Allosterically Modulates Nicotinic Receptors: Effects
on the Course of Alzheimer’s Disease
Joseph Coyle and Paul Kershaw
Despite the proven efficacy of acetylcholinesterase inhib-itors in Alzheimer’s disease, there is a need for new and more effective treatments. Galantamine is a novel treat-ment for Alzheimer’s disease that inhibits acetylcholines-terase and modulates nicotinic receptors. In randomized, double-blind, placebo-controlled studies of up to 6 months duration, galantamine significantly improved cognitive function. Galantamine also had beneficial effects on in-strumental and basic activities of daily living, and post-poned the progression of behavioral symptoms. Patients who completed one of the 6-month, placebo-controlled studies were eligible to enter a 6-month, open-extension study of the 24-mg/day dose of galantamine. At the end of 12 months, cognitive function and activities of daily living were preserved in those patients who had been treated throughout the study with galantamine 24 mg/day. At 12 months, this group of patients had significantly better cognitive functions than patients who had been treated with a placebo for 6 months before receiving galantamine. These studies indicate that galantamine postpones the progression of symptoms in Alzheimer’s disease. Since galantamine shows the greatest benefits when treatment is started early, its long-term benefits may result from an effect on the underlying disease process; such an effect might be mediated by galantamine’s concomitant action on nicotinic receptors. Biol Psychiatry 2001;49: 289 –299 © 2001 Society of Biological Psychiatry
Key Words: Galantamine, Alzheimer’s disease, nicotinic
receptors, activities of daily living, behavioral symptoms
Introduction
A
lzheimer’s disease (AD) has become a major public health issue (Jeste et al 1999). In the United States, AD is estimated to cost more than $100 billion annually in direct and indirect costs (Ernst and Hay 1994; Schumock1998); the aging of our population will escalate this cost. The economic burden of AD together with the impact of the illness on patients, family members, and other care-givers makes the search for effective long-term treatments a high priority.
The median survival for patients with AD is approxi-mately 8 years from the onset of symptoms (Barclay et al 1985). During this time, patients invariably exhibit func-tional as well as cognitive decline (Gauthier et al 1997). The majority of patients will also develop behavioral disturbances (Teri et al 1989). These noncognitive aspects of the illness often cause a change in patients’ level of care: independent living gives way to dependence on family members or assisted living, and often to skilled care or placement in a nursing home (Brodaty et al 1993; The Canadian Study of Health and Aging 1994; Teri et al 1989).
Central cholinergic deficits are thought to contribute to the development of cognitive impairment as well as some of the behavioral symptoms associated with AD (Bartus et al 1982; Coyle et al 1983; Cummings and Kaufer 1996). Symptomatic pharmacotherapy for AD has therefore been directed at enhancing cholinergic neurotransmission in the central nervous system (CNS). To date, the most success-ful pharmacologic strategy has been the inhibition of acetylcholinesterase (AChE), the enzyme responsible for catabolizing acetylcholine (ACh) in the synaptic cleft. Three cholinesterase inhibitors— donepezil, rivastigmine, and tacrine—are currently available in the United States for the treatment of AD. These treatments improve cog-nitive and global function in randomized controlled stud-ies of 6 months duration (Corey-Bloom et al 1998; Knapp et al 1994; Rogers et al 1998b; Ro¨sler et al 1999). Results from either open-label studies or retrospective analyses suggest that donepezil and tacrine have beneficial effects on behavioral symptoms (Kaufer et al 1996; Mega et al 1999; Raskind et al 1997); prospective, double-blind data are required to confirm these findings.
Since the nature of AD is progressive, treatments that slow the decline in cognitive and daily function and delay the development of behavioral symptoms are desirable. From the Harvard Department of Psychiatry, Belmont, Massacusetts (JC) and
Janssen Research Foundation, Titusville, New Jersey (PK).
Address reprint requests to Joseph T. Coyle, M.D., Chairman, Consolidated Department of Psychiatry, Harvard Medical School, McLean Hospital, 115 Mill Street, Belmont MA 02478.
Received May 30, 2000; revised September 28, 2000; accpted November 1, 2000.
© 2001 Society of Biological Psychiatry 0006-3223/01/$20.00
Galantamine is a novel treatment for AD, which inhibits AChE (Bores et al 1996) and modulates nicotinic ACh receptors (nAChRs) (Schrattenholz et al 1996). The main focus of this review is on the results of pivotal phase III studies to assess galantamine’s effects on the course of AD.
Pharmacology of Galantamine
Galantamine increases the availability of ACh in the cholinergic synapse by competitively inhibiting AChE, the enzyme responsible for its breakdown (Bores et al 1996; Thomsen et al 1991a). Galantamine has more than a 10-fold selectivity for AChE relative to butyrylcholinest-erase (Thomsen and Kewitz 1990), which is in contrast to nonselective agents such as tacrine and physostigmine (Thomsen et al 1991b). The inhibition of AChE ceases within 24 hours of discontinuing galantamine (Thomsen and Kewitz 1990), so that anesthetic agents and muscle relaxants, if required, can be safely administered within a short period of stopping galantamine.
Galantamine also potentiates cholinergic neurotrans-mission by positively modulating the response of nAChRs to ACh (Albuquerque et al 1997; Schrattenholz et al 1996). Galantamine is an allosteric potentiating ligand because it acts at a site on the nAChR that is different from the ACh-binding site (Maelicke, in press; Schrattenholz et al 1996). Galantamine appears to enhance both pre- and postsynaptic nAChR function by making nAChRs more sensitive to available ACh (Albuquerque et al 1997; Maelicke, in press; Schrattenholz et al 1996). Submicro-molar concentrations of galantamine increase the fre-quency of opening of nicotinic receptor ion channels, thereby potentiating submaximal ACh-activated currents. Studies have shown that the potentiating effect of galan-tamine on nAChRs is maintained in cell models that are devoid of AChE (Schrattenholz et al 1996), indicating that the modulatory effect of galantamine on nAChRs is not related to its AChE activity (Schrattenholz et al 1996).
