65,C. unshiu), Owari (72,C. unshiu), Fuzhu (67,C. erythrosa), Sunki (95,C. sunki), Szibat (91,C. suhuiensis), Szinkom (96,C. suhuiensis), Shekwasha (98,C. depressa), Sihue Gan (73,C. suhuiensis), Ladu Ordinary (77,C. paratangerina), Brickaville (64,C. tangerina), Peau lisse (76,C. deliciosa), Vohangisahy (60,C. tangerina), Beauty of Glen Retreat (61,C. tangerina), Ladu (69,C. paratangerina), Dancy (66,C. tangerina), Ougon (99,C. suavissima), Avana Apireno (93,C. deliciosa), de Chios (92,C. deliciosa), Willow leaf (90,C. deliciosa), Commune (79,C. deliciosa), Tardivo di Ciacculli (94,C. deliciosa).
Fig. 4. Dendrogram obtained from the cluster analysis of 58 mandarin leaf oils. Samples are clustered using Ward's technique with an Euclidean distance measure.
Sabinene/linalool chemotype: 17 cultivars (no. 58,59,62,63,68,74,75,78,80}88) be-longed to this group. Essential oils were characterized by a high content of sabinene (21.2}57.3%), associated with linalool (3.1}31.0%) and terpinen-4-ol (1.5}7.9%). This cluster could be divided into three subgroups. Subgroups IA and IB are di!erentiated on the basis of sabinene content: 21}43% for seven samples (no. 58,59,62,68,78,81,85, subgroup IA) and 46}57% for the nine others (no. 63,74,75,80,82-84,86,87, subgroup IB). For most samples of subgroup IA, linalool exhibited a higher percentage (17}31%) that those of subgroup IB (3}17%). It is noticeable that the samples no. 74 and 75 exhibited higher content of (E)-b-ocimene (14.0 and 16.0% vs 5.0}7.1%). The remaining sample (no. 88, subgroup IC) exhibited an atypical composition dominated by citronellal (47.8%) with an appreciable amount of sabinene (21.2%) and moderate contents of citronellol (7.7%) and linalool (6.6%).
c-terpinene/linalool chemotype: 19 essential oils (samples no. 60,61,64}67, 69}73,76,77}89,91,95}98) belonged to this chemotype (cluster II). This cluster could be readily divided into two subgroups on the basis of linalool/c-terpinene ratio. The"rst subgroup (IIA), to which belonged seven samples (no. 60,61,64,66,69,76,77) exhibited linalool as major component (37.6}59.3%) with appreciable contents ofc-terpinene
Fig. 5. Discriminant analysis scatterplott of 58 mandarin leaf oils.
(3.0}15.7%) and thymol (8.4}16.2%). The second subgroup (IIB), to which belonged the 12 other samples (no. 65,67,70}73,89,91,95}98) exhibited an appreciable amount of
c-terpinene (28.3}67.4%) except for the oil no. 67 (11.4%). The content of linalool was not over 27.6%.
We noticed that several components were sometimes important:
f (Z)-b-ocimene (9.8% vs 0.3}0.6%) associated with a low content of (E)-b-ocimene (0.5% vs 4.1}11.0%) in the sample no. 69 (subgroup IIA);
f b-pinene (13.7}17.1% vs 4.2}8.1%) andp-cymene (14.3}16.6% vs 1.3}6.6%) in the oils no. 65, 70}72 (subgroup IIB);
f myrcene (15.5}19.4% vs 1.0}1.4%) and limonene (7.7}15.9% vs 4.0}5.2%) asso-ciated with a slightly higher content of oxygenated acyclic monoterpenes: citronel-lal, neral, neryl acetate and geranyl acetate in the oils no. 89 and 97 (subgroup IIB); f thymol (20.5% vs 0}9.5%) in the sample no. 67 (subgroup IIB);
f thymyl methyl ether (8.0}17.3% vs 0}0.4%) in the sample no. 66 of subgroup IIA and samples no. 67, 95 and 98 of subgroup IIB.
Methyl N-methylanthranilate chemotype: only six oils (no. 79, 90, 92}94 and 99) were dominated by methylN-methylanthranilate (48.0}78.7%) with appreciable amounts ofc-terpinene (0.1}28.6%) and limonene (4.6}11.8%). It is noticeable that the sample no. 99 (C. suavissima) exhibited a higher percentage of methylN-methylanthranilate (78.7%), a very low content of c-terpinene (0.1%) and an appreciable amount of
b-sinensal (2.7%).
In the literature, three di!erent compositions were described: (i) cis- and trans -linalool oxide (12}60%), associated with linalool (14}36%) for`miyacawaa,`okitsua
(C. unshiu) and`dahongpaoacultivars (C. tangerina) from China (Lin and Hua, 1992); (ii) c-terpinene (11}38%) andp-cymene (14}41%) associated withb-pinene (6}14%) (Kamiyama, 1968; Lawrence, 1995b) or linalool (23%) (Kamiyama, 1967) for samples from Japan, or associated withb-elemene (11}25%) and linalool (3}15%) (Lawrence, 1995a) for the samples from unspeci"ed cultivars (C. unshiu); (iii) methyl N-methylan-thranilate (42}52%) associated withc-terpinene (24}29%) for commercial oils (Dugo et al., 1996; Mondello et al., 1996a, b, 1997). The c-terpinene/p-cymene/b-pinene composition described for our samples no. 65, 70}72 fromC. unshiu(`wasea,` clausel-linaa,`URSSa, and`owariacultivars) was close to that reported in the literature for three samples from the same species (Kamiyama, 1968).
4. Discussion
To our knowledge, this is the"rst time that the chemical composition of peel and leaf oils of nearly 100 cultivars of mandarin from 16 species has been reported.
Peel oils of mandarin belonged to three major chemotypes: limonene, limonene/c -terpinene and linalyl acetate/limonene. Fig. 7 shows that the limonene chemotype was more widespread than the limonene/c-terpinene chemotype (80 varieties from 11 species vs 16 varieties from 6 species). The monovarietalC. yatsushiroexhibited an atypical composition dominated by linalyl acetate and limonene.
Fig. 7. Inter- and intraspeci"c di!erentiation on the basis of three chemotypes distinguished for peel mandarin oils.
For"ve species (C. clementina, C. tangerina,C. unshiu, C. templeand C. paratan-gerina), all the varieties investigated exhibited the limonene chemotype. Conversely, a chemical intraspeci"c variability was observed for C. reticulata, C. deliciosa, C. nobilisandC. suhuensis. ConcerningC. reticulataandC. deliciosa(41 and 10 samples, respectively), the limonene and limonene/c-terpinene compositions appeared at a ra-tio51and32.C. nobilisis dominated by the limonene chemotype while two samples over three, belonging to C. suhuiensis, exhibited the limonene/c-terpinene composition. Concerning the monovarietal species, the situation appears more variable. The oils fromC. erythrosaandC. reshniexhibited limonene as a major component whereas the samples fromC. amblycarpaandC. sunkion the one hand, the sample fromC. yatsushiro on the other hand showed limonene/c-terpinene and linalyl acetate/ limonene compositions respectively.
It has to be pointed out that three samples, belonging to the limonene/c-terpinene chemotype, were distinguished by atypical compositions: limonene/b-pinene for the sample no. 88 from the monovarietalC. amblycarpa, limonene/myrcene for the oil no. 89 fromC. nobilisand limonene/p-cymene for the sample no. 96 fromC. suhuiensis. In our sampling, domination of the oils by limonene was common. This has been reported before for the oils of `dancya, `malvasioa, `ortaniquea, `ellendalea and
Fig. 8. Inter- and intraspeci"c di!erentiation on the basis of three chemotypes distinguished for leaf mandarin oils.
Shaw, 1974; Koketsu et al., 1983; Dellacassa et al., 1992; Calvarano et al., 1989),
`communea,`nulesa,`orovala,`monrealacultivars fromC. clementina(Verzera et al., 1997, 1998),`praecoxacultivar fromC. unshiu(Lawrence, 1989) and for some samples from unspeci"ed varieties and/or species (Shaw, 1979; Lawrence, 1989, 1995b). The limonene/c-terpinene chemotype has been described for a great number of commer-cial oils (Verzera et al., 1992; Dugo, 1994) and for three cultivars `sandersona,
`comunaand`malvasioafromC. reticulata(Ashoor and Bernhard, 1967; Dellacassa et al., 1992). To our knowledge, the linalyl acetate/limonene, the limonene/b-pinene and the limonene/myrcene compositions have never been reported for the mandarin peel oils.