There are theoretical reasons for expecting that galan-tamine’s effects on nAChRs might reduce the symptoms of AD. As well as controlling the release of ACh, presynaptic nAChRs also modulate the release of gluta-mate and monoamines, such as serotonin and norepineph-rine (Albuquerque et al 1997; Levin and Simon 1998; Alkondon et al 1999). These neurotransmitter systems are also affected in AD and appear to contribute to the cognitive and behavioral symptoms of the illness (Table 1). Thus, activating nAChRs may help to correct these neurochemical deficits, thereby alleviating symptoms of AD (Maelicke, in press). There are also some data to suggest that activation of nAChRs may modify disease progression in AD by reducing neuronal vulnerability to the cytotoxic effects of amyloid and by reducing its formation (Kihara et al 1998).
Enhancing nAChR function may be particularly impor-tant in the early stages of AD. Recent data suggest that in the early phase of the illness the symptoms of AD are not primarily caused by a loss of cholinergic function (Davis et al 1999). It has therefore been suggested that strategies aimed at enhancing the function of noncholinergic sys-tems, such as the glutamatergic system, may be effective for early cognitive decline (Davies 1999). One approach would be to activate nAChRs because they augment the release of glutamate (Albuquerque et al 1997; Levin and Simon 1998).
Combining pharmacologic mechanisms has become a popular and successful strategy for treating other CNS disorders, including depression (Stahl 1997) and schizo-phrenia (Worrel et al 2000). Moreover, for Parkinson’s disease combining two dopaminergic agents with different mechanisms (L-dopa and a dopamine agonist) often be-comes the mainstay of treatment as the disease progresses (Olanow and Koller 1998). Allosteric modulators, by themselves, have low intrinsic potential to activate nAChRs. Thus, together with AChE inhibition, allosteric modulation may produce a robust enhancement of phasic neurotransmission at nAChRs, thereby avoiding
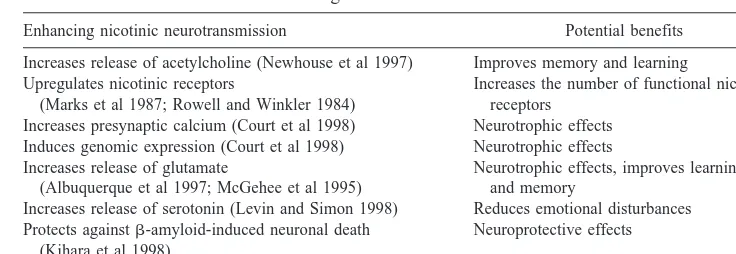
nonselec-Table 1. Potential Benefits of Enhancing Nicotinic Neurotransmission
Enhancing nicotinic neurotransmission Potential benefits
Increases release of acetylcholine (Newhouse et al 1997) Improves memory and learning Upregulates nicotinic receptors
(Marks et al 1987; Rowell and Winkler 1984)
Increases the number of functional nicotinic receptors
Increases presynaptic calcium (Court et al 1998) Neurotrophic effects Induces genomic expression (Court et al 1998) Neurotrophic effects Increases release of glutamate
(Albuquerque et al 1997; McGehee et al 1995)
Neurotrophic effects, improves learning and memory
Increases release of serotonin (Levin and Simon 1998) Reduces emotional disturbances Protects againstb-amyloid-induced neuronal death
(Kihara et al 1998)
Neuroprotective effects
tive overstimulation of nAChRs. An allosteric potentiating ligand, such as galantamine, may therefore circumvent the problem of nicotinic agonist–induced desensitization of the nAChR and consequently the development of toler-ance and loss of clinical efficacy (Maelicke, in press). This raises the possibility that combining allosteric modulation of nAChRs with inhibition of AChE may result in better long-term efficacy in the treatment of AD than AChE inhibition alone (Maelicke, in press).
Preclinical Evidence
Several lines of evidence indicate that galantamine has cognitive-enhancing effects. In particular, Sweeney and colleagues (Sweeney et al. 1988, 1989, 1990) have shown that galantamine attenuates cognitive deficits in mice. In these studies, galantamine has been shown to be highly specific for AChE and to have a relatively long duration of action (Sweeney et al 1988). In mice with selective excitotoxic lesions of the nucleus basalis that produced substantial cortical cholinergic deficits, treatment with galantamine reversed impairments in the 24-hour retention of passive avoidance and in the working memory compo-nent of the Morris water maze (Sweeney et al 1990). Notably, behavioral tolerance did not occur with repeated doses of galantamine; in fact, prior doses of galantamine appeared to have a priming effect on later performance. These characteristics led to the proposal that galantamine might be an effective drug for the treatment of AD (Sweeney et al 1988, 1990).
Methods
Patients
Patients who were included in galantamine studies had:
• A diagnosis of probable AD according to the
Na-tional Institute of Neurologic and Communicative
Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria (Mc-Khann et al 1984).
• Mild to moderate dementia, which was confirmed by
a Mini-Mental State Examination (MMSE) (Folstein et al 1975) score of 11–24 and a score of$12 on the Cognitive subscale of the Alzheimer’s Disease As-sessment Scale (ADAS-cog) at screening (Rosen et al 1984). In one study (GAL-USA-10), patients had to have an MMSE score of 10 –22 and an ADAS-cog score of $18 at screening.
• No clinical evidence of other causes of cognitive
impairment.
• A responsible caregiver who, together with the
pa-tient (or appropriate representative), provided written informed consent to participate in the study. To increase the external validity of the clinical data, patients with concomitant diseases such as hypertension, heart failure (New York Heart Association score I–II), non–insulin dependent diabetes mellitus, and hypothyroid-ism were included in these studies, provided their comor-bid condition was well controlled.
Design
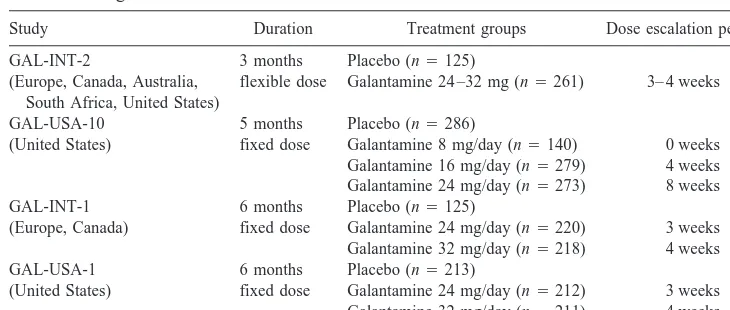
The design of these pivotal phase III studies are given in Table 2. These studies were randomized, double blind, and placebo controlled. They were multicenter studies that were conducted in the United States, Canada, Europe, Australia, and South Africa (Table 2). At the end of GAL-USA-1, patients who still met the inclusion criteria were eligible to enter a 6-month extension study using the 24-mg dose of galantamine (GAL-USA-3).
Outcome Measures
Cognitive function was the main measure of efficacy, in keeping with Food and Drug Administration guidelines
Table 2. Design of Pivotal Phase III Galantamine Studies
Study Duration Treatment groups Dose escalation period
GAL-INT-2 3 months Placebo (n5125) (Europe, Canada, Australia,
South Africa, United States)
flexible dose Galantamine 24 –32 mg (n5261) 3– 4 weeks GAL-USA-10 5 months Placebo (n5286)
(United States) fixed dose Galantamine 8 mg/day (n5140) 0 weeks Galantamine 16 mg/day (n5279) 4 weeks Galantamine 24 mg/day (n5273) 8 weeks GAL-INT-1 6 months Placebo (n5125)
(Europe, Canada) fixed dose Galantamine 24 mg/day (n5220) 3 weeks Galantamine 32 mg/day (n5218) 4 weeks GAL-USA-1 6 months Placebo (n5213)
(Leber 1990). This was measured by ADAS-cog, which assesses memory, attention, language, and orientation (Rosen et al 1984). An overall assessment of response to treatment was measured with the Clinician’s Interview-Based Impression of Change plus Caregiver Input (CIBIC-plus) (Schneider et al 1997). The CIBIC-plus was scored by a trained clinician based on separate interviews with the patient and the caregiver. Scores ranged from 1 to 7 (1, markedly improved relative to baseline; 7, markedly worse). Two different scales were used to assess patients’ activities of daily living (ADL): the disability assessment for dementia (DAD) scale (Ge´linas et al 1999) and a 23-item version of the Alzheimer’s Disease Cooperative Study Activities of Daily Living (ADCS/ADL) inventory (Galasko et al 1997). Behavioral symptoms were assessed by the Neuropsychiatric Inventory (NPI) (Cummings et al 1994).
Statistical Analyses
The primary statistical assessment for efficacy was ob-served case (OC) analysis, which includes data from assessments made while the patient is on the study drug at designated times. All the results that are reported in this review are for this analysis, unless otherwise stated. Also, intention to treat (ITT) analyses were performed using data from patients who provided postbaseline data while on treatment. Missing data in the ITT analyses were handled by the last observation carried forward method. For ADAS-cog, DAD, ADCS/ADL inventory, and NPI, efficacy assessments were based on mean change from
baseline score; for CIBIC-plus, efficacy was based on CIBIC-plus scores at 6 months.
Results
Galantamine: Effects on Cognitive Decline
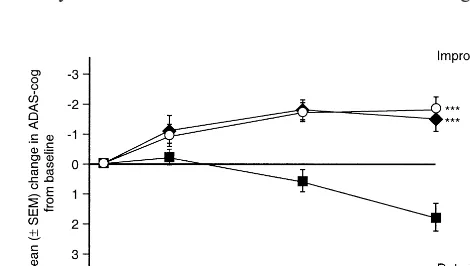
In all four phase III studies, galantamine produced signif-icant benefits on cognitive function relative to a placebo for both OC and ITT analyses (Table 3). In the 5-month study and both 6-month studies, the placebo groups experienced a significant decline in cognitive function relative to baseline (1.8 –2.4 points on ADAS-cog, p ,
.05) (Raskind et al 2000; Tariot et al 2000b; Wilcock et al, in press). In contrast, patients in the galantamine 16-, 24-, and 32-mg/day groups maintained a significant improve-ment in cognitive function relative to baseline (p,.05 for all three groups) (Figures 1 and 2). Furthermore, a pooled analysis of the two 6-month studies showed that
galan-Figure 1. Mean change in the Cognitive subscale of the Alzhei-mer’s Disease Assessment Scale (ADAS-cog) over 6 months (GAL-USA-1).E, galantamine 24 mg/day; Œ, galantamine 32 mg/day;■, placebo. ***p#.001 vs. placebo. (Reproduced with permission from Raskind et al 2000.)
Figure 2. Mean change in the Cognitive subscale of the Alzhei-mer’s Disease Assessment Scale (ADAS-cog) over 5 months (GAL-USA-10).E, galantamine 24 mg/day;l, galantamine 16 mg/day;■, placebo. ***p#.001 vs. placebo. (Reproduced with permission from Tariot et al 2000b.)