The chemical composition of the leaf oils was characterized by a great variability. The 99 oils investigated in this and the previous studies (Lota et al., 2000), exhibited three major chemotypes: sabinene/linalool, c-terpinene/linalool and methyl N-methylanthranilate. Fig. 8 shows that the sabinene/linalool composition was the most frequently observed (59 samples belonging to 8 species) followed by the c -ter-pinene/linalool chemotype (31 varieties belonging to 11 species) and the methyl N-methylanthranilate chemotype (9 varieties which belonged to 3 di!erent species). We observed a chemical variability for theC. reticulataandC. deliciosacultivars, which exhibited the three major compositions while two chemotypes were found in
C. nobilisandC. tangerinacultivars. Conversely, a great homogeneity was described forC. clementinaandC. temple(sabinene/linalool chemotype) as well asC. unshiuand
C. paratangerina(c-terpinene/linalool chemotype).
We noted that two samples fromC. nobilis(no. 74 and 75) were associated with the sabinene/linalool chemotype, but were distinguished by the presence of (E)-b-ocimene in appreciable amounts. The monovarietalC. amblycarpa (sample no. 88) was also di!erentiated by an original composition of their essential oil (citronellal/sabinene). Moreover, concerning thec-terpinene/linalool chemotype, it has to be pointed out that the four investigated samples from C. unshiu species (no. 65, 70}72) were characterized by the presence ofb-pinene at an appreciable content. The samples no. 89 and 97, belonging toC. nobilisandC. yatsushirorespectively, were discriminated by myrcene and limonene contents. The oils of C. erythrosa (sample no. 67) and C. suavissima (sample no. 99) contained appreciable amount of (E)-b-ocimene and thymyl methyl ether or (Z)-b-ocimene respectively. Finally, the oils ofC. sunkiandC. depressa (no. 95 and 98), one sample from C. tangerina (no. 66) and one from C. reticulata(no. 33) (Lota et al., 2000) produced thymyl methyl ether while one sample fromC. suhuiensis(no. 91) and six others fromC. reticulata(no. 19, 20, 25, 26, 33 and 36) (Lota et al., 2000) contained thymol.
Although the sabinene/linalool chemotype was widespread in our sampling, it was described in the literature only for `clementina, `michala, `nectarina, `suntaraa
cultivars (Fleisher and Fleisher, 1990, 1991; Karawya and Hifnawy, 1979) or for unspeci"ed cultivar (Kamiyama and Amaha, 1972) from C. reticulata. The c -ter-pinene/linalool chemotype was reported only for the oils of`dancyaor unspeci"ed cultivars fromC. reticulata(Attaway et al., 1967; Ekundayo et al., 1990) and for one sample of unspeci"ed cultivar fromC. unshiu(Kamiyama, 1967). Although the methyl N-methylanthranilate chemotype was scarse in our sampling, it is widely reported for commercial oils (Dugo et al., 1996; Mondello et al., 1996a, b, 1997) as well as for the
`baladyasample fromC. reticulata(Fleisher and Fleisher, 1990, 1991; Karawya and Hifnawy, 1979). Conversely, none of our samples contained cis- and trans-linalool oxide as major components.
Acknowledgements
The authors are indebted to the`DeHleHgueH ReHgional a` la Recherche et a` la Tech-nologie pour la Corseafor"nancial support (Convention no. 96-327), to the` Collec-tiviteH Territoriale de Corsea for a research grant (MLL) and to INRA-CIRAD of Corsica for welcome and availability of plant material.
References
Ashoor, S.H.M., Bernhard, R.A., 1967. Isolation and characterization of terpenes fromCitrus reticulata
Blanco and their comparative distribution among otherCitrus species. J. Agric. Food Chem. 15, 1044}1047.
Attaway, J.A., Pieringer, A.P., Barabas, L.J., 1967. The origin ofCitrus#avor components-III. A study of the percentage variations in peel and leaf of terpenes during one season. Phytochemistry 6, 25}32. Baaliouamer, A., Meklati, B.Y., Fraisse, D., Schar!, C., 1992. The chemical composition of some
cold-pressedCitrusoils produced in Algeria. J. Essent. Oil Res 4, 251}258.
Boelens, M.H., Jimenez, R., 1989. The composition of some mediterraneanCitrusoils. J. Essent. Oil Res. 1, 151}159.
Calvarano, I., Bovalo, F., Di Giacomo, A., 1974. L'olio essenziale di clementine. Essenze Deriv. Agrumari 44, 117}123.
Calvarano, M., Calvarano, I., Dellacassa, E., Menendez, P., Di Giacomo, A., 1989. Su alcune essenze di mandarino dell'Uruguay. Essenze Deriv. Agrumari 59, 397}406.
Castola, V., Bighelli, A., Casanova, J., 2000. Intraspeci"c chemical variability of the essential oil ofPistacia lentiscusL. from Corsica. Biochem. Syst. Ecol. 26, 79}88.