Table 3. Effects of Galantamine on Cognitive Function in Phase III Studies
Study Treatment groups
Mean change from baseline ADAS-cog score
ITT OC
GAL-INT-2 Placebo 10.6 10.5 Galantamine 24 –32 mg 21.1a 21.4a GAL-USA-10 Placebo 11.7 11.8
Galantamine 8 mg/day 10.4 10.1b Galantamine 16 mg/day 21.4c 21.5c Galantamine 24 mg/day 21.4c 21.8c GAL-INT-1 Placebo 12.2 12.4
Galantamine 24 mg/day 20.6c 20.7c Galantamine 32 mg/day 21.3c 21.7c GAL-USA-1 Placebo 12.0 12.2
Galantamine 24 mg/day 21.9c 21.7c Galantamine 32 mg/day 21.4c 21.6c
Negative change score on the Cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog) indicates improvement, positive change score indicates deterioration. ITT, intention-to-treat analyses; OC, observed case analy-ses.
ap
#.01 for galantamine-placebo difference. bp
#.05 for galantamine-placebo difference. cp
tamine maintained significant improvements in cognitive function regardless of whether patients had mild or mod-erate disease (Lilienfeld and Parys, in press) (Figure 3).
The CIBIC-plus is judged by an investigator who is blind to all other assessments and therefore is a means of confirming the clinical relevance of any benefits observed on cognitive function (Committee for Proprietary Medic-inal Products 1997). In all of these studies, galantamine produced a significantly better overall response to treat-ment than the placebo, as judged by CIBIC-plus (Raskind et al 2000; Tariot et al 2000b; Wilcock et al, in press; Wilkinson et al 2000).
The Long-Term Effects of Galantamine on Cognitive Decline
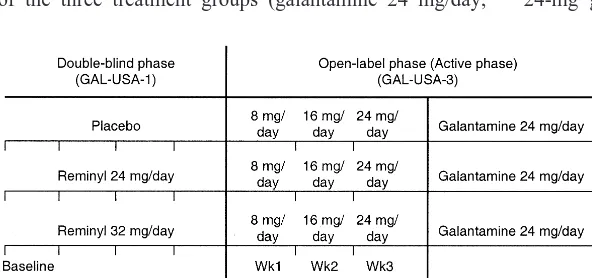
Patients who completed the 6-month United States study were eligible to enter a 6-month, open-label study (Ras-kind et al 2000). Approximately 80% of patients from each of the three treatment groups (galantamine 24 mg/day,
galantamine 32 mg/day, or placebo) who completed the double-blind study entered the extension phase (n5353), and 268 patients completed it. All of these patients received a 24-mg dose of galantamine regardless of the treatment they had received in the double-blind phase (Figure 4). During the extension phase, investigators remained blinded to the treatment patients had received during the double-blind phase. This design (staggered start design) has been put forward as a means of identifying whether a new treatment affects the course of AD (Leber 1996).
At 12 months the group of patients who had received the 24-mg dose of galantamine in the double-blind phase had preserved cognitive function, as indicated by a mean change from baseline ADAS-cog score that was not significant on both ITT and OC analyses (Figure 5). This stabilizing effect on cognitive function was seen for both mild (MMSE$18) and moderately (MMSE,18) severe stages of the illness. There was a significant difference in mean change from baseline ADAS-cog score between this 24-mg galantamine group and those patients who had
Figure 3. Mean change in the Cognitive subscale of the Alzhei-mer’s Disease Assessment Scale (ADAS-cog) at 6 months according to baseline disease severity (pooled data from GAL-INT-1 and GAL-USA-1). MMSE, Mini-Mental State Examina-tion;h, placebo;s, galantamine 24 mg/day;■, galantamine 32 mg/day. *p# .05 vs. baseline. (Reproduced with permission from Lilienfeld and Parys 2000b.)
Figure 4. Design of GAL-USA-1,3.
received a placebo in the double-blind phase (p5.03 for OC analysis, p5.05 for ITT analysis). Patients who had received galantamine 32 mg/day in the double-blind phase experienced a modest deterioration in ADAS-cog score by 12 months (1.8 points). The reduction in dose may have resulted in a rebound effect and therefore some loss of efficacy.
It has been proposed that if a treatment only has symptomatic effects, placebo-treated patients who are then placed on the active drug should approach the perfor-mance of those who were taking the active drug from the beginning of the trial (Grundman and Thal 1998; Leber 1996) (Figure 6). At the end of 12 months, patients who received galantamine 24 mg/day throughout the trial had significantly better cognitive function than those who received a placebo for the first 6 months. These results suggest that galantamine slows the underlying disease progress, which may reflect its concomitant action on nAChRs (Maelicke, in press). However, further galan-tamine studies, designed specifically to assess disease-modifying effects, are required to test this possibility.
Even allowing for the possible biases inherent in an open-label study, these results are very encouraging. A stable dose of galantamine 24 mg/day appears to postpone the decline in cognitive function for at least 12 months. This is a clinically important effect, particularly in a disease in which patients typically survive 8 years from the time of diagnosis (Barclay et al 1985). To quantify this benefit numerically, these data can be compared with either data from untreated patient cohorts or data extrap-olated from the placebo group that participated in the
double-blind phase of the study. The average annual decline in ADAS-cog for untreated AD patients is about eight points, but the rate of change is less for patients with mild AD (Stern et al 1994). In this 12-month study, linear extrapolation of the change in ADAS-cog in the placebo group at 6 months (2.2 points) suggests that the placebo group would have deteriorated by four to five points at 12 months. This latter estimate is similar to the annual change in ADAS-cog scores of four to six points reported for placebo groups from 12-month, placebo-controlled studies in patients with mild to moderate AD (Mulnard et al 2000; Torfs and Feldman 2000).
GALANTAMINE: EFFECTS ON FUNCTIONAL
DE-CLINE. In three out of four of the pivotal phase III studies galantamine showed significant benefits on ADL (Table 4). In the 3-month, phase III study (Wilkinson et al 2000), galantamine produced significant benefits on ADL as indicated by a drug–placebo difference in the mean change from baseline DAD score of 4.3 points (p5.004) (Figure 7). Moreover, in this study functional performance was preserved in galantamine-treated patients, as indicated by a DAD score that did not significantly differ from baseline. This preservation of functional activity was observed regardless of whether patients were maintained on a dose of 32 mg/day or one of 24 mg/day. In contrast, the decline in total DAD score for the placebo group was statistically significant (p , .001). Analysis of the DAD clusters showed that galantamine’s significant benefits on
Figure 6. Assessing disease modification: the randomized (stag-gered) start design.
Table 4. Effects of Galantamine on Activities of Daily Living in Phase III Studies
Study Treatment groups
Mean change from baseline ADL scorea
ITT OC
GAL-INT-2 Placebo 25.2 24.2 Galantamine 24–32 mg/day 20.4b 10.1c GAL-USA-10 Placebo 23.8 24.0
Galantamine 8 mg/day 23.2 23.1 Galantamine 16 mg/day 20.7b 20.5b Galantamine 24 mg/day 21.5c 21.6c GAL-INT-1 Placebo 25.8 25.2
Galantamine 24 mg/day 22.7 22.7 Galantamine 32 mg/day 22.4d 21.4e GAL-USA-1 Placebo 22.9 22.8
Galantamine 24 mg/day 22.7 22.9 Galantamine 32 mg/day 22.1 21.7
ADL, Activities of Daily Living; ITT, intention-to-treat analyses; OC, observed case analyses.
aFor GAL-INT-2, GAL-INT-1, and GAL-USA-1 the disability assessment for dementia scale was used. For GAL-USA-10 the Alzheimer’s Disease Cooperative Study ADL inventory was used. For both ADL scales, a positive change score indicates improvement.
bp
#.001 for galantamine–placebo difference. cp
#.01 for galantamine–placebo difference. dp
functional ability were seen for a wide range of daily activities including both instrumental and basic ADL (Figure 8).
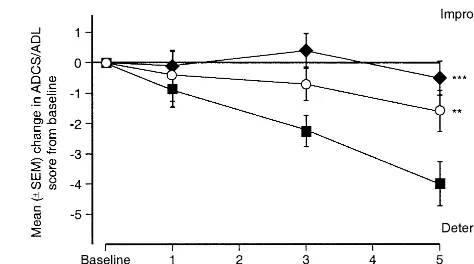
The 5-month study, conducted in the United States (Tariot et al 2000b) (Table 2), confirmed that galantamine 16- and 24-mg doses have beneficial effects on the course of ADL. The galantamine–placebo difference in the mean change from baseline ADCS/ADL score was 2.4 –3.5 points (p,.01 for both doses vs. placebo) (Table 4 and Figure 9). At the end of the study functional performance was preserved in galantamine-treated patients (16 mg/day group), whereas the placebo group experienced a signifi-cant decline (p, .05).
The positive ADL outcome in these studies provides consistent evidence of galantamine’s functional benefit. For donepezil, a significant effect on ADL has been reported in one out of four prospective, randomized, double-blind studies (Burns et al 1999; Rogers et al 1996, 1998a, 1998b). Tacrine has failed to show significant benefits on instrumental ADL, but favorable effects have
been observed on the Progressive Deterioration Scale (Knapp et al 1994). Rivastigmine has shown favorable effects on ADL, although specific benefits on basic ADL have not been reported (Corey-Bloom et al 1998; Ro¨sler et al 1999; Bentham et al 1999).
In the 6-month study conducted in the United States (Raskind et al 2000) there were no significant differences between galantamine and a placebo at 6 months on ADL. However, at the end of the open-extension phase, patients who had received galantamine 24 mg/day for 12 months had maintained their functional ability, as indicated by a mean DAD score that was not significantly different from baseline (21.7 points change from baseline). Analysis of the DAD clusters revealed that both instrumental and basic ADL had been preserved at 12 months. In contrast, patients who had received the placebo for the first 6 months experienced a significant decline in ADL (8.1 points, p,.001 vs. baseline). The failure of this group of patients to achieve the same level of functional benefit as
Figure 7. Mean change from baseline in disability assessment for dementia (DAD) scale score over 3 months (GAL-INT-2).Œ, galantamine 24 –32 mg/day;■, placebo. **p#.01 vs. placebo.
those patients who were treated continuously with tamine 24 mg provides additional evidence that galan-tamine may alter the course of AD. The group of patients who had received the 32-mg dose of galantamine in the double-blind phase also experienced functional decline by 12 months (6.2 points, p, .001 vs. baseline).
The deterioration in day-to-day functioning contributes to patients’ dependence on family members and other caregivers. The early stages of AD affect instrumental ADL, such as leisure, housework, and managing finances. In the later stages there is a progressive loss in the ability to undertake basic ADL, such as eating, dressing, and personal hygiene (Gauthier et al 1997). Galantamine’s stabilizing effects on instrumental and basic ADL for at least 12 months would therefore be expected to be an important benefit for patients and carers. Moreover, stud-ies of 12 months duration indicate that the annual decrease in DAD score in untreated patients is 11–13 points (Torfs and Feldman 2000), confirming the clinical relevance of maintaining ADL function at baseline levels.