Corticchiato, M., Tomi, F., Bernardini, A.F., Casanova, J., 1998. Composition and infraspeci"c variability of essential oil fromThymus herba baronaLois. Biochem. Syst. Ecol. 26, 915}932.
Dellacassa, E., Rossini, C., Menendez, P., Moyna, P., Verzera, A., Trozzi, A., Dugo, G., 1992.Citrusessential oils of Uruguay. Part I. Composition of oils of some varieties of mandarin. J. Essent. Oil Res. 4, 265}272. Di Giacomo, A., Calvarano, I., Mangiola, C., 1982. L'olio essenziale di petitgrain clementine. Essenze Deriv.
Agrumari LII, 139}146.
Dugo, G., 1994. The composition of the volatile fraction of the italianCitrusessential oils. Perfum. Flavor. 19, 29}51.
Dugo, G., Mondello, L., Cotroneo, A., 1996. Characterisation of italianCitruspetitgrain oils. Perfum. Flavor. 21, 17}28.
Ekundayo, O., Bakare, O., Adesomoju, A., Stahl-Biskup, E., 1990. Leaf volatile oil composition of mandarin (C. reticulataBlanco) from Nigeria. J. Essent. Oil Res. 2, 329}330.
Fleisher, Z., Fleisher, A., 1990. Mandarin leaf oil (Citrus reticulataBlanco). Aromatic plants of the Holy Land and the Sinai. Part III. J. Essent. Oil Res. 2, 331}334.
Fleisher, Z., Fleisher, A., 1991.Citruspetitgrain oils of Israel. Perfum. Flavor. 16 (1), 43}47.
FormaHcek, V., Kubeczka, K.-H., 1982. Essential oils analysis by capillary gas chromatography and Carbon-13 NMR spectroscopy. Wiley, Chichester.
Gazea, F., Calvarano, I., Calvarano, M., 1998. Characteristics of newCitrushybrids essential oil,Citrus clementina]C. limon. J. Essent. Oil Res. 10, 235}239.
Huet, R., 1991. Les huiles essentielles d'agrumes. Fruits 46, 551}576.
Kamiyama, S., 1967. Studies on the leaf oils ofCitrusspecies. Part I. Composition of leaf oils fromCitrus unshiu,Citrus kokitsu, andCitrus limon. Agric. Biol. Chem. 31, 1091}1096.
Kamiyama, S., 1968. Studies on the leaf oils ofCitrusSpecies. Part II. An examination of the seasonal variation of leaf oil composition. Bull. Brew. Sci. 14, 43}47.
Kamiyama, S., Amaha, M., 1972. Studies of leaf oils ofCitrusspecies VI. Composition of leaf oils from ten
Citrustaxa and some intrageneric hybrids. Bull. Brew. Sci. 18, 17}27.
Karawya, M.S., Hifnawy, M.S., 1979. Leaf essential oils of three di!erent varieties ofCitrus reticulataBlanco growing in Egypt. Perfum. Flavor. 4 (2), 27}30.
Koketsu, M., Magaihaes, M.T., Wilberg, V.C., Donaliso, M.G.R., 1983. Oleos essenciais de frutos citricos cultivados no Brazil. Bol. Presqui EMBRAPA Cent. Tecnol. Agric. Aliment. (7), p. 21 (cited by Lawrence 1995a).
Lawrence, B.M., 1989. Mikan oil. In: Essential Oils 1981}1987, Allured Publishing Corporation. Carol Stream, Il, USA, pp. 221}222.
Lawrence, B.M., 1995a. Mandarin or tangerine oil. In: Essential Oils 1992}1994. (a) p. 43 (1976}1977); (b) pp. 45}46 (1979}1980); (c) pp. 132}135 (1988}1991); (d) pp. 41}44, 110}113 (1992}1994). Allured Publishing Corporation, Carol Stream, Il, USA.
Lawrence, B.M., 1995b. Satsuma mandarin leaf oil. In: Essential Oils 1992-1994. Allured Publishing Corporation USA, Carol Stream, Il, pp. 125}126.
Lawrence, B.M., 1996. Progress in Essential Oils. Mandarin oil. Perfum. Flavor. 21 (2), 25}28.
Lin, Z.K., Hua, Y.F., 1992. Systematic evolutional relation of chemical components of the essential oils from 11 taxa ofCitrusleaves. Acta Botanica Sinica 34, 133}139.
Lota, M-L., De Rocca Serra, D., Tomi, F., Casanova, J., 2000. Chemical variability of peel and leaf essential oils of mandarins fromCitrus reticulataBlanco. Biochem. Syst. Ecol. 28, 61}78.