GALANTAMINE: EFFECTS ON THE PROGRESSION OF
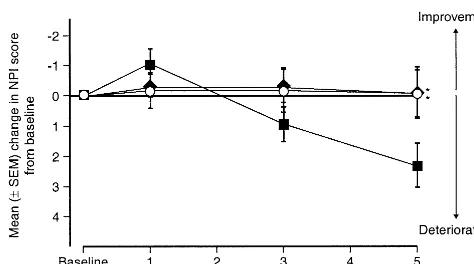
BEHAVIORAL SYMPTOMS. The available data suggest that improvements in cognitive function do not necessarily produce benefits in behavioral symptoms (Cummings 2000). The NPI scale was therefore used in the 5-month study conducted in the United States to investigate the effects of galantamine on behavioral symptoms (Tariot et al 2000a). At the end of the study galantamine 16 and 24 mg/day produced significant benefits on behavioral symp-toms, as indicated by a drug–placebo difference in the mean change from baseline NPI score of 2.4 points (p,
.05 for both doses vs. placebo) (Figure 10). Among the 10 items in the NPI scale, aberrant motor behavior, anxiety, disinhibition, and hallucinations appeared to be the main contributing factors to the overall significant therapeutic
benefits of galantamine relative to the placebo (Tariot et al 2000a). At the end of the study, the NPI scores for the 16-and 24-mg/day galantamine groups did not significantly differ from baseline values (0.1-point improvement for both doses), whereas the placebo group had experienced a significant decline (2.3 points, p,.05 vs. baseline). These data suggest that galantamine delays the progression of behavioral symptoms in patients with mild to moderate AD. Similar behavioral benefit, based on prospectively collected data from a randomized, controlled study, has to date only been reported for metrifonate (Morris et al 1998). An exploratory analysis of the noncognitive com-ponent of ADAS suggests that tacrine may also have favorable effects on behavioral symptoms (Raskind et al 1997).
Behavioral symptoms cause a great deal of distress to families and caregivers (Kaufer et al 1998) and are the most common reason for referral to a specialist and placement in a nursing home (Steele et al 1990). There-fore, postponing the progression of these symptoms is an important treatment goal that would be expected to benefit patients and their families and possibly delay nursing home placement. Although the central cholinergic system may, at least in part, mediate the behavioral symptoms of AD (Cummings and Kaufer 1996), there may be differ-ences in the psychotropic effects of different cholinergic treatments (Cummings 2000). Further studies are required to test this possibility.
GALANTAMINE: TOLERABILITY PROFILE. Galan-tamine is well tolerated. The most common adverse events are those expected from cholinergic stimulation (Table 5). Using a slow dose escalation of up to 8 weeks, the discontinuation rates due to adverse events in patients who received galantamine 16 and 24 mg were low (7% and 10%, respectively) and comparable to the discontinuation rate in the placebo group (7%) (Tariot et al 2000b). These data compare favorably with those from rivastigmine and tacrine studies (Knapp et al 1994; Ro¨sler et al 1999) and
Figure 10. Mean change from baseline in Neuropsychiatric Inventory (NPI) score over 5 months (GAL-USA-10).E, galan-tamine 24 mg/day;l, galantamine 16 mg/day;■, placebo. *p#
.05 vs. placebo. (Reproduced with permission from Tariot et al 2000b.)
Table 5. Proportion (%) of Patients with Adverse Events Occurring at Least 5% More Often during Treatment with Any Galantamine Dose than with a Placebo
Adverse event
Placebo (n5286)
Galantamine 16 mg/day (n5279)
Galantamine 24 mg/day (n5273)
Nausea 4.5 13.3 16.5
Vomiting 1.4 6.1 9.9
Anorexia 3.1 6.5 8.8
Diarrhea 5.9 12.2 5.5
Discontinuation due to adverse event (%)
7.0 7.0 10.0
are comparable with data from donepezil studies (Burns et al 1999; Rogers et al 1998b). Notably, nausea and vomit-ing, if they occurred, were generally associated with dose escalation and resolved rapidly in most subjects. Since allosteric modulators do not directly activate the nAChR and only potentiate submaximal ACh-induced responses, it has been suggested that they are less likely to cause significant unwanted effects (Maelicke, in press). The tolerability data for galantamine provide support for this hypothesis.
Conclusions
Cholinesterase inhibition has been the traditional approach to treating the symptoms of AD. Recently, nAChRs have received considerable attention as potential therapeutic targets for the treatment of AD. Activation of presynaptic nAChRs has been shown to augment the release of a number of neurotransmitters that are deficient in patients with AD, including ACh, monoamines, and glutamate (Albuquerque et al 1997; Levin and Simon 1998). More-over, there is evidence to suggest that activation of nAChRs may protect againstb-amyloid toxicity (Kihara et al 1998). Thus, treatments, such as galantamine, that activate nAChRs and inhibit AChE may have long-term efficacy superior to that of treatments that act on AChE alone.
For new medications to be effective in the long-term treatment of AD, they should have favorable and sustained effects on the cognitive, functional, and behavioral symp-toms of the illness. With these criteria as a measure of treatment success, galantamine is a very promising treat-ment for AD. In well-designed randomized, controlled studies of up to 6 months duration, galantamine signifi-cantly decreases the cognitive and functional symptoms of AD and appears to postpone the emergence of behavioral symptoms.
Historical placebo control subjects show a five- to eight-point decline in ADAS-cog scores over 1 year. In contrast, patients who received galantamine 24 mg/day for 12 months had maintained their baseline ADAS-cog score and showed only a three-point decline from their peak performance, suggesting that continuous treatment with galantamine slows the rate of cognitive decline. Moreover, patients treated with a placebo for 6 months who then receive galantamine 24 mg/day do not achieve the same level of cognitive function as those patients continuously treated with galantamine. These two observations raise the possibility that galantamine’s long-term benefits may be due to an effect on the underlying disease process. This potentially important effect, which may be due to galan-tamine’s concomitant activity on nAChRs, requires further assessment in future clinical studies.
Aspects of this work were presented at the symposium “Nicotine Mechanisms in Alzheimer’s Disease,” March 16 –18, 2000, Fajardo, Puerto Rico. The conference was sponsored by the Society of Biological Psychiatry through an unrestricted educational grant provided by Janssen Pharmaceutica LP.
References
Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrat-tenholz A, Barbosa CT, et al (1997): Properties of neuronal nicotinic acetylcholine receptors: Pharmacological character-ization and modulation of synaptic function. J Pharmacol Exp
Ther 280:1117–1136.
Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX (1999): Choline and selective antagonists identify two sub-types of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci 19:2693–2705.
Barclay LL, Zemcov A, Blass JP, Sansone J (1985): Survival in Alzheimer’s disease and vascular dementias. Neurology 35: 834 – 840.
Bartus RT, Dean RL, Beer B, Lippa AS (1982): The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408 – 414.
Bentham P, Gray R, Sellwood E, Raftery J (1999): Effectiveness of rivastigmine in Alzheimer’s disease. Improvements in functional ability remain unestablished. BMJ 319:640 – 641. Bores GM, Huger FP, Petko W, Mutlib AE, Camacho F, Rush
DK, et al (1996): Pharmacological evaluation of novel Alz-heimer’s disease therapeutics: Acetylcholinesterase inhibitors related to galanthamine. J Pharmacol Exp Ther 277:728 –738. Brodaty H, McGilchrist C, Harris L, Peters KE (1993): Time until institutionalization and death in patients with dementia. Role of caregiver training and risk factors. Arch Neurol 50:643– 650.
Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Moller HJ, et al (1999): The effects of donepezil in Alzheimer’s Disease– results from a multinational trial. Dement Geriatr Cogn
Disord 10:237–244.
The Canadian Study of Health and Aging (1994): Patterns of caring for people with dementia in Canada. Can J Aging 13:470 – 487.
Committee for Proprietary Medicinal Products (1997): Note for
Guidance on Medicinal Products in the Treatment of Alzhei-mer’s Disease. London: The European Agency for the
Eval-uation of Medicinal Products.
Corey-Bloom J, Anand R, Veach J (1998): A randomised trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr
Psychopharmacol 1:55– 65.
Court JA, Lloyd S, Thomas N, Piggott MA, Marshall EF, Morris CM, et al (1998): Dopamine and nicotinic receptor binding and the levels of dopamine and homovanillic acid in human brain related to tobacco use. Neuroscience 87:63–78. Coyle JT, Price DL, DeLong MR (1983): Alzheimer’s disease: A
Cummings JL (2000): Cholinesterase inhibitors: A new class of psychotropic compounds. Am J Psychiatry 157:4 –15. Cummings JL, Kaufer D (1996): Neuropsychiatric aspects of
Alzheimer’s disease: The cholinergic hypothesis revisited.
Neurology 47:876 – 883.
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994): The Neuropsychiatric Inven-tory: Comprehensive assessment of psychopathology in de-mentia. Neurology 44:2308 –2314.
Davies P (1999): Challenging the cholinergic hypothesis in Alzheimer disease. JAMA 281:1433–1434.
Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, et al (1999): Cholinergic markers in elderly patients with early signs of Alzheimer’s disease. JAMA 281:1401–1406. Ernst RL, Hay JW (1994): The US economic and social costs of
Alzheimer’s disease revisited. Am J Public Health 84:1261– 1264.
Folstein MF, Folstein SE, McHugh PR (1975): “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189 –198. Galasko D, Bennett D, Sano M, Ernesto C, Thomas R,
Grund-man M, et al (1997): An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Dis
Assoc Disord 11(suppl 2):S33–S39.
Gauthier S, Ge´linas I, Gauthier L (1997): Functional disability in Alzheimer’s disease. Int Psychogeriatr 9(suppl 1):163–165. Ge´linas I, Gauthier L, McIntyre M, Gauthier S (1999):
Devel-opment of a functional measure for persons with Alzheimer’s disease: The disability assessment for dementia. Am J Occup
Ther 53:471– 481.
Grundman M, Thal L (1998): Trial designs: Pharmacotherapy of Alzheimer’s disease. In: Gauthier S, editor. Pharmacotherapy
of Alzheimer’s Disease. London: Martin Dunitz, pp 185–196.
Jeste DV, Alexopoulos GS, Bartels SJ, et al (1999): Consensus statement on the upcoming crisis in geriatric mental health.
Arch Gen Psychiatry 56:848 – 853.
Kaufer DI, Cummings JL, Christine D (1996): Effect of tacrine on behavioral symptoms in Alzheimer’s disease: An open-label study. J Geriatr Psychiatry Neurol 9:1– 6.
Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, et al (1998): Assessing the impact of neuro-psychiatric symptoms in Alzheimer’s disease: The Neuropsy-chiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 46:210 –215.
Kihara T, Shimohama S, Urushitani M, Sawada H, Kimura J, Kume T, et al (1998): Stimulation of alpha4beta2 nicotinic acetylcholine receptors inhibits beta-amyloid toxicity. Brain
Res 792:331–334.
Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI, Tacrine Study Group (1994): A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA 271:985–991.
Leber P (1990): Guidelines for Clinical Evaluation of
Anti-Dementia Drugs. Washington, DC: U.S. Food and Drug
Administration.
Leber P (1996): Observations and suggestions on antidementia drug development. Alzheimer Dis Assoc Disord 10(suppl 1):31–35.
Levin ED, Simon BB (1998): Nicotinic acetylcholine
involve-ment in cognitive function in animals. Psychopharmacology 138:217–230.
Lilienfeld S, Parys W (in press): Additional benefits to patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. Maelicke A (in press): Allosteric modulation of nicotinic
recep-tors as a treatment strategy for Alzheimer’s disease. Dement
Geriatr Cogn Disord.
Marks MJ, Stitzel JA, Collins AC (1987): Influence of kinetics of nicotine administration on tolerance development and recep-tor levels. Pharmacol Biochem Behav 27:505–512.
McGehee DS, Heath MJ, Gelber S, Devay P, Role LW (1995): Nicotine enhancement of fast excitatory synaptic transmis-sion in CNS by presynaptic receptors. Science 269:1692– 1696.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984): Clinical diagnosis of Alzheimer’s dis-ease; report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34:939 –944. Mega MS, Masterman DM, O’Connor SM, Barclay TR,
Cum-mings JL (1999): The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch
Neurol 56:1388 –1393.
Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, Gulanski B (1998): Metrifonate benefits cognitive, behav-ioral, and global function in patients with Alzheimer’s dis-ease. Neurology 50:1222–1230.
Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, et al (2000): Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease. JAMA 283:1007–1015.
Newhouse PA, Potter A, Levin ED (1997): Nicotinic system involvement in Alzheimer’s and Parkinson’s diseases. Drugs
Aging 11:206 –228.
Olanow CW, Koller WC (1998): An algorithm (decision tree) for the management of Parkinson’s disease: Treatment guide-lines. American Academy of Neurology. Neurology 50(suppl 3):S1–S57.
Raskind MA, Peskind ER, Wessel T, Ding C (2000): Galan-tamine in Alzheimer’s disease—a 6-month randomized, pla-cebo-controlled trial with a 6-month extension. Neurology 54:2261–2268.
Raskind MA, Sadowsky CH, Sigmund WR, Beitler PJ, Auster SB (1997): Effect of tacrine on language, praxis, and non-cognitive behavioural problems in Alzheimer’s disease. Arch
Neurol 54:836 – 840.
Rogers SL, Doody RS, Mohs RC, Friedhoff LT, Donepezil Study Group (1998a): Donepezil improves cognition and global function in Alzheimer disease: A 15-week, double-blind, placebo-controlled study. Arch Intern Med 158:1021–1031. Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT,
Donepezil Study Group (1998b): A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzhei-mer’s disease. Neurology 50:136 –145.
Rogers SL, Friedhoff LT, Donepezil Study Group (1996): The efficacy and safety of donepezil in patients with Alzheimer’s disease: Results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia 7:293–303. Rosen WG, Mohs RC, Davis KL (1984): A new rating scale for
Ro¨sler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, et al (1999): Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomised controlled trial. BMJ 318:633– 638.
Rowell PP, Winkler DL (1984): Nicotinic stimulation of [3H]acetylcholine release from mouse cerebral cortical syn-aptosomes. J Neurochem 43:1593–1598.
Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al (1997): Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Im-pression of Change. Alzheimer Dis Assoc Disord 11(suppl 2):S22–S32.
Schrattenholz A, Pereira EF, Roth U, Weber KH, Albuquerque EX, Maelicke A (1996): Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol Pharmacol 49:1– 6. Schumock GT (1998): Economic considerations in the treatment and management of Alzheimer’s disease. Am J Health Syst
Pharm 55(suppl 2):S17–S21.
Stahl SM (1997): Are two antidepressant mechanisms better than one? J Clin Psychiatry 58:339 –340.
Steele C, Rovner B, Chase GA, Folstein M (1990): Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry 147:1049 –1051. Stern RG, Mohs RC, Davidson M, Schmeidler J, Silverman J,
Kramer-Ginnsberg E, et al (1994): A longitudinal study of Alzheimer’s disease: Measurement, rate and predictors of cognitive deterioration. Am J Psychiatry 151:390 –396. Sweeney JE, Bachman ES, Coyle JT (1990): Effects of different
doses of galanthamine, a long-acting acetylcholinesterase inhibitor, on memory in mice. Psychopharmacology 102: 191–200.
Sweeney JE, Hohmann CF, Moran TH, Coyle JT (1988): A long-acting cholinesterase inhibitor reverses spatial memory deficits in mice. Pharmacol Biochem Behav 31:141–147. Sweeney JE, Puttfarcken PS, Coyle JT (1989): Galanthamine, an
acetylcholinesterase inhibitor: A time course of the effects on performance and neurochemical parameters in mice.
Phar-macol Biochem Behav 34:129 –137.
Tariot PN, Kershaw P, Yuan W (2000a): Galantamine postpones
the emergence of behavioral symptoms in Alzheimer’s dis-ease: A 5-month, placebo-controlled study. Poster presented at the World Alzheimer’s Congress, Washington, DC.
Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C (2000b): A 5-month, randomized, placebo-controlled trial of galantamine in Alzheimer’s disease. Neurology 54: 2269 –2276.
Teri L, Borson S, Kiyak HA, Yamigashi M (1989): Behavioral disturbance, cognitive dysfunction and functional skill. Prev-alence and relationship in Alzheimer’s disease. J Am Geriatr
Soc 37:109 –116.
Thomsen T, Kaden B, Fischer JP, Bickel U, Barz H, Gusztony G, et al (1991a): Inhibition of acetylcholinesterase activity in human brain tissue and erythrocytes by galantamine, phy-sostigmine and tacrine. Eur J Clin Chem Clin Biochem 29:487– 492.
Thomsen T, Kewitz H (1990): Selective inhibition of human acetylcholinesterase by galanthamine in vitro and in vivo. Life
Sci 46:1553–1558.
Thomsen T, Zendeh B, Fischer JP, Kewitz H (1991b): In vitro effects of various cholinesterase inhibitors on acetyl- and butyrylcholinesterase of healthy volunteers. Biochem
Phar-macol 41:139 –141.
Torfs K, Feldman H (2000, July): 12-month decline in cognitive and daily function in patients with mild-to-moderate Alzhei-mer’s disease: Two randomized, placebo-controlled studies. Poster presented at World Alzheimer Congress, Washington, DC.
Wilcock GK, Lilienfeld S, Gaens E (in press): Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: A multicentre randomised controlled trial. BMJ.
Wilkinson D, Lilienfeld S, Truyen L (2000, April): Galantamine improves activities of daily living in patients with Alzhei-mer’s disease: A 3-month placebo-controlled study. Poster presented at the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy, Stockholm.
Worrel JA, Marken PA, Beckman SE, Ruehther VL (2000): Atypical antipsychotic agents: A critical review. Am J Health