Mondello, L., Dugo, P., Bartle, K.D., 1995. Automated HPLC-HRGC: a powerful method for essential oil analysis. Part V. Identi"cation of terpenes hydrocarbons of bergamot, lemon, mandarin, sweet orange, bitter orange, grapefruit, clementine and mexican lime oils by coupled HPLC-HRGC-MS (ITD). Flav. Fragr. J. 10, 33}42.
Mondello, L., Dugo, G., Dugo, P., Bartle, K.D., 1996a. On-line HPLC-HRGC in the analytical chemistry of
Citrusessential oil. Perfum. Flavor. 21, 25}49.
Mondello, L., Dugo, P., Dugo, G., Bartle, K.D., 1996b. On-line HPLC-HRGC-MS for the analysis of natural complex mixture. J. Chromatogr. Sci. 37, 174}181.
Mondello, L., Basile, A., Previti, P., Dugo, G., 1997. ItalianCitruspetitgrain oils. Part II. Composition of mandarin petitgrain oil. J. Essent. Oil Res. 9, 255}266.
Moshonas, M.G., Shaw, P.E., 1974. Quantitative and qualitative analysis of tangerine peel oil. J. Agric. Food Chem. 22, 282}284 (cited by Lawrence 1995a).
Ortiz Marcide, J.M., Tadeo Illuch, J.L., Diaz Illamos, F.J., Adam Estelles, A., 1983. Etudes des huiles essentielles de feuilles du groupe de la mandarine cleHmentine. Utilisation taxonomique. Fruits 38, 125}131.
Retamar, J.A., 1986. In: Verghese, J. (Ed.), Essential Oils from Aromatic Species, on Essential Oils,. Synthite Industrial Chemicals Pte Ltd., Kolenchery, India, pp. 123}279 (cited by Lawrence 1995a).
Ruberto, G., Biondi, D., Piattelli, M., Rapisarda, P., Starrantino, A., 1993. Pro"les of essential oils of new
Citrushybrids. Flav. Fragr. J. 8, 179}184.
Ruberto, G., Biondi, D., Piattelli, M., Rapisarda, P., Starrantino, A., 1994. Essential oils of newCitrus
hybrids,Citrus clementina]C. limon. J. Essent. Oil Res. 6, 1}8.
Ruberto, G., Renda, A., Piattelli, M., Rapisarda, P., Starrantino, A., 1997. Essential oil of two new pigmentedCitrushybrids,Citrus clementina]Citrus sinensis. J. Agric. Food Chem. 45, 467}471. Salgueiro, L., Vila, R., Tomi, F., Tomas, X., Can8igueral, S., Casanova, J., Proenc7a da Cunha, A., Adzet, T.,
1997. Composition and intraspeci"c variability of the essential oil ofThymus camphoratus. Phytochemis-try 45, 1177}1183.
Shaw, P.E., 1979. Review of quantitative analyses ofCitrusessential oils. J. Agric. Food Chem. 27, 246}257. Tanaka, T., 1961. In: Citologia: Semi centennial commemoration papers onCitrus studies. Citologia
supporting fondation. Osaka, Japan, pp. 114.
Tomi, F., Bradesi, P., Bighelli, A., Casanova, J., 1995. Computer-aided identi"cation of individual compo-nents of essential oils using carbon-13 NMR spectroscopy. J. Magn. Reson. Anal. 1, 25}34. Verzera, A., Cotroneo, A., Stagno d'Alcontres, I., Donato, M.G., 1992. On the genuineness of essential oils.
Part XXX. Detection of distilled essential oils added to cold-pressed mandarin essential oils. J. Essent. Oil Res. 4, 273}280.
Verzera, A., Mondello, L., Trozzi, A., Dugo, P., 1997. On the genuineness ofCitrusessential oils. Part LII. Chemical characterization of essential oil of three cultivars ofCitrus clementineHort. Flav. Fragr. J. 12, 163}172.
Verzera, A., Trozzi, A., Mondello, L., Dellacassa, E., Lorenzo, D., 1998. Uruguayan essential oil. Part X. Composition of the oil ofCitrus clementineHort. Flav. Fragr. J. 13, 189}195.
Yajima, I., Yanai, T., Nakamura, M., Hayashi, K., 1979. Compositions of the volatiles of peel oil and juice fromCitrus unshiu. Agric. Biol. Chem. 43, 259}264.
Yamanishi, T., Kobayashi, A., Mikumo, Y., Nakasone, Y., Kita, M., Hattori, S., 1968. Composition of peel oil fromCitrus unshiu. Agric. Biol. Chem. 32, 593}598.